1Department of Biotechnology, IMS Engineering College, Ghaziabad, UP, India
2Amity Institute of Biotechnology, Amity University Raipur, Chhattisgarh, India
3Department of Food Process Engineering, Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad India
Corresponding author email: skmiet@gmail.com
Article Publishing History
Received: 18/04/2020
Accepted After Revision: 18/06/2020
Now a days, many industries like textile, food and pharmaceuticals use solid dry wastes as substrate for fermentation. In the solid-state fermentation (SSF), solid surface matrix in the absence or nearly in the absence of available free water used to carry out fermentation process. In this, microorganisms are grown in the absence of free flowing aqueous phase. A traditional way for the production of α-amylase in the past was the use of submerged fermentation (SmF) which is now replaced with SSF. The alternative of SmF is SSF in a variety of industrial production like enzymes, biofuels, single cell proteins, organic acids, antibiotics, aroma, biopestistdes etc. Earlier, SSF was the choice of fermentation process only where the choice of microorganisms is fungi, but now variety of bacterial strains are also being used in such processes. SSF facilitates the natural habitat of microorganisms and has a better choice due to its simplicity, relatively low capital investment and operating cost and less water output, etc. SSF with an agro-industrial residues have also replaced the high cost media used in submerged fermentation for α-amylase production. Agro-industrial residue utilization by filamentous fungi provides an alternative source for utilization of these substrates as a solid substrate for production of amylase and other useful industrial products. In this review we tried to study various solid substrates used for fungal amylase production, their potential, and optimization strategies
Solid substrate, SSF, bio-processing, agricultural waste, α-amylases
Mishra S. K, Kumar P. R, Singh R. K, Singh A. K. Agro-Industrial Residues as Solid Substrate for Α-Amylase Production Using Solid State Fermentation by Filamentous Fungi: A Review. Biosc.Biotech.Res.Comm. 2020;13(2).
Mishra S. K, Kumar P. R, Singh R. K, Singh A. K. Agro-Industrial Residues as Solid Substrate for Α-Amylase Production Using Solid State Fermentation by Filamentous Fungi: A Review. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2ZP02RP
Copyright © Mishra et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Among many industrially important enzymes, one of the most commonly used enzymes is α-amylases, which is required for the production of many fermented foods. Apart from food and starch industries α-amylases are also having a wide application in other industries such as paper pulp and textile, etc. (Gupta et al., 2003). Amylase enzymes having a wide variety of industrial applications ranging from the conversion of starch to sugar, corn and maltose syrup for food and productions of cyclodextrins. (Kunamneni et al., 2005). Demand of amylases is also increasing in paper and pulp industry, textile industry These enzymes account for about 30% of the world’s total enzyme productions. Due to increasing demand in various industries, there is enormous interest in developing amylase with better properties, thermal stability and suitability for industrial application by using cost effective production techniques (Pandey et al., 2000). α-amylase are produced by microorganisms plants and animals, where they play a key role in carbohydrate metabolism. From centuries amylases from plant and microbial origin contatntly being used as food additives. Amylases isolated from barley have been used in brewery industries and fungal amylases have been widely used in the preparation and processing of oriental foods (Reddy et al., 2003).
Barley amylases have been used in brewery industries and fungal amylases have been widely used in the preparation and processing of oriental foods (Reddy et al., 2003). Amylases from microbial sources are used for the industrial production of various useful products due to the cost effectiveness, consistency, thermal stability and ease of process modifications and optimization. Amylases play key role in the carbohydrate metabolism and are produced by microbe, plants as well as animals also. From centuries amylases from plant and fungal origin are being constantly used as food additives. Bacteria and filamentous fungi such as Aspergillus are used for the production of α-amylase.(Ajayi et al., 2003). However the amylase of fungal origin has been found to be most stable than the bacterial enzyme. Filamentous fungi are attractive host organism for the production of commercially useful enzymes. Coproduction of two industrially important enzyme amylase and protease through SSF by Rhizopus oryzae using bread waste as a solid substrate have been studies and effect of various physical and chemical parameters were studied (Benabda et al., 2019).
Amylase production using Bacillus subtilis as a bacterial strain using solid residues such as wheat bran, rice bran, banana peel and other fruit peel for optimize the production of enzyme with minimal amount of supplementary carbon and nitrogen source (Almanaa et al., 2020). Combined effect of solid state fermentation to submerged fermentation was studied using the edible fungus Neurospora intermedia to biotransformation of ethanol and this study may be useful for the production of other industrially important products (Gmoser et al,2019).
All these types of fermentation processes agro-industrial solid wastes can be efficiently used in the form of solid substrates for SSF. The purpose of this review article is to summarize the information related to SSF for α-amylase production, which is distributed across various literatures to help scientific community. Various different types of solid substrates are used in this purpose and each substrate used to have their own merits and demerits. Hence this article will felicitate the researchers with the information regarding various solid substrates used for fungal amylase production, their potential, and optimization strategies.
Solid State Fermentation strategies for amylase production
In solid state fermentation solid matrix is used in presence of minimal water content and sometimes in absence of water to support growth and metabolic activity of microorganism. Solid matrix could be either used as support material or simply a source of nutrient content allows the growth and metabolism of microorganism. SSF provides natural habitats to the microorganisms therefore considered as a preferred choice over submerged fermentation. SmF is considered as a violation to natural living of microorganism, especially unfavorable for the growth of fungi. Scientific data reveals that more than 98% of microbial isolates from marine habitat have been obtained from the underwater surfaces of solid substrates (Kelecom et al., 2002, Holker et al., 2004).
This indicates that SSF is well adapted suited for the growth and metabolism of fungi. At laboratory level studies many research article have been published for observing various physical and chemical factors that affects on fungal metabolism. SSF provides better economic feasibility for production of amylase and other high end products by using low cost agricultural residues and resulting in solving the problem of waste disposal. Solid substrate not only supply nutrient for fungal growth it also play a key role as a support material which helps in effective growth of fungus due to its porosity, moisture holding capacity etc. These agricultural byproducts are rich in starch and lignocellulosic compound based on their origin in agricultural practices or agro-industrial sources (Pandey et al., 1992).
In present time enzyme production strategies are centered in the selection and screening of suitable solid substrate for maximum enzyme production and optimization of various physical and chemical parameters process parameters that affects the growth of fungus and enzyme production. Industrial production of amylase using SSF having advantages for growth of fungus which have capability to penetrate these solid substrate effectively. (Ramachandran et al., 2004). In addition to the utilization of these agro-industrial wastes as solid substrate in amylase production as solid substrate as well as in solving the waste disposal problem. With the improved production of lignocellulose degrading enzyme such as xylanase and cellulase SSF becoming most favorable process for cost effective production of industrially important enzymes and biofuels etc (Taherzadeh et al., 2019).
With the increasing harmful effect of traditional chemical pesticides now researchers are working on the production of biological control agents through SSF by using agro-industrial waste as substrates. Production of entomopathgens of fungal origin is being produced by SSF for adopting sustainable production method and for effective biological control method to reduce the pest eradication in the agricultural practices (Sala et al,. 2019). Now novel approaches are developing for agricultural waste treatment and further being used for up scaling of SSF processes using bacterial culture for the production of poly γ glutamic acid in continuously stirred solid state bioreactors ( Fang et al,. 2020)
Role of filamentous fungi for amylase production
However many fungal strains are commercially used for production of amylase but researchers are constantly trying to isolate novel fungal strains for amylase production. A team of researchers of Brazil isolated new fungus from the Atlantis forest region having ability to produce higher glucoamylase using agro-industrial waste as solid substrate under standardized conditions. The isolated fungi A. carbonarius showed maximum production of amylase at pH 6.0, temperature 30ºC and incubation time 96 hours when supplemented with 1% starch in the media (Pasin et al., 2020).
Since fungi have very efficient enzymatic system therefore fungi having ability to degrade lignocellulosic waste resulting in the formation of many valuable compounds that may be useful for production vast variety of useful products. Two types of extacellular enzymatic system are recognizable in fungi i.e. hydrolytic and ligninolytic system. Lignocellulosic residues from agricultural and municipal solid wastes are particularly abundant in nature and have a potential for bioconversion (Abdel-Azeem et al., 2020). .
Filamentous fungi have unique and excellent property to degrade cellulosic waste and resulting in the production of α-amylases and other industrially important enzymes, amino acids, antibiotics, organic acids etc. using SSF for past several decades. As these moulds are known for the production of extracellular products such as enzymes. Therefore filamentous fungi are widely exploited for various SSF processes for the production of a variety of industrial products including α-amylase. Production of enzymes as well as other industrially important products by solid-state fermentation (SSF) using agricultural wastes through moulds turned to be a cost-effective production technique and surprisingly most of these processes are optimized. Detailed literature is available on various fungal sources for the productions of amylases are given in the table.
Table 1. Different types of agro-substrate used for α-amylase production using SSF by Filamentous fungus
| S. No | Organism | Substrate | Activity(U/g) | References |
| 1 | Aspergillus oryzae | Spent brewing grain | 6583 | Francis et al., (2003) |
| 2 | Aspergillus oryzae | Coconut oil cake | 3388 | Vishwanathan et al., ( 2001) |
| 3 | Aspergillus flavus | Amaranthus grains | 1920 | Ramachandran et al., (2004) |
| 4 | Thermomyces lanuginosus | Wheat Bran | 534 | Kunamneni et al., (2005) |
| 6 | Aspergillus oryzae | Ground nut oil cake | 9196 | Ramachandran et al., (2004) |
| 7 | Aspergillus oryzae | Wheat Bran | 15095 | Gangadharan et al., (2007) |
| 8 | Aspergillus awamori | Corn streep liquor | 738 | Prakasham et al., (2006) |
| 9 | Penicillium fellutanum
|
Soluble starch with sea water | 157 | Kathiresan & Manivannan 2006 |
| 10 | Penicillium expansum | Loquat kernel flour | 1012 | Erdal & Taskin (2010) |
| 11 | Aspergillus sp. | wheat bran | 164 | Chimata et al., (2010) |
| 12 | Penicillium brevicompactum | Wheat bran | 666.6 | Balkan & Ertan (2010) |
| 13 | Trichothecium roseum | Wheat bran | 1048 | Balkan et al., (2011) |
| Aspergillus niger | Ipomoea batatas | 450 | Sunder et al., (2012) | |
| 15 | Aspergillus niger | Rice Bran | 334.51 | Rajasekar et al., (2013) |
| 16 | Aspergillus fumigatus | Pomegranate peel | 341.7 | Singh et al., (2014) |
| Aspergillus awamori | Casava Peel | 31.64 | Kalaiarasi & Pavatham (2015) | |
| 17 | Aspergillus oryzae | Agro-waste | 750 | Naili et al., (2016) |
| 18 | Aspergillus terreus | pearl millet | 19.19 | Sethi et al., (2016) |
| 19 | Pleurotus ostreatus
|
Potato peel waste | 2503.6 | Ergun et al., (2017) |
| 20 | Rhizopus delemar | Apple pomace | 21.03 | Pathania et al., (2018) |
| 21 | Rhizopus oryzae | Bread waste | 100 | O Benabda et al., (2019) |
| 22 | Aspergillus carbonarius | Wheat and brewing residues | 404 | Pasin et al,.(2019) |
| 23 | Aspergillus oryzae | soybean husk and flour mill | 47000 | Melnichuk et al.,(2020) |
Agricultural by products in amylase production
Microbial enzymes are versatile in nature and relatively more stable compared to plant and animal enzymes and having wide range of industrial applications. Since these agricultural residues are rich in cellulosic content along with other biologically active compounds. Therefore these agricultural wastes can be used as alternative solid substrate in SSF for the production of industrially important compounds like biofuel production, animal feed, mushroom cultivation, as well as for the production of industrial products such as enzymes, organic acids, amino acids etc.
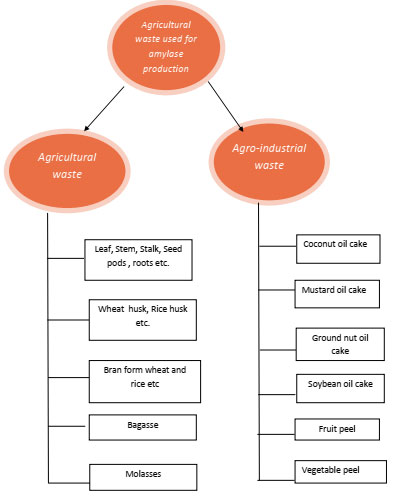
These agro industrial wastes can be used in industrial processes for the production of high value industrial products to reduce the production cost which also reduces the pollution load from the environment these agro industrial residues consist of molasses, rice husks, rice bran and wheat bran, bagasse, leaves, straw, stalk, shell, pulp, peel, roots, etc. These substrate traditionally used for animal feed, soil quality improvement, biofertilizers etc. from ancient times, But due to scientific innovations and technology development in agricultural practices huge amount of field residues are generated. Disposal of underutilized agricultural waste is problematic and non eco friendly practice. In oil exploration industries, huge amount of processed residues in the form of oil cake are produced. These residues contain high concentration of fat, carbohydrate suspended solids and as well as dissolved solids are also present. Oil cake is of different types like canola oil cake, sunflower oil cake, coconut oil cake, sesame oil cake, mustard oil 0cake, soy bean cake, groundnut oil cake etc. (Ramachandran et al., 2007). Different types of agricultural by products which have been used as solid substrate for production of various industrial products including enzymes in fermentation process are summarized in Fig-1
Figure 1: Different types of agricultural waste used in SSF as solid substrate for amylase production
These agricultural wastes are generated throughout the year through various agricultural practices and having various limiting nutrients for the growth of microorganism. Therefore, these substrates having an unlimited prospective to be used as alternative solid substrates in SSF. Researchers are constantly using these agro industrial residues for α-amylase production by using various microbial strains to optimize fermentation process parameters for enhanced production. A similar type of study was carried out by busing wheat bran as solid substrate for single cell protein and amylase production by using A. Niger as microbial strain. Maximum biomass was produced when media was supplemented with NH4Cl, NaNO3, KNO3 and (NH4)2SO4 as additional nitrogen source for enhanced growth of fungal mycelium in submerged fermentation. However media supplemented with (NH4)2SO4 showed maximum production of biomass and amylase activity i.e. 326 U/L with 6 days of fermentation
(Oshoma et al., 2019).
In many countries bread waste is easily available and disposal of these wastes is a problematic affair. To solve these issues researchers are trying to use such waste for production of enzymes i.e. amylase and protease. Rhizopus oryzae was used to degrade humidified bread waste by SSF with minimal salt solution for production of amylase and protease. Highest amylase activity was observed 100U/g and for protease 2400U/g (Benabda et al., 2019). Since fungi have very efficient enzymatic system therefore fungi having ability to degrade lignocellulosic waste resulting in the formation of many valuable compounds that may be useful for production vast variety of useful products. Two types of extracellular enzymatic system are recognizable in fungi i.e. hydrolytic and ligninolytic system. Lignocellulosic residues from agricultural and municipal solid wastes are particularly abundant in nature and have a potential for bioconversion (Abdel-Azeem et al., 2020).
Brazil is the major producer of citrus low cost source of hesperidin a polyphenolic compound having potential to restrict anti-glycation effects (AGEs) which constitute a vast variety of compounds synthesized due to interaction between amino acids of proteins and reducing sugars which induces chronic diseases through pathogenesis. This research work concludes that inhibition of AGEs results in the reduction of amylase activity 50% proving that there is strong correlation between anti-glycation with polyphenolic content and antioxidant capacity (Fernandes et al., 2020).
CONCLUSION
Agro-industrial waste are rich in various nutrient that contain bioactive compounds makes them favorable for the growth of filamentous fungi in solid state fermentation. Composition of these products ranges from sugars minerals proteins and amino acids etc. therefore these agro-industrial residues are considered as raw material in bio-conversion processes for the production of high value industrial products. The microorganisms having unique properties to use these solid substrate as raw materials for their growth and nutrition during fermentation processes and results in the formation a variety of biochemical of industrial application. SSF is relatively simpler but cost effective process for the industrial scale optimization and bio-processing. Alternatively, use of agro-industrial wastes as raw materials can help to reduce the production cost as well as recycling of waste make the environment more eco-friendly. Amylase is the most widely used enzymes among different enzymes and being constantly used in different industrial and medicinal purposes. With the development of new and novel techniques in bioprocess engineering such as Response Surface Methodology(RSM), Artificial Neural Network) ANN based optimization and Bioreactor designing for SSF leads the production of amylase at large scale for reducing the cost and efficient utilization of agro-waste. This review will facilitate for establishing the large scale fermentation process with the use of appropriate fungal strain.
REFERENCES
Abdel-Azeem, A. M., Hasan, G. A., & Mohesien, M. T. (2020). Biodegradation of Agricultural Wastes by Chaetomium species. In Recent Developments on Genus Chaetomium (pp. 301-341). Springer, Cham.
Adejuwon, A. O., Tsygankova, V. A., & Obayemi, O. S. (2019). α-Amylase Production Using Aspergilus vadensis Isolated From Pulverized Cocoa Seeds. Life Science Journal, 16(8).
Adejuwon, A. O., Tsygankova, V. A., & Obayemi, O. S. (2019). α-Amylase Production Using Aspergilus vadensis Isolated From Pulverized Cocoa Seeds. Life Science Journal, 16(8).
Ajayi, A.O. and Fagade, O.E. (2003). Utilization of corn starch as substrate for β- amylase by Bacillus sp., African J. Biomed. Res., 6 (1): 37-42.
Almanaa, T. N., Vijayaraghavan, P., Alharbi, N. S., Kadaikunnan, S., Khaled, J. M., & Alyahya, S. A. (2020). Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. Journal of King Saud University-Science, 32(2), 1555-1561.
Balkan, B and Ertan, F. (2005). Production and Properties of α‐amylase from Penicillium chrysogenum and its Application in Starch Hydrolysis. Preparative Biochemistry and Biotechnology, 35(2):169-178.
Benabda, O., M’hir, S., Kasmi, M., Mnif, W., & Hamdi, M. (2019). Optimization of Protease and Amylase Production by Rhizopus oryzae Cultivated on Bread Waste Using Solid-State Fermentation. Journal of Chemistry, 2019.
Benabda, O., M’hir, S., Kasmi, M., Mnif, W., & Hamdi, M. (2019). Optimization of Protease and Amylase Production by Rhizopus oryzae Cultivated on Bread Waste Using Solid-State Fermentation. Journal of Chemistry, 2019.
Chimata, N. K., Sasidhar, P., & Challa, S. (2010). Production of extracellular amylase from agricultural residues by a newly isolated Aspergillus species in solid state fermentation. African Journal of Biotechnology, 9(32), 5162-5169.
Erdal, S and Taskin, M. (2010). Production of α-amylase by Penicillium expansum MT-1 in solid-state fermentation using waste Loquat (Eriobotrya japonica Lindley) kernels as substrate. Romanian Biotechnological Letters, 15(3): 5342-5350.
Ergun, S. O., & Urek, R. O. (2017). Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Annals of Agrarian Science, 15(2), 273-277.
Fang, J., Huan, C., Liu, Y., Xu, L., & Yan, Z. (2020). Bioconversion of agricultural waste into poly-γ-glutamic acid in solid-state bioreactors at different scales. Waste Management, 102, 939-948.
Fernandes, A. C., Santana, Á. L., Martins, I. M., Moreira, D. K., Macedo, J. A., & Macedo, G. A. (2020). Anti-glycation effect and the α-amylase, lipase, and α-glycosidase inhibition properties of a polyphenolic fraction derived from citrus wastes. Preparative Biochemistry & Biotechnology, 1-9.
Francis, A. Sabu, K.M. Nampoothiri, S. Ramachandran, S. Ghosh, G. Szakacs, A. Pandey, Use of response surface methodology for optimizing process parameters for the production of a-amylase by Aspergillus oryzae, Biochem. Eng. J. 15 (2003) 107–115.
Francis, F., Sabu, A., Nampoothiri, K.M., Szakacs, G., Pandey, A.( 2002). Synthesis of a- amylase by Aspergillus oryzae in solid-state fermentation. J. Basic Microbiol. 42, 320–326.
Gangadharan, D., Nampoothiri, K. M., Sivaramakrishnan, S., & Pandey, A. (2009). Biochemical characterization of raw-starch-digesting alpha amylase purified from Bacillus amylolique faciens. Applied biochemistry and biotechnology, 158(3), 653-662.
Gmoser, R., Sintca, C., Taherzadeh, M. J., & Lennartsson, P. R. (2019). Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste Management, 97, 63-70.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V.K., Chauhan, B. (2003). Microbial α-amylases: A biotechnological perspectives. Process Biochemistry, 38: 1599-1616.
Kalaiarasi, K., & Parvatham, R. (2015). Optimization of process parameters for α-amylase production under solid-state fermentation by Aspergillus awamori MTCC 9997.
Kathiresan, K and Manivannan, S. (2006). Alpha-Amylase production by Penicillium fellutanum isolated from mangrove rhizosphere soil. African Journal of Biotechnology, 5 (10):829-832.
Kelecom, A. (2002). Secondary metabolites from marine microorganisms. Anais da Academia Brasileira de Ciências, 74(1), 151-170.
Kunamneni A., Permaul K., Singh S., Amylase production of solid state fermentation by the thermophilic fungus Thermomyces lanuginose. J. Bioscience and Bioengineering, 100 (2), 168-171, (2005).
Kunamneni, A., Kumar, K.S., Singh, S. (2005). Response surface methodological approach to optimize the nutritional parameters for enhanced production of a-amylase. African J. Biotechnol. 4 (7), 708–716.
Kunamneni, A., Perumal, K. and Singh, S. (2005). Amylase production in solid state fermentation by the thermophilic fungus Thermomyces Lanuginosus. J. Biosci. Bioeng. 100:168-171.
Melnichuk, N., Braia, M. J., Anselmi, P. A., Meini, M. R., & Romanini, D. (2020). Valorization of two agro industrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Management, 106, 155-161.
Naili, B., Sahnoun, M., Bejar, S., & Kammoun, R. (2016). Optimization of submerged Aspergillus oryzae S2 α-amylase production. Food science and biotechnology, 25(1), 185-192.
Oshoma, C. E., Eguakun-Owie, S. O., & Obuekwe, I. S. (2019). Utilization of Banana Peel as a Substrate for Single Cell Protein and Amylase Production by Aspergillus niger. African Scientist, 18(3), 143-150.
Oshoma, C. E., Okonkwo, P. O., & Obasuyi, C. (2019). Influence of nitrogen sources on the production of single cell protein and amylase by Aspergillus niger using wheat bran. SAU Science-Tech Journal, 4(1), 75-84.
Pandey, A. (1992). Recent process developments in solid-state fermentation. Process biochemistry, 27(2), 109-117.
Pandey, A., Nigam, P., Soccol, C. R., Soccol, V. T., Singh, D., & Mohan, R. (2000). Advances in microbial amylases. Biotechnology and applied biochemistry, 31, 135-152.
Pandey, A., Selvakumar, P., and Ashakumary, L. (1994), Glucose production by Aspergillus niger on rice bran is improved by addition of nitrogen sources, World J. Microbiol. Biotechnol. 10, 348-349.
Pasin, T. M., dos Anjos Moreira, E., de Lucas, R. C., Benassi, V. M., Ziotti, L. S., Cereia, M., & de Moraes, M. D. L. T. (2020). Novel amylase-producing fungus hydrolyzing wheat and brewing residues, Aspergillus carbonarius, discovered in tropical forest remnant. Folia Microbiologica, 65(1), 173-184.
Pathania, S., Sharma, N., & Handa, S. (2018). Utilization of horticultural waste (Apple Pomace) for multiple carbohydrase production from Rhizopus delemar F2 under solid state fermentation. Journal of Genetic Engineering and Biotechnology, 16(1), 181-189.
Prakasham, R. S., Subba Rao, C., Sreenivas Rao, R., & Sarma, P. N. (2007). Enhancement of acid amylase production by an isolated Aspergillus awamori. Journal of applied microbiology, 102(1), 204-211.
Rajasekar, A., & Dhamodharan, R. G. (2013). Production and optimazation of amylases using Aspergillus niger. International Journal of Scientific and Engineering Research, 4(7), 2497.
Ramachandran, S., Patel, A. K., Nampoothiri, K. M., Francis, F., Nagy, V., Szakacs, G., (2004). Coconut oil cake-a potential raw material for the production of a-amylase. Bioresource Technology, 93, 169–174
Reddy N.S., Nimmagadda A., Sambasiva Rao K.R.S. (2003), An overview of the microbial α-amylase family, African Journal of Biotechnology, 2 (12), 645-648.
Sala, A., Barrena, R., Artola, A., & Sánchez, A. (2019). Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Critical Reviews in Environmental Science and Technology, 49(8), 655-694.
Sethi, B. K., Jana, A., Nanda, P. K., DasMohapatra, P. K., Sahoo, S. L., & Patra, J. K. (2016). Production of α-amylase by Aspergillus terreus NCFT 4269.10 using pearl millet and its structural characterization. Frontiers in plant science, 7, 639.
Singh, S., & Gupta, A. (2014). Comparative fermentation studies on amylase production by Aspergillus flavus TF-8 using Sal (Shorea robusta) deoiled cake as natural substrate: Characterization for potential application in detergency. Industrial Crops and Products, 57, 158-165.
Sundar, R., Liji, T., Rajila, C., & Suganyadevi, P. (2012). Amylase production by Aspergillus niger under submerged fermentation using Ipomoea batatas. Int. J. Appl. Biol. Pharmaceut. Technol, 3(2), 175-82.
Taherzadeh-Ghahfarokhi, M., Panahi, R., & Mokhtarani, B. (2019). Optimizing the combination of conventional carbonaceous additives of culture media to produce lignocellulose-degrading enzymes by Trichoderma reesei in solid state fermentation of agricultural residues. Renewable Energy, 131, 946-955.
UHölker, M. Höfer, J. Lenz,(2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi, Appl. Microbiol. Biotechnol. 64 ,175–186.
Viswanathan, P., & Surlikar, N. R. (2001). Production of α‐amylase with Aspergillus flavus on Amaranthus grains by solid‐state fermentation. Journal of basic microbiology, 41(1), 57-64.