Department of Zoology, Adarsha Science, J. B. Arts and Birla Commerce College, Dhamangaon Railway, Amravati, Maharashtra. 444709 India
Corresponding author email: drsoqureshi@gmail.com
Article Publishing History
Received: 09/05/2020
Accepted After Revision: 20/06/2020
Although utility of insect cell lines for production of proteins of human interest has been scaled up from experiment-level to industrial production level, use of vertebrate sera is discouraged during industrial production of therapeutics. DZNU-Bm-1, a larval ovarian cell line is highly susceptible to BmNPV in MGM-448 medium supplemented with 10% Foetal Bovine Serum and can be used as IC-BEVS platform for foreign gene expression. The high cost of this medium is a serious bottleneck for use of DZNU-Bm-1 in production of therapeutics. This study reports reduction in serum dependence of this cell line and its successful adaptation to a low-cost Mitsuhashi and Maramorosch (MM) medium supplemented with 3% FBS to bring down the cost of maintaining the cell line. Adaptation of MM medium was achieved by decreasing the volume of original medium by 20% and increasing the volume of the new medium -to which the cell line was being adapted- by 20% at each passage. The initial cell density, final cell density, number of days taken to attain final cell density and population doubling time were noted at each passage. The cell line was slow to reach sufficient cell density during initial steps but showed reduced population doubling time during subsequent passages
DZNU-Bm-1; insect cell line; adaptation; population doubling time
Qureshi S. O. Adaptation of an Indigenous Insect Cell Line DZNU-Bm-1 to Mitsuhashi and Maramorosch (MM) Culture Medium Supplemented with 3% Foetal Bovine Serum. Biosc.Biotech.Res.Comm. 2020;13(2).
Qureshi S. O. Adaptation of an Indigenous Insect Cell Line DZNU-Bm-1 to Mitsuhashi and Maramorosch (MM) Culture Medium Supplemented with 3% Foetal Bovine Serum. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3dWHMtw
Copyright © Qureshi This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Insect cell lines have found application in a wide array of fields including cell biology, genetics, virology and agriculture. Insect cells have many advantages over mammalian cells in culture, being able to tolerate a broad range of environmental conditions, to proliferate and maintain physiological function in variable pH, temperature, and oxygen conditions (Akiyama et al., 2013). Their ability to grow without carbon dioxide supplementation has further simplified culture systems and has made them an economically viable alternative to mammalian cells (Irons et al., 2018).Insect cells exhibit many additional desirable characteristics like easy adaptation to suspension and serum-free culture as well as low media requirements. These qualities point to possibility of their large-scale culture (Neermann and Wagner, 1996; Donaldson and Shuler, 1998; Lynn, 2001; Ikonomou et al., 2003; Mitsuhashi and Goodwin, 2018).
Recently, Rubio et al., (2019) have suggested that pursuing development of insect cell-based foods may lead to new sustainable food products thereby accelerating the field of cellular agriculture. Trager’s (1935) success in using inorganic salts, maltose, digested egg albumin and B. mori haemolymph to maintain ovarian tissues of B. mori spawned a wave of experiments to formulate growth media for culture of insect cells. Mitsuhashi (1982, 1984) successfully modified Grace’s medium several times for cultivation of different cell lines. Supplementation with vertebrate sera like foetal bovine serum (FBS) or other proteinaceous materials has been successfully used to get high growth rate (Weiss and Vaughn, 1986). Gradually with increase in interest of researchers in insect cell culture, many serum-free media have been developed (Hink, 1991; Donaldson et al., 1998). Caron et al. (1990) reported high growth rate by increasing total Pluronic F-68 concentration to 0.3% with IPL/41 serum-free medium. Lepidopteran cell lines are now being viewed as an attractive alternative to mammalian cell lines for biomanufacturing of proteins of human interest, biopesticides and vaccines (Kost et al., 2005; Drugmand et al., 2012; Airenne et al., 2013; Van Oers et al., 2015).
Over the years the utility of insect cell lines for production of proteins of human interest has been scaled up from experiment-level to industrial production level (Elias, 2007). Use of vertebrate sera is discouraged during industrial production of therapeutics because of inherent potential risk for transmission of infectious agents as well as the heterogeneity and lack of reliability (Grillberger et al., 2009). Insect cell lines that have high susceptibility to a baculovirus can be used to develop efficient Insect Cell-Baculovirus Expression Vector Systems (IC-BEVS) (Agathos, 2010). An efficient IC-BEVS production platform can be used for foreign gene expression, production of therapeutic compounds and vaccines. DZNU-Bm-1, a larval ovarian cell line has been shown to be highly susceptible to BmNPV in MGM-448 medium (Khurad et al., 2006) and can be used as IC-BEVS platform for foreign gene expression (Khurad et al., 2013).
However, the high cost of MGM-448 medium with supplementation of 10% Foetal Bovine Serum (FBS) is a serious bottleneck for its use in production of therapeutics or other proteins of interest. The present study describes successful reduction in the serum dependence of the cell line DZNU-Bm-1 by first adapting it to MGM 448 growth medium supplemented with 3% FBS and then to a low-cost Mitsuhashi and Maramorosch (MM) medium supplemented with 3% FBS to reduce the cost of maintaining this cell line.
MATERIALS AND METHODS
Culture Media:Modified Grace’s medium MGM-448 (Mitsuhashi, 1984) and Mitsuhashi and Maramorosch medium (Mitsuhashi and Maramorosch, 1964) were prepared in laboratory. Modified Grace’s Medium (MGM-448): MGM-448 is a complex medium composed of six salts, twenty amino acids, three sugars, four organic acids, ten vitamins and four additives, inosine, cytochrome-c, fetuin and bovine plasma albumin fraction-V. Five stock solutions viz. MGM O.S.A., GMA-salt-mix-A, GMA-salt-mix-B, GMA-Vita-mix-IA, GMA-Vita-mix-IIB were prepared as described by Mitsuhashi (1984).
In order to prepare 250 ml of complete MGM-448 medium, required quantities of respective stock solutions were mixed and the double distilled water was added to make up the volume. To this solution bovine plasma albumin fraction-V, fetuin, cytochrome- c, inosine were added. The medium was supplemented with required quantity (3% or 10%) of FBS. The pH was adjusted to 6.37-6.40 with saturated KOH. The medium was sterilized by passing through 0.2 μm pore size Millipore membrane filter using negative pressure through Sartorius filter unit. No antibiotics were added to the medium.
Mitsuhashi and Maramorosch medium (MM Medium): It is among the simplest insect tissue culture media. It is a mixture of salts used in Carlson’s Balanced Salt Solution (CBSS), lactalbumin hydrolysate, TC-yeastolate and glucose supplemented with 3% Foetal Bovine Serum (FBS). For preparation of this medium, stock solution A and stock solution B of CBSS were prepared. 25 ml of each stock solution was mixed. The additives were added and the volume was made up to 250 ml by adding double distilled water. The medium was supplemented with 3% FBS. Sterilization was carried out by passing through 0.2 µm pore size Millipore membrane filters using negative pressure through Sartorius filter unit. No antibiotics were added to the medium.
Maintenance of cell line and subculturing: DZNU-Bm-1 a larval ovarian cell line from B. mori was kindly provided by Dr. A. M. Khurad. The cell line was being cultured in MGM-448 medium supplemented with 10% FBS. The cell line was maintained in the laboratory in glass tissue culture flasks, incubated at 25± 10C and passaged regularly. After some time, the cell line could be subcultured regularly by splitting the cultures in a ratio of 1:2 at an interval of 4-5 days.
Adaptation to MGM-448 medium supplemented with 3% FBS: The cell line was first adapted to MGM-448 supplemented with 3% FBS to reduce its serum dependence as described by Mitsuhashi and Grace (1969) through passaging of the cells. This was done by decreasing the volume of original medium (MGM-448 supplemented with 10% FBS) by 20% and increasing the volume of MGM-448 medium supplemented with 3% FBS by 20% at each passage. The initial cell density, final cell density, number of days taken to attain final cell density and population doubling time were noted at each passage.
Adaptation to MM Medium supplemented with 3% FBS: The cell line was subsequently adapted to MM medium supplemented with 3% FBS. The volume of MGM-448 supplemented with 3% FBS was reduced while the volume of MM medium supplemented with 3% FBS was increased during through regular passaging. During each passage, the volume of original medium was reduced by 20% while the volume of MM medium supplemented with 3% FBS was increased by 20%. The process was repeated till MGM-448 supplemented with 3% FBS was completely replaced with MM medium supplemented with 3% FBS. The initial cell density, final cell density, number of days taken to attain final cell density and population doubling time were noted at each passage.
Population Doubling Time:Cell suspension was sampled from culture flasks at the time of seeding the culture and on attainment of final cell density. The cell suspension was allowed to flow in the chambers of Neaubauer haemocytometer chamber and cells were counted in four large corner squares each of which is divided into 16 small squares. The volume of one large square is 0.1 mm3. Therefore, average cell count was multiplied by 104 to give number of cells per ml. The cell number was determined as an average of readings from two culture flasks. The population doubling time (PDT) was calculated using the exponential formula given by Hayflick (1973).
r = 3.32 (Log x2/x1)/t2-t1
where, r = Multiplication rate, x1 = Initial cell number at selected time, t1x2 = Final cell number at selected time t2, Generation time (g) = 1/r
Cell Morphology:The cell cultures were observed under MAGNUS INVI inverted phase contrast microscope and photographed regularly after they were adapted to MM medium supplemented with 3% FBS. Cell population comprised of different types. The cell size of each type was determined by ocular micrometer in the population taking about 50-100 readings. The relative percentage of each type in population was determined by counting different cell types in optical fields under the inverted microscope.
Karyotype Analysis: Karyotypic studies were carried out in healthy cell cultures during exponential cell growth phase after their successful adaptation to MM medium supplemented with 3% FBS. Demicolcine (Sigma) having a concentration of 50 μg/ml was added to culture flasks to arrest the cell division at metaphase. Final concentration of demicolcine in the culture flask was 1 μg/ml of medium. The cultures were allowed to stand at 25oC for 24-72 hours. The cells were harvested by centrifugation and the supernatant was removed. The pellet was resuspended in hypotonic solution (0.6% KCl) for 15-20 minutes. The cells were again centrifuged and the cell pellet was fixed in 50% glacial acetic acid for 10-15 minutes. The fixed cells were spread on glass slides and allowed to air dry. The slides were washed in distilled water, transferred to Acetorcein stain for 20 minutes, washed briefly in running tap water, air-dried for 12 hours, cleared in two changes of xylene and mounted in DPX. About 50 chromosome spreads were counted and spreads were photographed to determine the range of chromosomes.
RESULTS AND DISCUSSION
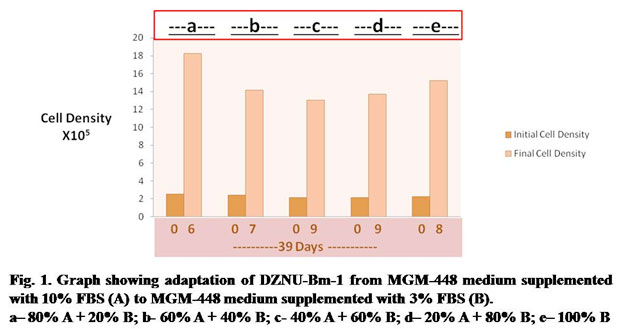
In the first stage of experiment, DZNU-Bm-1 cell line originally cultured in MGM-448 medium supplemented with 10% FBS was successfully adapted to MGM-448 medium supplemented with 3% FBS through several passages. The cell line took about 39 days for adaptation (Fig.1). DZNU-Bm-1 has been reported to take more than 10 months for adaptation to haemolymph-free MGM-448 (Bahekar, 2018). The cells remained freely suspended in culture medium. Cell clumping reported in earlier studies (Bahekar and Qureshi, 2013) was not observed. During step b of adaptation the cells showed reduced growth (Fig. 1). The growth of cells during step c and step d was slowest (Fig. 1). During each of these steps cells required 9 days to reach sufficient cell density for subculturing. The population doubling time was also highest during these steps showing a peak value of about 83 hours (Table 1). Belloncik et al. (1990) have reported slow growth rate and other difficulties during adaptation. In the present study, by step e the population doubling time was reduced to about 70 hours. After further passages in the same medium population doubling time was reduced to about 49 hours.
Table 1. Population Doubling Time of DZNU-Bm-1 during adaptation from MGM-448 medium supplemented with 10% FBS (A) to MGM-448 medium supplemented with 3% FBS (B).
| No. of Days after Seeding of Cultures | Step | Medium Composition | Population Doubling Time in Hours |
| 06 | a | 80% A + 20% B | 52 |
| 07 | b | 60% A + 40% B | 68 |
| 09 | c | 40% A + 60% B | 83 |
| 09 | d | 20% A + 80% B | 81 |
| 08 | e | 100% B | 70 |
(A)= MGM-448 medium supplemented with 10% FBS
(B)= MGM-448 medium supplemented with 3% FBS
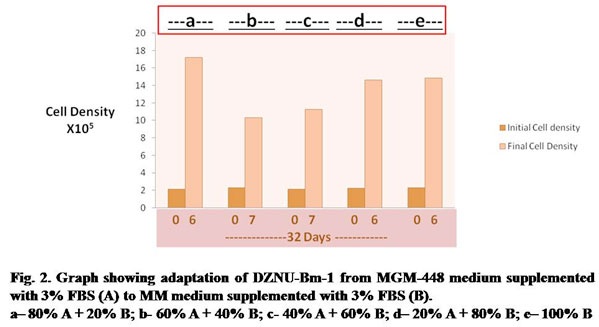
Table 2. Population Doubling Time of DZNU-Bm-1 during adaptation from MGM-448 medium supplemented with 3% FBS to MM medium supplemented with 3% FBS (B)
| No. of Days after Seeding of cultures | Step designation | Medium composition | Population Doubling time in Hours |
| 06 | a | 80% A + 20% B | 48 |
| 07 | b | 60% A + 40% B | 81 |
| 07 | c | 40% A + 60% B | 70 |
| 06 | d | 20% A + 80% B | 53 |
| 06 | e | 100% B | 54 |
(A)= MGM-448 medium supplemented with 3% FBS
(B)= MM medium supplemented with 3% FBS
The cell line was later adapted to more cost-effective MM culture medium supplemented with 3% FBS. The cell line was easily adapted to MM medium taking about 32 days for adaptation (Fig. 2). Growth of cells was slow during step b and c requiring about 7 days each for attaining sufficient cell density for subculture. Population doubling time was maximum (about 81 hours) during step b (Table 2). During next subcultures the cell line could be passaged by day 6 with population doubling time being reduced to about 54 hours. There was no significant change in cell morphology after adaptation to MM medium supplemented with 3% FBS. The population of Bm-1 cell line is heterogeneous and can be differentiated into four cell types- large round, small round, giant cells and spindle shape – on the basis of their shape and size.
Large round cells: They are round, measuring about 31 ± 0.62 μm in diameter. Their relative percentage in the population is about 30.2%. Initially, they showed partial attachment to the surface of culture flask immediately after subculture.
Small round cells: The small round cells measure about 13.74 ± 0.16 μm. They form 53.4% of the total cell population.
Giant cells: These are very large round cells having a diameter of about 69 ± 3.22 μm. They form 4.7% of the total cell population.
Spindle-shape cells:These cells have protoplasmic processes on the sides giving them appearance of a spindle (Fig. 1.11). Their size is about 51 ± 3.42 μm × 11 ± 0.51 μm. Their percentage in cell population is 11.7%.
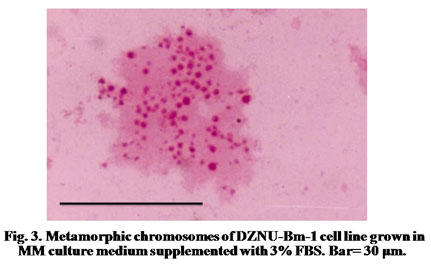
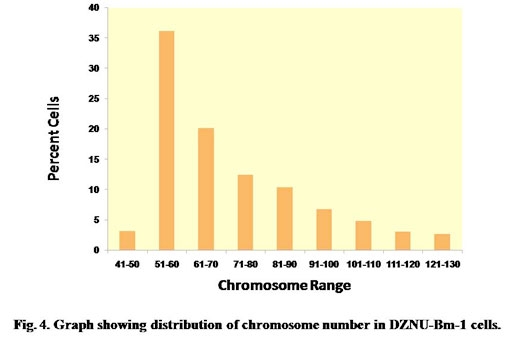
The chromosome spreads of cells exhibited numerous microchromosomes that resembled the chromosomes of DZNU-Bm-12 cell line (Khurad et al., 2009). The chromosomes appeared as numerous dot-like, darkly stained bodies (Fig. 3). The diploid number of B. mori chromosomes has been known to be 56. The chromosome number in the cell line ranged from 45 to 126 with a mode towards diploidy. A few cells also possessed more than 100 chromosomes indicating the presence of polyploid cells (Fig. 4). Grace (1967) has reported more than 100 chromosomes in the cells of B. mori ovarian cell line. Chromosome range of 35 to 150 in larval ovarian cell line and 60 to 180 in the pupal ovarian cell line of B. mori have also been reported (Sudeep et al., 2002).
CONCLUSION
In the present study DZNU-Bm-1 cell line being cultured in MGM-448 medium supplemented with 10% FBS was first adapted to MGM-448 medium supplemented with 3% FBS. The cell line was slow to reach sufficient cell density during initial steps but showed reduced population doubling time during subsequent passages. The cell line was then successfully adapted to cost effective MM medium supplemented with 3% FBS. The morphological and cultural characteristics as well as the chromosome range of the cells did not exhibit any significant change from those cultured in MGM-448 (10% FBS). DZNU-Bm-1 has earlier been shown to be able to produce foreign proteins. The current study establishes its reduced serum dependence and ability to grow in low-cost culture media raising hopes of adapting it to other serum-free media.
ACKNOWLEDGMENTS
Author is thankful to Professor Dr. A.M. Khurad, Former Head, P.G.T.D. Zoology, RTM, Nagpur University Campus, Nagpur for providing cell line and insightful guidance for this study.
REFERENCES
Agathos, S., 2010. Insect cell culture. In: Baltz, R., Demaine, A., Davies, J., Bull, A., Junker, B., Katz, L., Lynd, L., Masurekar, P., Reeves, C., Zhao, H. (Eds.), Manual of Industrial Microbiology and Biotechnology, 3rd edn. ASM Press, Washington DC, pp. 212-222.
Akiyama, Y., Sakuma, T., Funakoshi, K., Hoshino, T., Iwabuchi, K., and Morishima, K., 2013. Atmospheric-operable bioactuator powered by insect muscle packaged with medium. Lab Chip 13, 4870–4880.
Airenne, K.J., Hu, Y.C., Kost, T.A., Smith, R.H., Kotin, R.M., Ono, C., Matsuura, Y, Wang, S., Seppo, Y., 2013. Baculovirus: An insect-derived vector for diverse gene transfer applications. Molecular Therapy 21(4), 739–749.
Bahekar, R.S., 2018. Growth kinetics and baculovirus productivity of indigenously developed cell line DZNU-Bm-1 of Bombyx mori (L) in different medium. Research Directions 6(5), 67-76.
Bahekar, R.S., Qureshi, S. O., 2013. Study of some morphological and chromosomal parameters for the characterization of three indigenously developed ovarian cell lines of Bombyx mori (L). Bioscience Biotechnology Research Communications 6(1), 28-31.
Belloncik, S., Allard, C., Rouleau, D., 1991. Adaptation of a Bombyx mori cell line to serum-free media. In: Proceedings of the 8th International Conference on Invertebrate and Fish Tissue Culture, Anaheim, CA, USA, 178-180.
Caron, A.W., Archambault, J. and Massie B., 1990. High level recombinant protein production in bioreactors using the baculovirus-insect cell expression system. Biotechnol. Bioeng. 36, 1133-1140.
Donaldson, M.S., Shuler, M.L., 1998. Low serum-free medium for the BTI-Tn5B1-4 insect cell line. Biotechnol. Prog. 14, 573-579.
Drugmand, J.C., Schneider, Y.J., Agathos, S.N., 2012. Insect cells as factories for biomanufacturing. Biotechnol. Adv. 30(5), 1140–1157.
Elias, C.B., Jardin, B., Kamen, A., 2007. Recombinant protein production in large-scale agitated bioreactors using the baculovirus expression system. In: Murhammer D.W. (Ed.) Methods in molecular biology series. Baculovirus and insect cell expression protocols. Springer, New York, pp. 225-245.
Grace, T.D.C., 1967. Establishment of a cell line from the silkworm, Bombyx mori. Nature 216, 613.
Grillberger, L., Kreil, T.R., Nasr, S., Reiter, M., 2009. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol. J. 4, 186–201.
Hayflick, L., 1973. Subculturing human diploid fibroblast cultures. In: Kurse, P.F, Patterson, M. K. Jr. (Eds.), Methods and Applications. Academic Press, New York, pp. 220-223.
Hink, W.F., 1991. A serum free medium for the subculture of insect cells and production of recombinant proteins. In Vitro Cell. Dev. Biol. 27 A (5), 397-401.
Ikonomou, L., Schneider, Y.-J., and Agathos, S. N., 2003. Insect cell culture for industrial production of recombinant proteins. Appl. Microbiol. Biotechnol. 62, 1–20.
Irons, S.L., Chambers, A.C., Lissina, O., King, L.A., and Possee, R.D., 2018. Protein production using the baculovirus expression system. Curr. Protoc. Protein Sci. 91, 5.5.1–5.5.22.
Khurad, A.M., Kanginakudru, S., Qureshi, S.O., Rathod, M.K., Rai, M. M., Nagaraju, J., 2006. A new Bombyx mori larval ovarian cell line highly susceptible to nucleopolyhedrovirus. Journal of Invertebrate Pathology 92, 59-65.
Khurad, A.M., Zhang, M.J., Deshmukh, C.G., Bahekar, R.S., Tiple, A.D., Zhang, C.X., 2013. A new continuous cell line from larval ovaries of silkworm, Bombyx mori. In Vitr. Cell. Dev. Biol. Anim. 45, 414–419.
Khurad, A.M., Bahekar, R.S., Zhang, M.J., Tiple, A.D., Lee, J. M., Zhang, C.X., Kusakabe, T., 2013. Development and characterization of a new Bombyx mori cell line for protein expression. Journal of Asia-Pacific Entomology 16, 17–22.
Kost, T.A., Condreay, J.P. and Jarvis, D.L., 2005. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 23, 567-575.
Lynn, D. E., 2001. Novel techniques to establish new insect cell lines. In Vitr. Cell. Dev. Biol. Anim. 37, 319–321.
Mitsuhashi, J., 1982. Media for insect cell cultures. In: Maramorosch, K. (Ed.), Advances in Cell Culture. Academic Press, New York, Vol. 2. pp. 133-196.
Mitsuhashi, J., 1984. Isolation of a continuous cell line from larval fat bodies of an Arctiid moth, Spilarctia seriatopunctata (Insecta, Lepidoptera, Arctiidae). Zool. Sci. 1, 415-419.
Mitsuhashi, J., Maramorosch, K., 1964. Leafhopper tissue culture: embryonic, nymphal and imaginal tissues from aseptic insects. Contrib. Boyce Thompson Inst. 22, 435-460.
Mitsuhashi, J. and Grace, T.D.C., 1969. Adaptation of established cell lines to a different medium. Appl. Ent. Zool. 4(3), 122-125.
Mitsuhashi, J., and Goodwin, R. H., 2018. The Serum-Free Culture of Insect Cells In Vitro. In: Mitsuhashi, J. (Ed.), Invertebrate Cell System Applications, CRC Press, Boca Raton, Florida, pp. 31–43.
Neermann, J., and Wagner, R., 1996. Comparative analysis of glucose and glutamine metabolism in transformed mammalian cell lines, insect and primary liver cells. J. Cell. Physiol. 166, 152–169.
Rubio, N.R., Fish, K.D., Trimmer, B.A., Kaplan D.L., 2019. Possibilities for Engineered Insect Tissue as a Food Source. Front. Sustain. Food Syst. 3, 1-13.
Sudeep, A.B., Mishra, A.C., Shouche, Y.S., Pant, U., Mourya, D.T., 2002. Establishment of two new cell lines from Bombyx mori (L.) (Lepidoptera: Bombycidae) and their susceptibility to baculoviruses. Indian J. Med. Res. 115, 189-193.
Trager, W., 1935. Cultivation of the virus of grasserie in silkworm tissue culture. J. Exp. Med. 61, 501-513.
Van Oers, M.M., Pijlman, G.P., Vlak, J.M., 2015. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J Gen Virol. 96(Pt 1), 6-23.
Weiss, S.A., Vaughn, J.L., 1986. Cell culture methods for large-scale production of baculoviruses. In: Granados, R.R. and Federici, B.A. (Eds.), The Biology of baculoviruses, CRC Press, Boca Raton, Florida, pp. 63-87.