1Department of Zoology, Gauhati University, Guwahati, Assam, India
2Department of Zoology, Cotton University, Guwahati, Assam, India
Corresponding author email: titikkshadas.89@gmail.com
Article Publishing History
Received: 17/04/2020
Accepted After Revision: 24/06/2020
Toxic metals get accumulated in our environment due to anthropogenic activities. Arsenic, a metalloid, disturbs haematological and biochemical factors of an organism as well as depletes cells major antioxidants and enzymes. The present work was designed to observe behavioural changes, histopathological and ultrastructural changes of kidney and intestine of Channa punctatus exposed to sub-lethal concentration (10% and 50% of LC50) sodium arsenite and its revival with aqueous garlic extract (AGE). The traditional LC50 test is used to measure the potential risk of sodium arsenite and the static renewal technique has been used to observe the morphological and behavioural changes of Channa punctatus. Histopathological lesions were examined and photographed with the help of Bright Field Microscope. Scanning Electron Microscope was used to observe the ultrastructural changes in the tissues of Channa punctatus. Histopathological and ultrastructural studies of kidney and intestine tissues reveal various alterations in the tissues. Hypertrophy of the epithelial cells of renal tubules along with reduction in the size of the tubular lumens was seen in the kidney tissue of the fishes with acute exposure. Hypertrophied nuclei, dilation, oedema were seen in the renal tubules. Vacuolisation due to disintegration of cytoplasm was quite evident. The revival group with aqueous garlic extract showed a lesser extent of vacuolisation and comparatively low enlargement of renal tubules. The intestine of the arsenic treated group revealed severe degenerative changes in the intestinal mucosa, elongated lumen in villi, fusion of villi and atropy in the muscularis. The cytoplasm demonstrated vacuolisation, apoptotic, and necrotic cells. The degenerative changes were found to be less severe in the arsenic + AGE treated group than the group treated with only arsenic. Hence the protective role of garlic can be established from the normalization of the behavioural changes and restoration of histological and surface ultrastructural damages in arsenic induced Channa punctatus.
Kidney, Intestine, Arsenic, Toxicity, Garlic, Revival
Das T, Goswami M. Acute Toxicity Impact of Sodium Arsenite on Behavioural Changes and Histopathology of Kidney and Intestine of the Freshwater Fish, Channa Punctatus and its Revival with Aqueous Garlic Extract. Biosc.Biotech.Res.Comm. 2020;13(2).
Das T, Goswami M. Acute Toxicity Impact of Sodium Arsenite on Behavioural Changes and Histopathology of Kidney and Intestine of the Freshwater Fish, Channa Punctatus and its Revival with Aqueous Garlic Extract. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/2UnrDWK
Copyright © Das and Goswami This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Arsenic, a metalloid, is an important environmental toxicant. Arsenic exists in aquatic environment either in arsenite (As3+) or arsenate (As5+) form that interconverts through redox and methylation reactions (Ghosh et al., 2006). However, the arsenite form has been found to be more toxic both in in vivo and in vitro experiments than the arsenate form (Cervantes et al., 1994). Fishes are continuously exposed to arsenic through gills and intake of arsenic contaminated food. In fish, arsenic varies in their toxicity by remaining in two oxidation states, methylated species, arseno-sugars and arseno lipids (Bears et al., 2006). The pathophysiology of arsenic in fish tissue takes place due to the combination of these forms (Wrobel et al., 2002).
Fish population is an important component of the food chain. Hence, any effect of such pollution would have adverse influence on the nutritive value of fish and on man through their ingestion. Some amount of histopathological works are available on the effects of different pollutants on fish kidneys (Gupta and Srivastava 2006) but much is not known about the effect of arsenic on the histopathology of exposed fishes.It has been found from previous literatures that acute toxicity of arsenic compounds varies from one species to another species and even in strains of the same species. It was also seen that toxicity of arsenic compounds depends on species, sex, age, dose, exposure period, nature, concentration and organic and inorganic form of the arsenic (Luh et al., 1973). In aquatic toxicology among different types of toxicity tests the traditional LC50 test or the acute toxicity test is often used to measure the potential risk of a particular chemical (Roy et al., 2006; Samanta et. al., 2020).
Garlic (Allium sativum L.) is one of the most important vegetables and one of the most ancient medicinal plants which originated from Central Asia over 6000 years ago. The products derived from garlic such as aged garlic extract (AGE) is found to have a higher antioxidant activity due to the presence of stable and highly bio available water soluble organosulfur compound like S-allyl cysteine sulphoxide (SAC) and S-allyl mercapto cysteine (SAMC), with highly potent antioxidant activity (Imai et al., 1994). The protective or ameliorative effect of dietary garlic and its oil was found on common carp (Cyprinus carpio) (Yousefi et. al., 2020), growing rabbits (Attia et. al., 2020) and Rohu fish (Labeo rohita) (Khan et. al., 2020) against the toxicity of ambient ammonia, lead and silver nanoparticles respectively.
Therefore, the present work was mainly aimed to study the behavioural changes as well as histopathological and ultrastructural studies of kidney and intestine of Channa punctatus exposed to sodium arsenite and its revival with aqueous garlic extract (AGE).
MATERIALS AND METHOD
Fish
Healthy and disease free Channa punctatus were collected from local markets in Guwahati. Their weight was approximately in between 25-45 gm and average length was 14 cm. Fishes were brought to ambient laboratory condition.
Acclimatization of the Fishes
Fishes were disinfected with a dip of 2% potassium permanganate (KMnO4) solution and were acclimatised in aquaria for two weeks before initiation of experiment. The water provided in the aquaria was from the tap in the laboratory and was changed on the following day. The fishes were fed everyday with fish food available in the market during acclimatization period. Proper aeration was done in all the processes. Following the standard procedure given by APHA the physicochemical parameter of the test water such as dissolved oxygen, carbon dioxide concentration, total ammonia, temperature, pH, and hardness were monitored.
Preparation of Stock Solution of Arsenic and its Treatment:Sodium Arsenite (NaAsO2), molecular weight- 129.91 Merck, India (Ltd.) was procured for performing the experiment. A stock solution was prepared by adding 5 mg of sodium arsenite to 100 ml of distilled water. The test concentration was prepared by diluting the stock solution with appropriate amount of distilled water. Physicochemical parameters of the water used in test solution were maintained according to the standard procedures given by APHA, 2005. The control group of fishes were kept in similar conditions without adding sodium arsenite.
Preparation of Garlic extract:The outer layer of garlic (Allium sativum L.) cloves were removed, and crushed mechanically in a mortar-pestle with 100 ml of autoclaved distilled water for 1 gm of garlic. The homogenate was shaken for 20 minutes, filtered successively through gauze and 0.22 micron membrane filter to obtain the aqueous garlic extract (AGE) (Chowdhury et al., 2008). 10 ml of AGE was added in each plastic pool during the experimental procedure.
Determination of 96 h- LC 50 for Arsenic: Fishes were transferred to different plastic buckets and exposed to five ascending concentrations of sodium arsenite, such as 5, 15, 25, 35 and 45 ppm (parts per million). In all cases, control groups of fishes were maintained. Each experimental trial was carried out for a period of 96 hours. The mortality rate of the fish was recorded at logarithmic time intervals that is, after 6, 12, 24, 48, 72 and 96 hours of exposure. The LC50 was determined statistically to different concentration of sodium arsenite in water which killed 50 percent of the test species during a specific time interval and under similar experimental conditions (Sparaque 1973). The test media was renewed daily during the experimental period. The data obtained in course of the investigation were analysed statistically to see whether there is any influence of different treatment concentrations on the mortality of the fish.
Experimental Design :To study the histopathological and ultrastructural changes of different tissues of C. Punctatus two experiments had been designed. In the first experiment fishes were exposed to sub lethal concentration i.e. 12ppm (approximately 50% of LC50 value) of sodium arsenite along with a control group for 96 hours. In the second experiment fishes were exposed to sub lethal concentration i.e. 2.5ppm (10% of LC50 value) of sodium arsenite along with a control group for 15 days. Six fishes were used for each set and two sets of experiments were carried out for 96 hours and four sets of experiments were carried out for 15 days. In the 96 hours experiment first set was taken as control without any dose and the second set was given the sub-lethal dose (12 ppm) of sodium arsenite. In the 15 days experiment, first set was taken as control without any dose and the second set was given the sub-lethal dose (2.5 ppm) of sodium arsenite. Another set of fishes received the same dose of sodium arsenite (2.5 ppm) mixed with 10 ml of Aqueous Garlic Extract (AGE). The water, arsenic and garlic treatments of each plastic pool were changed after 48 hour. An experiment was also performed by treating a group of fish only with aqueous garlic extract for 15 days.
Morphological and Behavioural Studies: Morphology and the behaviour of the fishes were observed thoroughly in all the experiments. A control experiment was run parallel by taking same number of fishes and same quantity of water for comparison. The fishes in each group were examined within 96 hours of experimental duration for any morphological and behavioural changes. Their behaviours were also recorded in control and garlic treated groups. In this study behaviours of the fishes such as hyper excitability, fin movements, yawn, fin flickering, S jerk, nudge, nip, cough, opercular movements, imbalanced swimming were considered as observational parameter for all the experiments. Together with the behavioural changes the alteration observed in the skin of the fishes were recorded.
Histological studies: Fishes from control and treated group were randomly selected and the kidney and intestine tissues were isolated for histopathological examinations. Physiological saline solution (0.75% NaCl) was used to rinse and clean the tissue. They were fixed in aqueous Bouin’s solution for 24 hours, processed through graded series of alcohols, cleared in xylene and embedded in paraffin wax. Sections were cut at 4 micron thickness and stained with Hematoxylin and eosin stain. Histopathological lesions were examined and photographed with the help of computer attached Bright Field Microscope (LeicaDM3000).
Scanning Electron Microscopic Analysis: Kidney and intestine tissues of control and treated groups were rapidly removed and processed routinely for scanning electron microscopic studies. The tissues were cut into small pieces of 1 mm thickness and fixed in 2.5 % glutaraldehyde prepared in cacodylate (sodium phosphate) buffer adjusted to pH 7.4 for 24 hours and afterward washed in phosphate buffer for 15 min. After dehydration in ascending series of acetone, samples were immersed in Tetra Methyl Silane for 10 minutes at 4 degree centrigrate. Then they were brought to room temperature to dry. The specimens were mounted on Aluminium Stubs coated with gold and observed through scanning electron microscope in Sophisticated Analytical Instrument Facility (SAIF), North-Eastern Hill University (NEHU), Shillong – 793022.
RESULTS AND DISCUSSION
The observed physico-chemical parameters of the tap water used in the experiment were temperature, pH, dissolved oxygen, dissolved carbon dioxide, total hardness and ammonia. These parameters were monitored throughout the acclimatization period and the trial periods in which the fishes were exposed to sodium arsenite and garlic. The observed physico-chemical parameters of the test water are listed in Table 1. The parameters of the test water were measured according to the experimental procedure described by APHA (1998) and according to their recommendations the fluctuation in temperature should not exceed 4°C and the oxygen content must not fall below 4 mg/L for the warm water fish.
Table 1. Physico-chemical parameters of the test medium.
| Physico-chemical parameter | Value | Mean |
| Temperature (°C) | 25-27 | 25.8 ± 0.4 |
| PH | 7.1-7.4 | 7.14 ± 0.18 |
| Dissolved oxygen (mg/L) | 6.5-7.3 | 6.96 ± 0.12 |
| Dissolved Carbon dioxide (mg/L) | 10.3-10.9 | 10.56 ± 0.18 |
| Total Hardness (mg/L) | 189-197 | 193.2 ± 0.68 |
| Ammonia (mg/L) | 0.15-0.18 | 0.164 ± 0.02 |
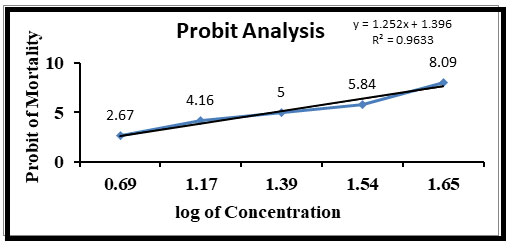
In the present study, it was observed that 45 ppm sodium arsenite in water induced death of all the exposed fishes within 96 hours. The 96 hours LC50 of sodium arsenite for Channa punctatus was found to be 25 ppm and its probit analysis graph can be seen in the Figure 1. Fishes treated with a concentration of 5, 10 and 12 ppm survived for more than 90 days with zero mortality rates.
Figure 1: Relationship between concentration of sodium arsenite and probit mortality of Channa punctatus after 96 hours.
In the present study it was observed that (Table 2) during the initial period of sodium arsenite exposure fishes at all the tested concentrations became restless, moved faster, showed rapid opercular movements and hyper excitability and had the tendency of jumping out from the arsenic contaminated water. Frequency of erratic swimming, jumping and gulping of air at the water surface was found to be increased during the initial period (30 hours) of exposure in both the two test concentrations but during the later period of exposure, these activities reduced. This might be due to their adaptation in the arsenic contaminated environment or because of reduction of their enzymatic activity. It was seen that, the changes of above activities depended on the concentration of sodium arsenite. When the fish were exposed to median lethal concentration (25 ppm) the observed changes in the behavioural activities were relatively increased initially (of initial 10 hours period of exposure time) and then reduced afterwards. It was also observed that for median lethal arsenic concentration fish were seen with increased body depigmentation along with lot of mucus all over the body. Towards the end of exposure period the fish struggled for breathing and had lost their swimming ability. The fish gradually lost their sense of equilibrium and became lethargic. These behavioural changes were more prominent for higher concentration of sodium arsenite than in the lower concentrations.
Table 2. Effect of Sodium arsenite on the behavioural activity of Channa Punctatus during 96 hours exposure to the sub-lethal and medium lethal concentration of Sodium arsenite and AGE (Aqueous Garlic Extract)
| Nature of Behaviour | Control
Group |
Arsenic exposure concentrations | AGE treated group | Post arsenic-AGE treated group | |
| 2.5 ppm | 25 ppm | ||||
| Opercular movement | + | ++ | +++ | + | + |
| Hyperexcitability | ˗˗ | ++ | +++ | + | ˗˗ |
| Fin movement | + | ++ | +++ | + | + |
| Imbalance swimming | ˗˗ | + | +++ | ˗˗ | ˗˗ |
| Mucus secretion | ˗˗ | + | +++ | ˗˗ | ˗˗ |
| Depigmentation | ˗˗ | + | ++ | ˗˗ | ˗˗ |
| Mortality | ˗˗ | ˗˗ | + | ˗˗ | ˗˗ |
˗˗ = Normal effect; + = mild effect; ++ = Moderate effect; +++ = Strong effect
When the fishes were exposed to Garlic treated water for the first 30 hours the fishes showed a little of excitability, opercular movement and fin flickering but they did not show any imbalanced swimming, depigmentation and mucus secretion during entire 96 hours exposure period. Another group of fishes first exposed to sublethal concentration (2.5 ppm) of sodium arsenite for 96 hours and then they were transferred to only AGE treated water for 96 hours. In this case the fishes showed a sign of relaxation and found to have less excitability, opercular movements, fin flickering, and depigmentation. It was observed that the test fishes showed almost similar behavior as of the fishes of control group towards the end of 96 hours of observation. At the end of the exposure period of median lethal concentration (25 ppm) of arsenic almost fifty percent of the fishes were found dead, scattered at the bottom of the tank with their mouth open. In the other case fish remained alive and relatively active throughout the experimental period.
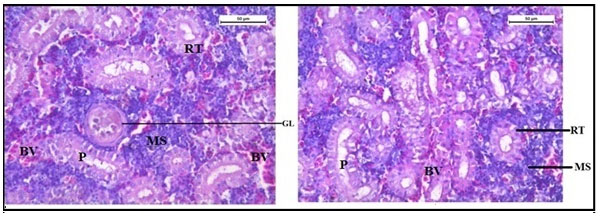
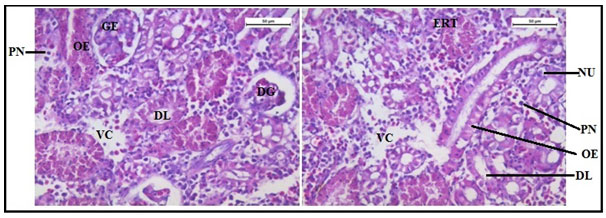
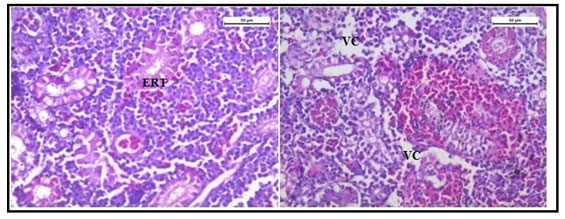
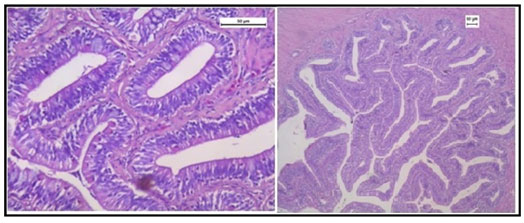
The histology of the kidney tissue of the control group of Channa punctatus, is shown in the Figure 2. The kidney tissue of C. punctatus revealed normal arrangement of the renal tubules and Bowman’s capsules with glomeruli distributed in the interstitium of haematopoietic tissue. Regular arrangement of renal tubules, proximal tubule and blood vessels were clearly visible. Histopathology of the kidney tissue exposed to sodium arsenite for 15 days revealed hypertrophy of the epithelial cells of renal tubules along with reduction in the size of the tubular lumens was seen in the acute exposure (Figure 3). Hypertrophied nuclei (NU), dilation (DG), oedema (OE) were prominent in the renal tubules. Vacuolisation (VC) due to disintegration of cytoplasm was quite evident. Pyknotic nuclei (PN) could be observed in mesenchymal tissue. Vacuolization (VC) and disorganized blood capillaries could be seen in Glomeruli.
Figure 2: Histology of kidney tissue in the control group of Channa punctatus. (GL- Glomerulus, RT- Renal Tubule, MS-Mesenchyma, P-Proximal Tubule, BV- Blood Vessels)
Figure 3: Histology of kidney tissue in the group of Channa punctatus treated with sodium arsenite for 15 days. (VC- Vaculisation, NU-Hypertrophied Nuclei, DS- Dequamation of Epithelial Lining, ERT- Enlargment of Renal Tubules, PN- Pyknotic Nuclei, DL-Dilation of Renal Tubules, OE-Qedema od Renal Tubules, DG-Dilation of Glomerulus, GE- Glomerular Expansion)
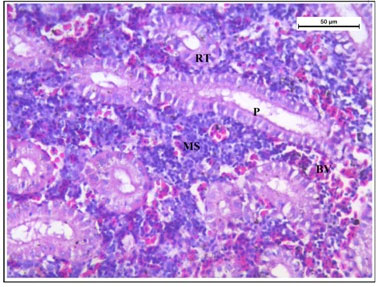
Histology of the kidney tissue of the fishes treated with only garlic for 15 days (Figure 4) represented normal structure of the kidney tissue similar to the structure of the control fishes. Histology of kidney tissue exposed simultaneously to sodium arsenite and AGE for 15 days (Figure 5) revealed that the histoarchitechture of proximal tubule, glomeruli and mesenchyma remained unaffected whereas few vacuolisation and comparatively low enlargement of renal tubules could be seen (Figure 5). Ruptures of few blood vessels were also prominent. A comparison of the histological changes of sodium arsenite treated kidney tissue with sodium arsenite + AGE treated group revealed lesser damage in the histoarchitechture of the kidney tissue. This is probably due to the protective effect of garlic which may prevent the accumulation of arsenic in the kidney tissue.
Figure 4: Histology of kidney tissue in the group of Channa punctatus treated with only garlic for 15 days (RT- Renal Tubule, P- Proximal Tubule, BV-Blood vessels, MS-Mesenchyma)
Figure 5: Histology of kidney tissue in the group of Channa punctatus simultaneously treated with sodium arsenite and aqueous garlic extract (AGE).
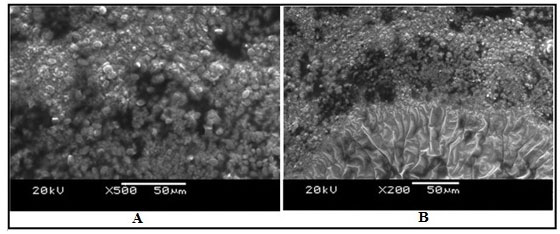
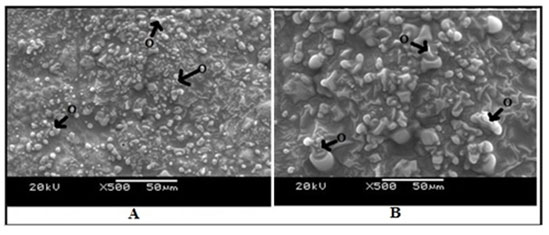
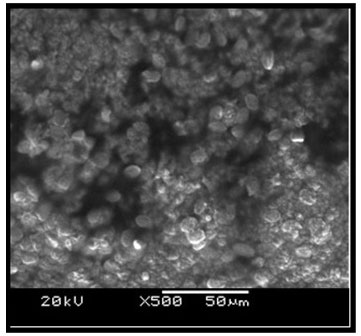
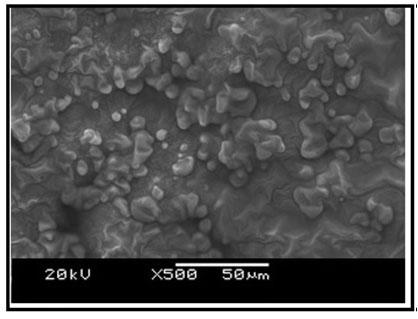
Ultrastructure of the kidney tissue of control group of Channa punctatus for 96 hours and 15 days experiments showed normal surface structure and regular arrangement of different components of the tissue (Figure 6). SEM images of the kidney tissue exposed to sodium arsenite for 96 hours as well as for 15 days (Figure 7) revealed many outgrowths in the surface of the tissue exposed to sodium arsenite in both 96 hours and 15 days of treatment. Although, it could be seen that the extent of the growth of the tumour like structures were more in the 15 days exposure as compared to 96 hours exposure. It was also observed that when the Channa punctatus were treated with only garlic extract the ultrastructure of the kidney tissue appeared to be similar to that of control group (Figure 8). SEM images of the kidney tissue exposed simultaneously to sodium arsenite and AGE for 15 days duration (Figure 9) showed significant recovery in the surface ultrastructure as compared to the deformation observed in the kidney tissue exposed only to sodium arsenite (Figure 7).
Figure 6: SEM images of kidney tissue in the control group of Channa punctatus. (A- 96 hours observation, B- 15 days observation)
Figure 7: SEM images of kidney tissue in the treated group of Channa punctatus. (A- 96 hours treatment, B-15 days treatment, O-Outgrowth or tumour like structure)
Figure 8: SEM images of kidney tissue in the group of Channa punctatus which was treated with only garlic for 15 days.
Figure 9: SEM image of kidney tissue in the group of Channa punctatus exposed with sodium arsenite and aqueous garlic extract simultaneously.
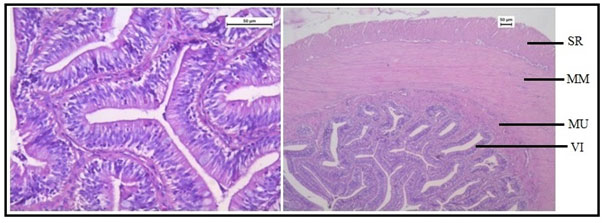
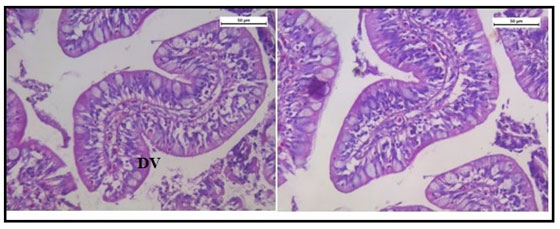
Histologically, the intestinal wall of C. punctatus is comprised of mucosa, submucosa, muscularis and serosa. The mucosal layer extends into prominent slender folds called villi which has intestinal glands. Figure 10 shows the normal histology of intestine in the control group of Channa punctatus.The intestinal histology of the treated groups showed severe degenerative changes in the intestinal mucosa, elongated lumen in villi, fusion of villi and atropy in the muscularis. On days upwards of the exposure, the cytoplasm demonstrated vacuolization, apoptotic, and necrotic cells in greater numbers in 15 days arsenic treated fishes (Figure 11).
Figure 10: Optical Micrograph of the intestine in the control group of Channa punctatus. (SR-Serosa, MM-Muscularis Mucosa, MU-Mucosa, VI-Villi)
Figure 11: Optical Micrograph of the intestine tissue of Channa punctatus treated with arsenic. (EL- Elonggated Lumen in Villi, VC – Vacoulisation, N- Necrosis)
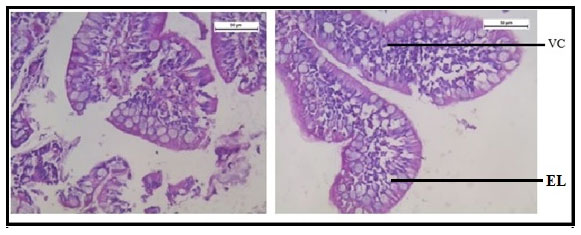
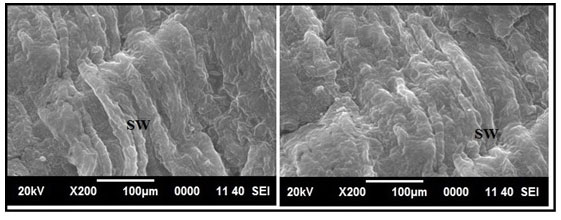
Figure 12 shows normal histology of the intestine in the group of fishes treated with only garlic extract. The histological study of intestine in the arsenic and AGE treated group revealed vacuolisation, partially damaged mucosa and distorted villi. However the damages were partially recovered (Figure 13).SEM photograph in the control group revealed normal ultrastructure of the intestine tissue (Figure 14). However in the treated group surface wrinkling and bulbous epithelium were observed (Figure 15). Intestinal mucosal folds were found to be damaged and epithelial cells were distorted. The degenerative changes were found to be less severe in the arsenic + AGE treated group than the group treated with only arsenic (Figure 17). The SEM images of intestine tissue treated with only garlic which was in accordance to the structure of the control group (Figure 16).
Figure 12: Optical micrograph of intestine tissue of Channa punctatus treated with only garlic extract.
Figure 13: Optical micrograph of intestine tissue of Channa Punctatus treated with arsenic and garlic extract simultaneously for 15 days duration (DV- Distorted Villi).
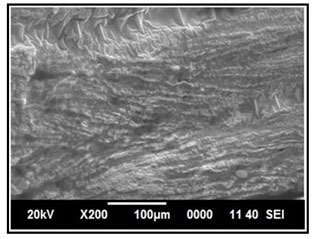
Figure 14: SEM image of intestine tissue in the control group of Channa punctatus
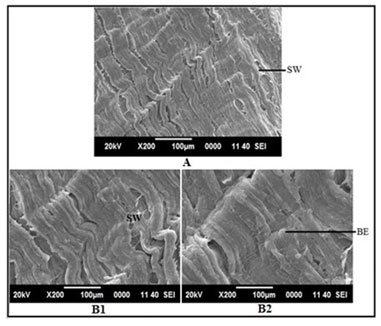
Figure 15: SEM images of intestine tissue of Channa punctatus treated with arsenic. (A- 96 hours treatment, B1 & B2 – 15 days treatment, BE- Bulbous Epithelium, SW- Surface Wrinkling).
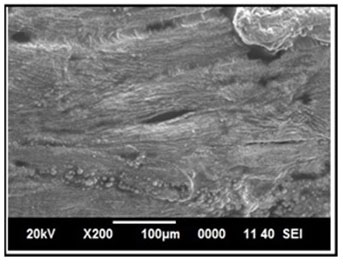
Figure 16: SEM image of intestine in Channa punctatus treated with only Aqueous Garlic Extract (AGE).
Figure 17: SEM images of intestine tissue in the group of Channa punctatus treated with arsenic and AGE for 15 days duration (SW-Surface Wrinkling).
This study was aimed to analyse behavioural and histological alterations on acute exposure of sodium arsenite on Channa punctatus and to study the ameliorative effect of garlic against arsenic toxicity. An excellent tool to assess the well-being of a particular organism has been the behaviour. It was observed that the different concentration of sodium arsenite initiated various pattern of behavioural changes in the test fish. The cause of such abnormal behaviour and altered movements might have been due to some enzymatic and ionic imbalances in blood and tissues (Larsson et al., 1981). It was also noted that, the changes of above activities depended on the concentration of sodium arsenite. The group of fishes exposed to sublethal concentration (2.5 ppm) of sodium arsenite for 96 hours was transferred to only AGE treated water for 96 hours. In this case the fishes showed a sign of relaxation and were found to have less excitability, opercular movements, fin flickering, and depigmentation. Many researchers observed abnormal behaviours in different fishes treated with various heavy metals (Santha et al., 2000; Karuppasamy 2001; Subathra and Karuppasamy 2003; Sivakumar et al., 2006).
The stressful toxic environment along with sensory stimulus might have increased the opercular movement for proper ventilation of gills to cope up with hypoxia (Lata et al., 2001). The neurotoxic effects and the disturbances to receptor system of the body might be the cause of abnormal behaviours in the fishes (Bhavani and Karuppasamy 2014). Behavioural responses, abnormal swimming, restlessness, fin flickering, hyper excitability, jumping out and to and fro movement of the fish observed in the present study might be the avoidance responses of the fishes towards the toxicant. The avoidance response may be because of changes in sensitivity of chemoreceptors. Similar observations were reported by researchers like Svecevieus (2001) and Agarwal (1991). Lethargy observed in this study towards the end of exposure period might be because of loss of energy resulted due to erratic swimming, jumping and restlessness. The impairment of nervous system might cause the lateral swimming and loss of equilibrium in the fishes (Sinha and Kumar, 1992).
The histology of the kidney tissue exposed to sodium arsenite represented expansion of renal tubules and distinct separation of their epithelial lining from the tubular cells. Most of the renal tubules were observed to lose their cellular integrity. Hypertrophied nuclei (NU), Dilation (DG), oedema (OE) were prominent in the renal tubules. Vacuolisation (VC) due to disintegration of cytoplasm was quite evident. Pyknotic nuclei (PN) could be observed in mesenchymal tissue. Vacuolization (VC) and disorganized blood capillaries could be seen in Glomeruli. Similar histological changes in the kidney of Channa punctatus (Bloch) due to the effects of sub-lethal concentrations of zinc were reported earlier (Gupta and Srivastava 2006). Pathological changes in the kidney of different fishes exposed to various other toxicant have earlier been reported by many researchers (Hadi and Alwan 2012; Anitha Kumari and Sree Ram Kumar 1997; Prashanth 2011; Banerjee and Bhatacharya 1994., Wang et. al., 2020).
The degenerative damages takes place due to the presence of high concentration of heavy metals in the kidney tissue (Anitha Kumari and Sree Ram Kumar 1997; Venkataramana and Radhakrishnaiah 1987).In the present study it has been observed that the extent of damages in the kidney tissue reduced to some extent in the fishes treated simultaneously with sodium arsenite and the Aqueous Garlic Extract (AGE) for 15 days duration. Histology of the kidney tissue of the fishes treated with only garlic for 15 days represented normal structure of the kidney tissue similar to the structure of the control fishes. The histopathology of the kidney of the group of fishes treated with sodium arsenite and AGE for 15 days showed that the histo-architecture of proximal tubule, glomeruli and mesenchyme remained unaffected. The histological alterations observed were vacuolisation, enlargement of renal tubules and ruptures in few blood vessels. A comparison of the histological changes of sodium arsenite treated kidney tissue with the revival group showed that there was a lesser extent of damage in the micro structure of the kidney tissue. This is probably due to the protective effect of garlic which might have prevented the accumulation of arsenic in the kidney tissue.
Ultrastructural changes of the kidney tissue exposed to sodium arsenite showed many tumour like outgrowths in the surface of the tissue. It was also observed that when the Channa punctatus were treated with only garlic extract the SEM images of the kidney tissue appeared to be similar to that of the control group. Massar et al., (2014) observed similar abnormal outgrowths at the surface of the kidney tissue of Common Carp (Cyprinus carpio L.) inhabiting a polluted reservoir. Kidney tissue of different fishes exposed to Glyphosate‑based Herbicide, Excel Mera 71, Cadmium Nanoparticles and the insecticide monocrotophos showed abnormalities in its ultrastucture (Samanta et al., 2018; Sangeetha et al., 2017; Singla 2015). Recently it has been observed that exposure of toxicant like copper significantly disorganized the morphology of kidney in rat (Wan et. al., 2020).
Ultrastructural changes of the kidney tissue exposed simultaneously to sodium arsenite and AGE showed a lesser extent of deformation as compared to the deformation observed in the kidney tissue exposed only to sodium arsenite. The extent of the growth of the tumour like structure was found to be more in the arsenic treated fishes than the ones treated with arsenic +AGE. These results indicate that there is a protective role of AGE that prevented the adverse effects of sodium arsenite on kidney tissue.The histology of the intestinal tissue of arsenic treated group of Channa punctatus showed severe degenerative changes in the intestinal mucosa, elongated lumen in villi, fusion of villi and atropy in the muscularis. The cytoplasm demonstrated vacuolization, apoptotic, and necrotic cells in greater numbers. The damages in the intestinal villi might be due to the defensive mechanism of fish against arsenic toxicity. Lifting of columnar epithelium of villi could be a defence mechanism of the fish against the toxic environment.In the arsenic + AGE treated group partially damaged mucosa, vacuolisation and epithelial lifting of intestinal villi were observed. The damages were less severe than the arsenic treated group. Normal histology of the intestine in the group of fishes treated with only garlic extract was seen.
The present findings are in accordance with the findings of earlier researchers. Similar histopathological changes in the intestine of arsenic treated Channa punctatus was reported by Hossain (2012). Dilation of vascular elements, oedema, and increased width of the sub mucosa, lumen and serosa were noted. The effect of different toxicants on the intestine of Tilapia fish were observed by Shah et al., (2009). The findings of Kaoud et al., (2011) revealed that the intestine tissue of Oreochromis niloticus when exposed to cadmium showed necrosis in mucosa and sub mucosal hemorrhage. The intestinal histology of chicken was also found to be altered by subchronic exposure of mercuric chloride (Zhou et. al., 2020).
The surface ultrastructural changes of the intestine of the arsenic treated group showed surface wrinkling and bulbous epithelium. Intestinal mucosal folds were found to be damaged and epithelial cells were distorted. The degenerative changes were found to be less severe in the arsenic + AGE treated group than the group treated with only arsenic. The SEM image of intestine tissue treated with only garlic was in accordance to the structure of the control group. Samanta et al., (2018) revealed that A. testudineus after glyphosate based herbicide exposure in laboratory condition under scanning electron microscopic showed severe damages in columnar epithelial cells and excess mucus secretion in the mucosal folds. Ghosh et al., (2001) observed similar changes in intestine tissue through SEM investigation in arsenic treated Notopterus notopterus. Histopathological Examination and Transmission Electron Microscopy mercury-exposed mice showed intestinal injury (Zhao et. al., 2020)
CONCLUSION
The ameliorative effect of garlic against arsenic toxicity was shown in the normalisation of behavioural changes and histopathological damages in arsenic induced Channa punctatus. From this study it can be concluded that the sensitivity of the fish Channa punctatus to sodium arsenite is very high and it can be regarded as a toxicant. It can also be concluded that garlic as a natural anti-oxidant do not cause any type of excitability or irritant nature in Channa punctatus. Hence it has been used as a treatment for revival against arsenic toxicity. The protective effect of garlic was shown in the normalisation of lesions in the histopathological and surface ultrastructural studies of arsenic induced tissues of Channa punctatus. Hence from the present investigation it can be concluded that garlic extract could be an effective natural agent to reduce the cellular damages in the arsenic exposed tissues and it definitely have firm role in the recovery of fish tissues after exposure to sodium arsenite.
Authors’ contributions
Titikksha Das contributed to the study design, performed the experimental work, analyzed the results and wrote the paper; Mamata Goswami contributed to the study design and analyzed the results.
REFERENCES
Agarwal, S. K. (1991). Bioassay evaluation of acute toxicity levels of mercuric chloride to an air breating fish, Channa Punctatus (Bloch.): Mortality and behaviour Study. J Envirn Biol, 12, 99-106.
Anitha Kumari, S., & Sree Ram Kumar, N. (1997). Histopathological alterations induced by aquatic pollutants in Channa punctatus from Hussain Sagar lake (AP). J Environ Biol, 18, 11-16.
Attia K. M., Assar M. H., Farouk Z. M., & Basuney H. A. (2020). Possible Protective Effects of Black Seed (Nigella sativa) or Garlic (Allium sativum) Against Lead-Induced Toxicity in growing rabbits. AJVS. 64, 52-65.
Banerjee, S., & Bhattacharya, S. (1994). Histopathology of kidney of Channa punctatus exposed to chronic non-lethal levels of elsan, mercury and ammonia. Ecotoxicol Environ Saf, 29, 265-275.
Bears, H., Richards, J. G., & Schulte, P. M. (2006). Arsenic exposure alters hepatic arsenic species composition and stress mediated gene expression in the common Killifish (Fundulus heterclitus). Aqua Toxico, 77, 257-266.
Bhavani, K., & Karuppasamy, R. (2014). Acute toxicity bioassay and behavioural changes on zebra fish, Danio rerio (Hamilton) under arsenic trioxide. Int J Mod Res Rev, 2, 40-46.
Cervantes, C., Ji, G., Ramirez, J. L., & Silver, S. (1994). Resistance to arsenic compounds in microorganisms. FEMS Microbiol Rev, 15, 355-367.
Chowdhury, R., Dutta, A., Ray Chaudhuri, S., Sharma, N., Giri, A. K, & Chaudhuri, K. (2008). In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem Toxicol, 46, 740–751.
Ghosh, A. R., & Chakrabarti, P. (2001). Impact of inorganic arsenic on the mucosal surface of stomach, intestine and intestinal caeca of Notopterus notopterus (Pallas). ICIPACT, 469-473.
Ghosh, D., Datta, S., Bhattacharya, S., & Mazumder, S. (2006). Perturbation in the catfish immune responses by arsenic: organ and cell specific effects. Comp Biochem physiol, 143, 455-463.
Gupta, P., & Srivastava, N. (2006). Effects of sub-lethal concentrations of zinc on histological changes and bioaccumulation of zinc by kidney of fish Channa punctatus (Bloch). J Environ Bio, 27, 211-215.
Hadi, A. A., & Alwan, S. F. (2012). Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminium. Inter J Pharma Life Sci, 3, 2071-2081.
Hossain, M. (2012). Effect of arsenic on the histological change of snakehead fish, channa punctata. J Life Earth Sci, 7, 67-70.
Imai, J., Ide, N., Nagae, S., Moriguchi, T., Matsuura, H., & Itakura, Y. (1994). Antioxidants and free radical scavenging effects of aged garlic extract and its constituents. Plant Medic, 60, 417-420.
Karuppasamy, R. (2001a). Evaluation of acute toxicity levels and behavioral responses of Channa punctatus (Bloch.) to phenyl mercuric acetate. Ecol Env Cons, 7, 75-78.
Kaoud, H. A., Zaki, M. M., El-Dahshan, A. R., Saeid, S., & El Zorba, H. Y. (2011). Amelioration of the toxic effect of Cadmium exposure in Nile Tilapia (Oreochromis Niloticus) by using Lemna gibba L. Life Sci J, 8, 185-195.
Khan M. S., Qureshi N. A., Jabeen F., Wajid M., Sabri S., & Shakir M. (2020). The role of garlic oil in the amelioration of oxidative stress and tissue damage in rohu Labeo rohita treated with silver nanoparticles. Fisheries Sc. 86, 255–269.
Larsson, A., Bengtsson, B. E., & Haux, C. (1981). Disturbed ion balance in flounder, Platichthys fiesus L. exposed to sublethal levels of cadmium. Aquat Taxicol, 1, 19-35.
Lata, S., Gopal, K., & Singh, N. N. (2001). Toxicological evaluations and morphological studies in a catfish, Clarias batrachus exposed to carbaryl and carbofuran. J Ecophysiol Occup Hlth, 1, 121-130.
Luh, M. D., Baker, R. A., & Henley, D. E. (1973). Arsenic analysis and toxicity-a review. Sci Total Environ, 2, 1-12.
Massar, B., Dey, S., & Dutta, K. (2014). Structural Changes in Kidneys of Common Carp (Cyprinus carpio L.) Inhabiting a Polluted Reservoir, Umiam in Meghalaya, India. J Adv Micro Res, 9, 105–109.
Prashanth, M. S. (2011). Histopathological Changes Observed in the Kidney of Freshwater fish, Cirrhinus Mrigala (hamilton) exposed to Cypermethrin. Recent Res Sci Tech, 3, 59-65.
Roy, S., Chattoraj, A., & Bhattacharya, S. (2006). Arsenic induced changes in optic tectalhistoarchitecture and acetyleholinesterase acetylcholine profile in Channa punctatus: amelioration by selenium. Comp Biochem Physiol, 144, 16-24.
Samanta, P., Pal, S., Mukherjee A. K., & Ghosh A. R. (2020). Acute toxicity assessment of arsenic, chromium and almix 20WP in Euphlyctis cyanophlyctis tadpoles. Ecotoxicol. Environ. Saf, 191, 110209.
Santha, K. M., Balaji, M., Saravanan, K. R., Soumady, D., & Ramudu, K. (2000). Effect of monocrotophos on the optomotor behaviour of an air breathing fish Anabas testudineus (Bloch). J Environ Biol, 21, 65-76.
Sangeetha, S., Deepa Rani, S., & Padma Priya, V. (2017). Histopathological changes in the liver and kidney of Indian major carp Catla catla (Hamilton, 1822) exposed to cadmium nanoparticles. Int J App Env Sci, 12, 1913-1926.
Samanta, P., Kumari, P., Pal, S., Mukherjee, A. K., Ghosh, A. R. (2018). Histopathological and ultrastructural alterations in some organs of Oreochromis niloticus exposed to glyphosate-based Herbicide, Excel Mera 71. J Microsc Ultrastruct, 6, 35-43.
Shah, A. Q., Kazi, T. G., Arain, M. B., Jamali, M. K., Afridi, H. I., Jalbani, N., Baig, J. A., & Kandhro, G. A. (2009). Accumulation of arsenic in different fresh water fish species – potential contribution to high arsenic intakes. Food Chem, 112, 520–524.
Singla, N. (2015). SEM studies in kidney of Ctenopharyngo donidellus (Cuvier and Valenciennes) induced by monocrotophos. Euro J Zoo Res, 4, 52-56.
Sinha, T. K. P., & Kumar, K. (1992). Acute toxicity of mercuric chloride to Anabas testudineus (Bloch). Environ Ecol, 10, 720–722.
Sivakumar, S., Karuppassamy, R., & Subhathra, S. (2006). Acute toxicity and behavioural changes in fresh water fish, Mystus vittatus (Blouch) exposed to chromium (VI) oxide. Nature Environ Poll Tech, 5, 381-388.
Subathra, S., & Karuppasamy, R. (2003). Bioassay evaluation of acute toxicity levels of cadmium on mortality and behavioural responses of an air-breathing fish, Channa punctatus (Bloch.). J Experi Zool India, 62, 245-250.
Svecevieus, G. (2001). Avoidance response of rainbow trout, Oncorhyncus mykiss to heavy metal model mixtures. A comparison with acute toxicity tests. Bull Environ Contam Toxicol, 67, 680-687.
Sparaque, J. B. (1973). The ABCs of pollutant bioassay using fish biological method for assessment of water quality. ASTM STM. 528. American Soc Tes Materials, 6-30.
Venkataramana, P., & Radhakrishnaiah, K. (1987). Lethal and sub-lethal effects of copper on the protein metabolism of the fresh water fish Labeo rohita (Ham.). Trends Life Sci, 2, 82-86.
Wan F., Zhong G., Ning Z., Liao J., Yu W., Wang C., Han Q., Li Y., Pan J., Tang Z., Huang R., & Hu L. (2020). Long-term exposure to copper induces autophagy and apoptosis through oxidative stress in rat kidneys. Ecotoxicol. Environ. Saf, 190, 110158.
Wang J., Zhang G., Lin Z., Luo Y., Fang H., Yang L., Xie J., & Guo L. (2020). Determination of arsenicals in mouse tissues after simulated exposure to arsenic from rice for sixteen weeks and the effects on histopathological features. Ecotoxicol. Environ. Saf, 200, 110742.
Wrobel, K., Wrobel, K., Parker, B., Kannamkumarath, S. S., & Caruso, J. A. (2002). Determination of As (III), As (IV), monomethylearsonic acid, dimethyl arsenic acid and arseno betaine by HPLC-ICP-MS analysis of reference material, fish tissue and urine. Talanta, 58, 899-907.
Yousefi M., Vatnikov Y. A., Kulikov E. V., Plushikov V. G., Drukovsky S. G., Hoseinifar S. H., & Doan H. V. (2020). The protective effects of dietary garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture, 526, 735400.
Zhou C., Xu P., Huang C., Liu G., Chen S., Hu G., Li G., Liu P., & Guo X. (2020). Effects of subchronic exposure of mercuric chloride on intestinal histology and microbiota in the cecum of chicken. Ecotoxicol. Environ. Saf, 188, 109920.
Zhao Y., Zhou C., Wu C., Guo X., Hu G., Wu Q., Xu Z., Li G., Cao H., Li L., Latigo V., Liu P., Cheng S., & Liu P. (2020). Subchronic oral mercury caused intestinal injury and changed gut microbiota in mice. Sci. Total Environ, 721, 137639.