1Fishery and Ecotoxicology Research Laboratory (Vice-Chancellor’s Research Group), Department of Zoology,
The University of Burdwan, Burdwan 713104, West Bengal, India.
2Hooghly Mohsin College, Department of Zoology, Chinsurah, West Bengal, India.
Coresponding author email :research.ncsaha@gmail.com
Article Publishing History
Received: 15/10/2022
Accepted After Revision: 05/12/2022
The present study was assessed to determine the acute toxicity and the changes in oxidative stress enzymes and some other biochemical parameters at the sublethal level of a thiocarbamate pesticide cartap hydrochloride on freshwater fish Oreochromis mossambicus. The study reveals that the 96h median lethal concentration (LC50) value of cartap hydrochloride is 20.7 µg/l. Besides, the exposed fish also exhibited erratic behavioral responses at the acute level. The effects of cartap hydrochloride at the sublethal concentration (30% of 96h LC50 value) after 15d and 30d exposure induces alterations in biochemical parameters of freshwater fish Oreochromis mossambicus. Moreover, the modulatory effects of Ocimum sanctum powder (20 gm/kg feed) on the toxicity of cartap hydrochloride were investigated. The investigation demonstrated that sublethal concentrations of cartap hydrochloride increased the levels of liver catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), malondialdehyde (MDA), aspartate aminotransferase (AST), and alanine aminotransferase (ALT).
Additionally, the exposed fish treated with dietary Ocimum sanctum abridged the toxic effects of the pesticide. Moreover by using integrated biomarker response (IBR) and biomarker response index (BRI) the change in the health status of pesticide exposed fish upon addition of Ocimum sanctum supplemented diet over control diet was determined. These results indicate that cartap hydrochloride alters the survivability and behavioral responses of Oreochromis mossambicus at the acute level and changes the biochemical parameters at the sublethal level which was modulated by the additament of Ocimum sanctum.
Cartap Hydrochloride, Oreochromis Mossambicus, Biomarker Response Index, Ocimum Sanctum,
Oxidative Stress Enzymes, Biochemical Parameters
Medda S, Saha N.C, Chatterjee A, Ghosh S, Pal S.Acute Toxicity Alterations in Oxidative Stress Enzymes and Biochemical Parameters in Oreochromis mossambicus, Induced by Cartap Hydrochloride and the Modulatory Effects of Ocimum sanctum Supplementation. Biosc.Biotech.Res.Comm. 2022;15(4).
Medda S, Saha N. C, Chatterjee A, Ghosh S, Pal S. Acute Toxicity Alterations in Oxidative Stress Enzymes and Biochemical Parameters in Oreochromis mossambicus, Induced by Cartap Hydrochloride and the Modulatory Effects of Ocimum sanctum Supplementation. Biosc.Biotech.Res.Comm. 2022;15(4). Available from: <a href=”https://bit.ly/3EHs7hV“>https://bit.ly/3EHs7hV</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The increasing density of the human population over the past few decenniums, rapid urbanization and industrial development lead to pollution in the freshwater ecosystem. Every year 80 percent of residential wastewater and commercial wastewater are dumped in natural water bodies worldwide which disrupt the aquatic ecosystems (Carazo-Rojas et al., 2018). It is reported that agrochemicals were estimated at around US$7.55 billion in 2017 and are anticipated to hit US$ 9.8 billion by 2050 (Nishimoto 2019).
This indicates that pesticide utilization has been gradually increasing (Maurya et al., 2019). Extensive utilization of pesticides lingers in the soil and finally enters the aquatic ecosystem by agricultural runoff thereby inducing water pollution and adversely affecting several non-target aquatic organisms like fish (Özkara et al., 2016). The pesticides alter endocrine processes and cause developmental anomalies with subsequent death of organisms (Maurya 2019 and Zhang 2017, Ogunnupebi et al., 2020).
Amongst the pesticides, carbamates are water-soluble and actively utilized for both residential and agricultural applications (Tsagkaris et al., 2020). Cartap hydrochloride is a thiocarbamate pesticide and is widely utilized in agriculture in India (Costa 2015 & Gilden 2016). It has been routinely used against pests of rice, sugarcane, fruit trees, vegetable crops, tea plantations, rice-fish farms and tea farms (Boorugu & Chrispal 2012). The WHO relegates it as a moderately hazardous (Class II) technical grade active pesticide. The maximal acceptable daily intake (ADI) level is 0.05 mg/kg but in severe poisoning, death can occur (Kalyaniwala 2016 & Vivek 2016).
Fish are considered as bioindicators for aquatic contamination and serve as a potential model for environmental monitoring as they accumulate contaminants water (Ali et al., 2020). Recent studies denoted that fish are consequential sources of proteins and lipids (Balami et al., 2020). In our investigation, Oreochromis mossambicus is selected as a model fish species due to its high growth rate, prosperous adaptation to different diets, susceptibility to diseases, and effective tolerance to a wide range of environmental conditions (Ghane et al., 2020). Several experiments have been carried out in apes, rabbits, plants, and humans regarding the adverse effects of cartap hydrochloride (Gupta et al., 2015). However, research regarding the toxic effects of cartap hydrochloride on aquatic organisms especially fish Oreochromis mossambicus is scarce.
Pesticide contaminants are toxic to non-target species in the aquatic ecosystem (Dar et al., 2015). Their propensity for the formation of free radicals and reactive oxygen species (ROS) is potent to cause oxidative stress in aquatic organisms, leading to a disruption of ROS and antioxidant defense, and therefore can contribute to organism stress (Bhattacharya et al., 2021; Chatterjee et al., 2021). In addition, ROS affects most bio-molecules, including DNA, proteins, and lipids (Bhattacharya et al., 2021).
Thus the evaluation of alterations in biochemical enzyme activity is a paramount approach for the evaluation of stress and may, consequently, serve as a possible implementation for aquatic toxicology (Tan et al., 2018). Several studies have been carried out on alterations of stress enzymes in fish exposed to pesticides (Bhattacharjee 2020 & Yang 2020). However, evidence regarding the toxic effect of this pesticide on alterations of biochemical stress enzymes in Oreochromis mossambicus is limited.
Medicinal plant science has been gaining great interest globally in recent years. Tulsi (Ocimum sanctum) is a shrub of the Lamiaceae family that has been established in northern Central India and is now native to the Eastern tropics. In a variety of research trials, tulsi has been recorded to possess immunomodulatory and antioxidant properties and are significantly effective towards several diseases (Smita 2018 & Sethi 2004).
Integrated biomarker response (IBR) provides a methodology that combines all the biomarker responses and plays a vital role in determining the toxicity of contaminants (Beliaeff and Burgeot, 2002; Serafim et al., 2012). Moreover, Biomarker Response Index (BRI) has been widely utilized in recent years to integrate multiple biomarker responses (Hagger et al., 2008). It is rudimentarily focussed on the alteration level (AL) of biomarker responses in contamination treatments relative to those in the control. The AL of each biomarker is graded into four stages. The score of each biomarker is subsequently multiplied by the corresponding weighting to compose an integrated index for evaluating the general impact and health status of the organism (Hagger et al., 2008).
The objective of the present study is to evaluate the acute toxicity and alterations in oxidative stress enzymes and some other biochemical parameters in Tilapia (Oreochromis mossambicus) exposed to cartap hydrochloride and the modulatory effects of Ocimum sanctum supplementation to treated fish. The entire biomarker dataset was evaluated using integrated biomarker response (IBR) and biomarker response index (BRI) to assess and compare the health status of exposed fish fed with control diet and Ocimum sanctum supplemented diet.
MATERIAL AND METHODS
Ethical Approval: The experimental bioassay was conducted as per the guidelines approved by Institutional Animal Ethics Committee.
Test chemical :The test chemical cartap hydrochloride, used in the study was collected from the local market. Its stock solutions (1% w/v) and subsequent dilutions were made following a standard protocol (APHA. (2005).
Test organism: Adult Oreochromis mossambicus (Class: Actinopterygii, Family: Cichlidae) of mean length 7.2±0.49 cm and mean weight 17.4±0.68 g was used for acute and sublethal bioassay. The specimens were given prophylactic treatment by bathing them in 0.05% potassium permanganate (KMnO4) solution for 2 min to prevent any dermal infections.
Maintenance condition: Fish of different sizes were placed in outdoor cement vats for acclimatization for 7 days and were provided with commercial feed. During this acclimation period, continuous aeration and daily water exchange were conducted for all the tanks. The values of the different physicochemical parameters of water used in the study were as follows: temperature 29.5 ± 0.5°C, pH 7.1 ± 0.5, free CO2 18.3 ± 2.0 mg/l, dissolved oxygen 6.2 ± 1.5 mg/l, total alkalinity 164 ± 7.6 mg/l, and hardness 120 ± 4.5 mg/l as CaCO3.
Acute toxicity bioassay: The static replacement bioassays were conducted in 15l glass aquaria with 10l of non-chlorinated tap water each containing 10 fish. The values of the physicochemical parameters of water used in the study were as follows: temperature 29.7 ±0.8°C, pH 7.2 ±0.3, free CO2 26.7 ±2.4 mg/l, dissolved oxygen 5.3 ± 0.5 mg/l, total alkalinity 174 ± 13.9 mg/l as CaCO3, hardness 125 ± 3.8 mg/l as CaCO3. Each test was accompanied by three replicates with a control consisting of tap water without any toxicant.
The fish were not fed for 24h before the commencement of the test. Initial range-finding tests were conducted to estimate the spectrum of concentrations of the test chemical. Then the selected concentrations of cartap hydrochloride (00, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60 µg/l) were used to estimate the 24, 48, 72, and 96 LC50 to Oreochromis mossambicus. The number of dead organisms was counted at every 24h of exposure during the experiment and was removed immediately to avoid any organic decomposition. From each aquarium, 10% of water was removed every 24h and replaced with the desired quantity of cartap hydrochloride to assure a fixed concentration of the toxicant in the solution.
The safe level of cartap hydrochloride was calculated based on application factors (AF) using standard protocols (Burdick 1967 & Edwards et al., 1967). The ratio of the maximum harmless concentration of a toxicant to the concentration that is lethal, after a given exposure period, to 50 percent of test animals (median lethal concentration, LC50) has been termed the “application factor. Application factors are used to establish acceptable toxicant concentration ranges depending on water, quality, species under study and life stage (Edwards & Brown, 1967).
Collection of plant material and preparation of experimental diet.:Fresh leaves of Ocimum sanctum were collected from the campus of The University of Burdwan, Golapbag, West Bengal. They were washed thoroughly in running tap water and subsequently dried for 10 days. After 10 days of sun drying, the leaves were crushed and ground using a mixture grinder. The powdered form of Ocimum sanctum was then added to the control diet (containing fish meal, wheat flour, wheat bran, fish oil, sunflower oil, vitamins, and minerals) at an amount of 20 g/kg of fish feed. The amount of 20 g of Ocimum sanctum powder/kg feed was selected in our investigation as this particular amount of Ocimum sanctum powder/kg feed was reported to stimulate growth rates in fish (Sikotariya & Yusufzai, 2019).
Experiments on biochemical parameters during the sublethal bioassay: Bioassays on biochemical parameters were also conducted in 20l glass aquaria, each containing 10 l of water and five fish. 6 µg/l (30% of 96 h LC50 value)] was employed for the experiment along with a control. The design of the bioassay is depicted in Table 1
Table 1. Experimental design
| Group | Treatment |
| I | Control without any contaminant + control diet |
| II | Cartap hydrochloride (6µg/l) + control diet |
| III | Cartap hydrochloride (6µg/l) + 20 gm of Ocimum sanctum powder/ kg diet |
| IV | Control without any contaminant + 20 gm of Ocimum sanctum powder/ kg diet |
Fish was fed at 10% of the bodyweight daily. Amid the experiment, 20% of the test medium was renewed and replaced with the required amount of pesticide. After 15 and 30 days respectively, fish was anesthetized by immersing them in 0.1% 2-phenoxyethanol. Then the fish were decapitated, and the liver was immediately collected for biochemical examination.
Tissue Homogenization and centrifugation: The liver tissue was homogenized in 2ml of phosphate buffer saline (PBS). The homogenized tissues were spun in a refrigerated centrifuge (REMI C Model, India) at 5000rpm for 15 minutes at 4o C. After centrifugation the supernatants were used directly as aliquots and were stored at -20o C for enzymatic analysis
Protein estimation :The protein content in liver tissue was measured by using the method of Lowry et al. (1951). Bovine serum albumin (BSA, Sigma) was used as a standard.
Oxidative stress enzymes analysis :Catalase (CAT) activity was measured following the reduction of hydrogen peroxide to water and molecular oxygen using a standard protocol (Aebi 1984). The estimation of the superoxide dismutase enzyme (SOD) was carried out by the protocol of Kakkar et al. (1984). Glutathione S-transferase (GST) activity was measured through the conjugation of GSH with 1- chloro-2,4-dinitrobenzene (Habig et al., 1974). Standard protocol was employed for the analysis of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (Bergmeyer, 1965) was followed with some minor modifications. The colorimetric assay of lipid peroxidation (LPO) was performed following the standard protocol (Buege & Aust 1978). The units of CAT, SOD, GST AST, and ALT were expressed as units of activity per milligram of protein (U/mg protein). MDA level was expressed as nmol TBARS per min per milligram of protein (nmol TBARS/min/mg protein). All the parameters were measured using a UV-Vis spectrophotometer (Cecil Aquarius CE 7400) at room temperature (28oC). All assays were run in triplicate.
Determination of IBR and BRI: IBR was determined by utilizing standard protocol with minor modifications (Beliaeff & Burgeot 2002). The IBR analysis for each biomarker was performed as follows:
Estimation of the mean and standard deviation for each treatment
Standardization of the results for each treatment as Fi′ = (Fi – mean F)/S, where Fi′ is the standardized value of the biomarker, Fi is the mean value of a biomarker, F is the mean of the biomarker and S is the standard deviation (SD) calculated for the treatment-specific values of each biomarker
Using the standardized data, X was calculated as + Fi′ in the case of activation and –Fi′ in the case of suppression and the minimum value for each biomarker for all treatments was obtained and then added to X.
Eventually, the score S was measured as B = |min Fi′| + Z, where B is the actual value of the minimum Fi′ and |min Fi′| is the actual value of the minimum Fi′.
Finally, IBR was determined by multiplying the obtained value of each biomarker (Bi) by the value of the next biomarker and dividing each measurement by 2, and thereafter summing all the values.
Moreover, the biomarker response index (BRI) for determining the health status of the organism was performed using a standard protocol (APHA. 2005).
Statistical methods : Finney’s probit analysis method was employed for estimating LC50 values. The Shapiro-Wilk test was used to assess normal distributions and Levene’s test was employed to evaluate homogeneity. All data obtained from our study fulfilled the parametric criteria and were analyzed using One-way ANOVA followed by Tukey multiple comparisons test to compare the means among the different treatment groups within each exposure period. The correlation matrix and principal component analysis were performed using software Graphpad prism 9 and JMP Pro 14. p< 0.05, p < 0.01 and p < 0.001 and p<0.0001 were accepted as levels of statistical significance. Data are presented as mean ± SEM.
RESULTS AND DISCUSSION
Acute toxicity and Behavioural Responses:The 24, 48, 72, and 96 h LC50 values of cartap hydrochloride to Oreochromis mossambicus with 95% confidence limits are depicted in Table 1. Based on the 96h LC50 value, the safe permissible limit of cartap hydrochloride was determined which is depicted in Table 2 and is reported to be within the range of 2.0 – 8.3 µg/l.
Table 2. Lethal concentration values and 95% confidence limits of cartap hydrochloride to Oreochromis mossambicus
| Point | Exposure concentration (µg/l) | |||
| 24h | 48h | 72h | 96h | |
| LC 50 | 38.6
(34.1-43.7) |
33.4
(28.7-38.8) |
26.8
(22.1-32.4) |
20.7
(16.0-26.9) |
Table 3. Safe concentrations of cartap hydrochloride to Oreochromis
mossambicus at 96h exposure period
| Pesticide | 96h LC50 (µg/l) | Method | Application Factor (AF) | Safe Level (µg/l) |
| Cartap hydrochloride |
20.7
|
Edwards & Brown, 1967 | 0.4 | 8.3 |
| Burdick, 1967 | 0.1 | 2.0 |
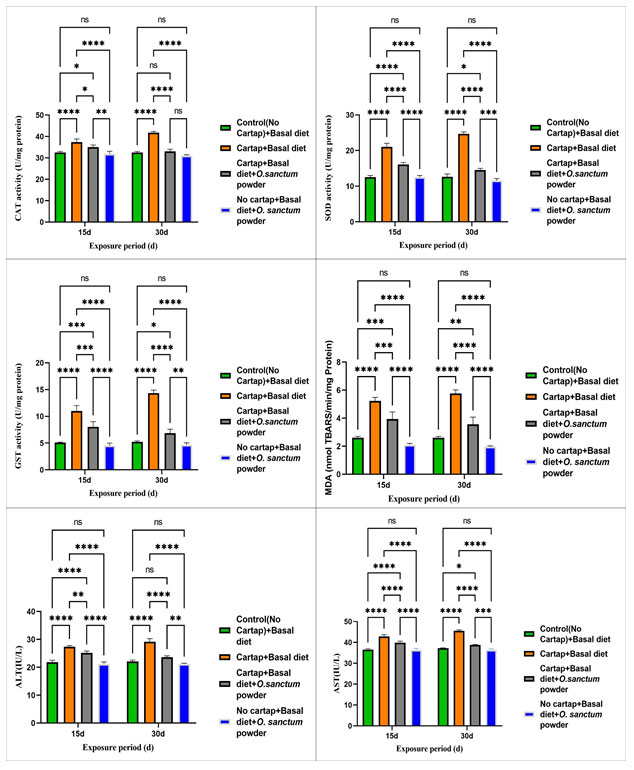
Oxidative stress and biochemical parameters:The effects of cartap hydrochloride on oxidative stress and biochemical parameters as well as their modulation by using Ocimum sanctum supplemented diet are depicted in Fig.1 respectively.
CAT activity :In 15d and 30d exposure period, catalase activity increased significantly (p< 0.05) in pesticide exposed fish supplemented with a control diet with respect to control. But the activity was reduced significantly (p< 0.05) upon addition of Ocimum sanctum supplemented diet to the fish exposed to 6 µg/l of pesticide on 15 and 30d exposure period.
SOD activity:SOD activity significantly increased (p< 0.05) in fish exposed to 6 µg/l of pesticide and provided with control diet after 15d and 30d with respect to control. However, the activity was significantly reduced (p< 0.05) when the Ocimum sanctum supplemented diet was incorporated to pesticide fish exposed on both 15d and 30d exposure periods.
GST activity” :In 15d and 30d exposure period, the activity of GST increased significantly (p<0.05) in fish exposed to 6 µg/l of cartap hydrochloride provided with a control diet with respect to the control. However, the activity was reduced significantly (p< 0.05) upon addition of Ocimum sanctum supplemented diet to the fish exposed to 6 µg/l of pesticide on both 15d and 30d exposure periods.
MDA activity : MDA activity increased significantly (p<0.05) in fish exposed to 6 µg/l of pesticide provided with a control diet in both 15d and 30d exposure periods with respect to the control. However, a significant reduction in MDA activity was observed upon the addition of an Ocimum sanctum supplemented diet to the exposed fish on both exposure periods.
AST and ALT activity: ALT and AST activities in fish exposed to 6 µg/l of pesticide combined with a control diet increased significantly (p<0.05) in 15d and 30d exposure periods compared to the control. However, adding an Ocimum sanctum enriched diet to the fish exposed to 6 µg/l of pesticide on both 15d and 30d treatment periods decreased the ALT and AST activities significantly (p<0.05).
Figure 1:Effects of cartap hydrochloride on A) CAT, B) SOD, C) GST, D) MDA, E) AST and F) ALT levels in Oreochromis mossambicus at different exposure periods. CH indicates cartap hydrochloride and OS indicates Ocimum sanctum. The values are represented as mean ±SEM, ns indicates non-significant and * indicates level of significance (* = p<0.05, ** = p<0.01, *** = p<0.001 and **** = p<0.0001).
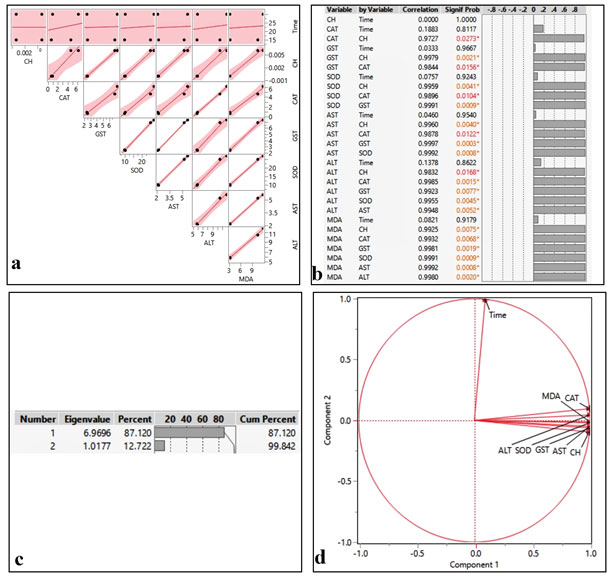
Chemometrics:The results of the correlation matrices between the concentration of cartap hydrochloride and all the tested parameters are depicted in Fig 2 a-b that was quantified by principal component analysis (Fig. 2 c-d). The statistical analysis demonstrated that CAT, SOD, MDA, AST, and ALT are positively and significantly correlated with cartap hydrochloride concentration (CH) (p <0.05).
Figure 2:Correlation matrix (a), pairwise comparison (b) and ordination diagram of PCA (c-d) on biochemical parameters of the liver in Oreochromis mossambicus. after exposure to cartap hydrochloride (CH)
IBR and BRI :IBR was utilized to determine the overall stress of the exposed fish Oreochromis mossambicus upon addition of a control diet vs supplemented diet. Higher IBR values indicate high stressful conditions while lower IBR values indicate the low stressful condition to the organism. In our investigation, the IBR value of pesticide-exposed Oreochromis mossambicus provided with a control diet is greater than the IBR value of pesticide-exposed Oreochromis mossambicus provided with Ocimum sanctum supplemented diet (Fig. 3).
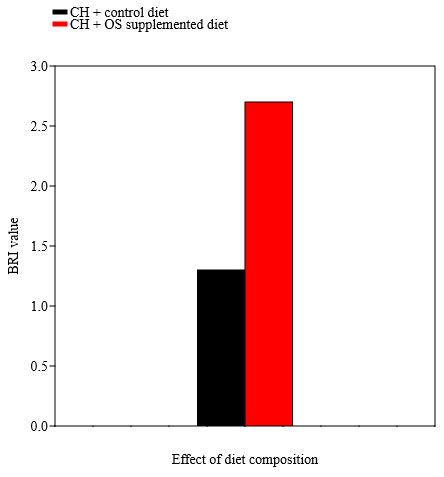
BRI designated the health status of pesticide exposed fish upon addition of control diet vs Ocimum sanctum supplemented diet. According to BRI, there are four classifications: no or slight effect (BRI>3), moderate effect (BRI between 2.75 and 3.0), major effect (BRI between 2.5 and 2.75), and severe effect (BRI ≤ 2.5). Our results indicated that based on BRI values severe adverse effect was observed in Oreochromis mossambicus exposed to cartap hydrochloride and supplemented with a control diet while the severity of the adverse effect was reduced in Oreochromis mossambicus exposed to cartap hydrochloride when provided with Ocimum sanctum supplemented diet (Fig. 4).
Figure 3:IBR values of cartap hydrochloride (CH) fish Oreochromis mossambicus upon addition
of control diet vs Ocimum sanctum supplemented diet
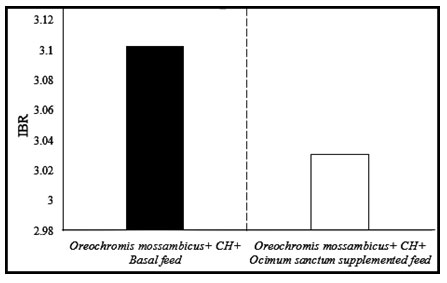
Figure 4: BRI values of cartap hydrochloride (CH) exposed Oreochromis mossambicus provided
with a control diet vs Ocimum sanctum (OS) supplemented diet.
In the present analysis, the 96h LC50 value of cartap hydrochloride to Oreochromis mossambicus is 20.7μg/l, suggesting that it is extremely toxic and is lower than the LC50 value of other fish species such as 0.376 mg/l in Cirrhinus mrigala and 0.3551 mg/l in Labeo rohita (Ali 2020 & Vani 2020). These variations between different fish species in the LC50 value of cartap hydrochloride depend on species, age, bodyweight water physiochemical parameters and duration of exposure (Saravanan et al., 2011). The result of the present study showed that the difference in species, water physiochemical parameters and duration of exposure is responsible for variations in LC50 value reported in our study from the study of previous researchers.
Biochemical stress indices are considered as potential biomarkers and are utilized as diagnostic tools to quantify the effects of environmental stress (Faheem 2019 & Iheanacho 2020). Superoxide dismutases (SOD) are a group of metalloenzymes that initially protects the cell against injury mediated by reactive oxygen species (ROS) (Bhattacharya et al., 2021; Chatterjee et al., 2021). These enzymes catalyze the dismutation of superoxide anion free radical (O2-) into molecular oxygen and hydrogen peroxide (H2O2) thereby damaging the cells (Bafana et al., 2011).
In the present study, SOD activity in the liver of exposed fish increased significantly which is due to the induction of superoxide ion that prevents the cell against oxidative stress (Zhang et al., 2013). A similar increase in SOD activity was reported in Pseudetroplus maculatus, Cyprinus carpio, and Ctenopharyngodon idellus, upon addition to Chlorpyrifos (Raibeemol, Chitra 2018 & Kaur 2017). CAT is an significant enzyme in the antioxidant system that is primarily involved in ROS detoxification and degradation of H2O2 into molecular oxygen and water. In the current study, the increase in catalase activity in pesticide exposed fish is likely due to the neutralization of the inimical effect of the toxicant-induced increased ROS generation.
Moreover, the greater increase in CAT activity revealed its efficient scavenging capabilities in eliminating H2O2 caused by pesticide-induced oxidative damage. An increase in CAT activity has also been observed in the studies with C. carpio after quinalphos exposure (Hemalatha et al., 2016). This upregulation of enzyme production might be a defense mechanism, providing the first line of defense against pesticide toxicity. To make xenobiotic chemicals more hydrophilic for excretion, GST promotes the conjugation of electrophilic substances or groups into tripeptide glutathione (Pontes et al., 2016). In the present analysis increased GST level in the liver of exposed fish is possibly due to the high rate of formation of glutathione disulfide (Li et al., 2010). Native gel electrophoresis study revealed that a significant increase in GST activity occurred in Mugil cephalus and Chanos chanos upon the addition of chlorpyriphos (Marigoudar et al., 2018).
One of the principal processes caused by oxidative stress is lipid peroxidation. Lipid peroxides are produced from the oxidative degradation of polyunsaturated lipids in the membranes of cells and organelles (Grotto et al., 2009). Bi-products of lipid peroxidation, such as malondialdehyde (MDA), are utilized as markers of incremented cellular reactive oxygen species concentration and symptoms of cellular injury (Grotto 2009 & Ayala 2014). Increased MDA in pesticide-exposed fish in the present study was likely due to induced oxidative cell injury and increased ROS generation (Faheem et al., 2019). In integration to chlorpyrifos and dichlorvos, similar induction of the MDA level has additionally been reported in Ctenopharyngodon idellus and juvenile Clarias gariepinus (Kaur 2017 & ON 2018).
Increased hepatic aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activity as examined in the current study is indicative of active amino acid catabolism to slake immediate energy demand under toxicant stress Hence, cellular damage caused by the toxicant was accompanied by increasing cell membrane permeability and enzyme leakage (Majumder & Kaviraj 2017). A high level of MDA coupled with the increase in the activities of hepatic enzymes in the liver was observed in our investigation. This positive correlation between MDA concentration as well as ALT and AST activities, suggests that the enhanced lipid peroxidation may be linked to hepatic damage caused by cartap hydrochloride. A similar induction in hepatic AST and ALT was reported when Oreochromis niloticus and Cyprinus carpio was exposed to chlorpyrifos (Stoyanova 2020 & Majumder 2017).
One of the oldest aromatic herbs, the leaves of tulsi (Ocimum sanctum) have great medicinal value, which keeps our body safe and averts the toxic effects of different environmental and chemical-induced injuries and damage by modulating the levels of anti-oxidant bio-molecules in the body (Sah et al., 2018). The leaf of tulsi, contains many bioactive compounds, including eugenol, ursolic acid, β-caryophyllene, linalool, and 1,8-cineole that might act as a potential immunostimulant. (Yang et al., 2020).
Several scientific studies reported that tulsi is a paramount remedy for chronic lifestyle-cognate diseases such as diabetes, metabolic syndrome, and psychological stress (Jamshidi & Cohen 2017). In our research, the Ocimum sanctum supplemented diet (20 g/kg diet) provided to cartap hydrochloride exposed Oreochromis mossambicus resulted in the substantial restoration of biochemical stress biomarkers by minimizing stress and thereby improving the health status of the fish as revealed from our IBR and BRI values. This shows that Ocimum sanctum possesses intrinsic antioxidant activity that resulted in the suppression of pesticide-induced oxidative stress.
Administration of Ocimum sanctum supplemented diet (20 g/kg diet) in the pesticide-exposed group not only restored oxidative stress biomarkers but also hepatic enzymes. The increased activity of ALT and AST was ameliorated in the group that received Ocimum sanctum supplemented diet (20 g/kg diet), suggesting that Ocimum sanctum can reduce hepatic enzyme activities after exposure to cartap hydrochloride. It seems that the enhanced antioxidant defense mechanism and diminished lipid peroxidation, resulting from Ocimum sanctum treatment, was able to protect the liver from oxidative damage caused by cartap hydrochloride, as evidenced by decreased hepatic enzyme activities.
This may be attributed to the presence of linolenic acid in the Ocimum sanctum, which can suppress the cycloxygenase and lipoxygenase pathways of arachidonic acid synthesis, resulting in anti-inflammatory action (Upadhyay 2017). Furthermore, the presence of beta carotene in the Ocimum sanctum aids in the prevention of cellular damage. (Upadhyay 2017). A similar type modulatory property of tulsi was found against arsenic toxicity in fish (Bhattacharya, 2017).
CONCLUSION
The present investigation revealed that Oreochromis mossambicus exhibited alterations in survivability and behavioral responses at the acute level as biochemical stress responses at sublethal level upon addition of pesticide. Thus, it is exposed from the work that cartap hydrochloride is prodigiously toxic to aquatic organisms. Therefore, the present findings on the toxicity of cartap hydrochloride to Oreochromis mossambicus may be used as a potential tool for creating awareness among people regarding the excessive use of agrochemicals. Furthermore, our studies also demonstrated that the addition of tulsi to the diet potentially abridged the toxic responses in fish induced by the addition of pesticide. Therefore, special attention should also be given to manufacturing the feed of the fish by including medicinal plant extracts in the feed to reduce the stress responses in fish induced by exposure to several contaminants in water. Further studies are required to elucidate the toxic effect of cartap hydrochloride on Oreochromis mossambicus and its modulation using Ocimum sanctum supplemented diet at the molecular and ultrastructural level.
Funding:The research did not receive any specific grant from funding agencies in the public, commercial or nonprofit sectors.
Conflict of interest:The authors have no conflict of interest.
ACKNOWLEDGEMENT
The authors are thankful to the Department of Zoology, The University of Burdwan and DST PURSE PHASE II for providing infrastructural facilities to carry out the work.
Data Availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Sharmistha Medda: Writing original draft, Validation, Methodology, Ritwick Bhattacharya: Software, validation, Formal Analysis, Data curation, Sarmila Pal: Investigation, Resources, Editing; Nimai Chandra Saha: Conceptualization, Writing Review and Editing, Visualization, Supervision
REFERENCES
Aebi, H. (1984). Catalase in Vitro. Methods in Enzymology, 105(C), 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ajima, M. N. O., Pandey, P. K., Kumar, K., & Poojary, N. (2017). Neurotoxic effects, molecular responses and oxidative stress biomarkers in Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) exposed to verapamil. Comparative Biochemistry and Physiology Part – C: Toxicology and Pharmacology, 196, 44–52. https://doi.org/10.1016/j.cbpc.2017.03.009
Ali, D., Almarzoug, M. H. A., Al Ali, H., Samdani, M. S., Hussain, S. A., & Alarifi, S. (2020). Fish as bio indicators to determine the effects of pollution in river by using the micronucleus and alkaline single cell gel electrophoresis assay. Journal of King Saud University – Science, 32(6), 2880–2885. https://doi.org/10.1016/j.jksus.2020.07.012
APHA. (2005). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA:Washington, DC, USA, 2005. American Water Works Association/American Public Works Association/Water Environment Federation, 552. https://doi.org/10.2105/AJPH.51.6.940-a
Ayala, A., Muñoz, M. F., & Argüelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. Landes Bioscience. https://doi.org/10.1155/2014/360438
Bafana, A., Dutt, S., Kumar, A., Kumar, S., & Ahuja, P. S. (2011, February). The basic and applied aspects of superoxide dismutase. Journal of Molecular Catalysis B: Enzymatic. https://doi.org/10.1016/j.molcatb.2010.11.007
Balami, S., Sharma, A., & Karn, R. (2020). Significance Of Nutritional Value Of Fish For Human Health. Malaysian Journal of Halal Research, 2(2), 32–34. https://doi.org/10.2478/mjhr-2019-0012
Beliaeff, B., & Burgeot, T. (2002). Integrated biomarker response: A useful tool for ecological risk assessment. In Environmental Toxicology and Chemistry (Vol. 21, pp. 1316–1322). SETAC Press. https://doi.org/10.1002/etc.5620210629
Bergmeyer, H.-U. (1965). Principles of Enzymatic Analysis. In Methods of Enzymatic Analysis (pp. 3–13). Elsevier. https://doi.org/10.1016/b978-0-12-395630-9.50008-6
Bhattacharjee, P., Borah, A., & Das, S. (2020). Quercetin-induced amelioration of deltamethrin stress in freshwater teleost, Channa punctata: Multiple biomarker analysis. Comparative Biochemistry and Physiology Part – C: Toxicology and Pharmacology, 227. https://doi.org/10.1016/j.cbpc.2019.108626
Bhattacharya, R., Chatterjee, A., Chatterjee, S., & Saha, N. C. (2021). Oxidative stress in benthic oligochaete worm, Tubifex tubifex induced by sublethal exposure to a cationic surfactant cetylpyridinium chloride and an anionic surfactant sodium dodecyl sulfate. Comparative Biochemistry and Physiology Part – C: Toxicology and Pharmacology, 240. https://doi.org/10.1016/j.cbpc.2020.108906
Bhattacharya, S. (2017). Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharmaceutical Biology. Taylor and Francis Ltd. https://doi.org/10.1080/13880209.2016.1235207
Boorugu, H. K., & Chrispal, A. (2012). Cartap hydrochloride poisoning: A clinical experience. Indian Journal of Critical Care Medicine, 16(1). https://doi.org/10.4103/0972-5229.94443
Buege, J. A., & Aust, S. D. (1978). Microsomal Lipid Peroxidation. Methods in Enzymology, 52(C), 302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Burdick, G. E. (1967). Use of bioassays in determining levels of toxic wastes harmful to aquatic organisms. In A symposium on water quality criteria to protect aquatic life (pp. 7–12). Trans. Am. Fish. Soc. Washington.
Carazo-Rojas, E., Pérez-Rojas, G., Pérez-Villanueva, M., Chinchilla-Soto, C., Chin-Pampillo, J. S., Aguilar-Mora, P., … Vryzas, Z. (2018). Pesticide monitoring and ecotoxicological risk assessment in surface water bodies and sediments of a tropical agro-ecosystem. Environmental Pollution, 241, 800–809. https://doi.org/10.1016/j.envpol.2018.06.020
Chatterjee, A., Bhattacharya, R., Chatterjee, S., & Saha, N. C. (2021). Acute toxicity of organophosphate pesticide profenofos, pyrethroid pesticide λ cyhalothrin and biopesticide azadirachtin and their sublethal effects on growth and oxidative stress enzymes in benthic oligochaete worm, Tubifex tubifex. Comparative Biochemistry and Physiology Part – C: Toxicology and Pharmacology, 242. https://doi.org/10.1016/j.cbpc.2020.108943
Costa, A. I. G., Queiroz, M. E. L. R., Neves, A. A., De Sousa, F. A., & Zambolim, L. (2015). Determination of pesticides in lettuce using solid-liquid extraction with low temperature partitioning. Food Chemistry, 181, 64–71. https://doi.org/10.1016/j.foodchem.2015.02.070
Dar, S. A., Yousuf, A. R., Balkhi, M. ul H., Ganai, F. A., & Bhat, F. A. (2015). Assessment of endosulfan induced genotoxicity and mutagenicity manifested by oxidative stress pathways in freshwater cyprinid fish crucian carp (Carassius carassius L.). Chemosphere, 120, 273–283. https://doi.org/10.1016/j.chemosphere.2014.07.031
Edwards, R. W., & Brown, V. M. (1967). Pollution and fisheries: a progress report. Wat. Pollut. Control, 66, 63–78.
Elia, A. C., Giorda, F., Pacini, N., Dörr, A. J. M., Scanzio, T., & Prearo, M. (2017). Subacute toxicity effects of deltamethrin on oxidative stress markers in rainbow trout. Journal of Aquatic Animal Health, 29(3), 165–172. https://doi.org/10.1080/08997659.2017.1349006
Faheem, M., Khaliq, S., & Lone, K. P. (2019). Effect of bisphenol-A on serum biochemistry and liver function in the freshwater fish, Catla catla. Pakistan Veterinary Journal, 39(1), 71–75.
Ghane, M. N., Dhamagaye, H. B., Meshram, S. J., & Salunkhe, A. (2018). Toxic Effects of Paclobutrazol in Oreochromis mossambicus Fingerlings. Pesticide Research Journal, 30(1), 112. https://doi.org/10.5958/2249-524x.2018.00019.5
Gilden, R., Plisko, M., Hiteshew, K., Friedmann, E., & Milton, D. (2016). Pesticide monitoring on soccer fields via shoe wipes and urine samples. Environmental Research, 147, 294–296. https://doi.org/10.1016/j.envres.2016.02.027
Grotto, D., Santa Maria, L., Valentini, J., Paniz, C., Schmitt, G., Garcia, S. C., … Farina, M. (2009). Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quimica Nova. Sociedade Brasileira de Quimica. https://doi.org/10.1590/S0100-40422009000100032
Gupta, M., Handa, D., Chaturvedi, A., Singh, R., & Lehl, S. S. (2015). Cartap poisoning: an unusual poisoning in North India. International Journal of Scientific Reports, 1(1), 99. https://doi.org/10.18203/issn.2454-2156.intjscirep20150214
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249(22), 7130–7139.
Hagger, J. A., Jones, M. B., Lowe, D., Leonard, D. R. P., Owen, R., & Galloway, T. S. (2008). Application of biomarkers for improving risk assessments of chemicals under the Water Framework Directive: A case study. Marine Pollution Bulletin, 56(6), 1111–1118. https://doi.org/10.1016/j.marpolbul.2008.03.040
Hemalatha, D., Amala, A., Rangasamy, B., Nataraj, B., & Ramesh, M. (2016). Sublethal toxicity of quinalphos on oxidative stress and antioxidant responses in a freshwater fish Cyprinus carpio. Environmental Toxicology, 31(11), 1399–1406. https://doi.org/10.1002/tox.22145
Iheanacho, S. C., & Odo, G. E. (2020). Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environmental Science and Pollution Research, 27(17), 21159–21173. https://doi.org/10.1007/s11356-020-08611-9
Jamshidi, N., & Cohen, M. M. (2017). The Clinical Efficacy and Safety of Tulsi in Humans: A Systematic Review of the Literature. Evidence-based Complementary and Alternative Medicine. Hindawi Limited. https://doi.org/10.1155/2017/9217567
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry and Biophysics, 21(2), 130–132.
Kalyaniwala, K., Abhilash, K. P. P., & Victor, P. J. (2016). Cartap hydrochloride poisoning. Journal of Association of Physicians of India, 64(AUGUST), 91–92.
Kaur, M. (2017). Oxidative Stress Response in Liver, Kidney and Gills of Ctenopharyngodon Idellus (Cuvier & Valenciennes) Exposed To Chlorpyrifos. MOJ Biology and Medicine, 1(4). https://doi.org/10.15406/mojbm.2017.01.00021
Li, X. Y., Luo, Y. R., Yun, M. X., Wang, J., & Wang, J. J. (2010). Effects of 1-methyl-3-octylimidazolium bromide on the anti-oxidant system of earthworm. Chemosphere, 78(7), 853–858. https://doi.org/10.1016/j.chemosphere.2009.11.047
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. The Journal of biological chemistry, 193(1), 265–275. https://doi.org/10.1016/0922-338X(96)89160-4
Majumder, R., & Kaviraj, A. (2017). Cypermethrin induced stress and changes in growth of freshwater fish Oreochromis niloticus. International Aquatic Research, 9(2), 117–128. https://doi.org/10.1007/s40071-017-0161-6
Marigoudar, S. R., Nagarjuna, A., Karthikeyan, P., Mohan, D., & Sharma, K. V. (2018). Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere, 211, 89–101. https://doi.org/10.1016/j.chemosphere.2018.07.137
Maurya, P. K., Malik, D. S., & Sharma, A. (2019). Impacts of pesticide application on aquatic environments and fish diversity. In Contaminants in Agriculture and Environment: Health Risks and Remediation (pp. 111–128). Agro Environ Media – Agriculture and Ennvironmental Science Academy, Haridwar, India. https://doi.org/10.26832/aesa-2019-cae-0162-09
Nishimoto, R. (2019). Global trends in the crop protection industry. Journal of Pesticide Science. Pesticide Science Society of Japan. https://doi.org/10.1584/jpestics.D19-101
Ogunnupebi, T. A., Oluyori, A. P., Dada, A. O., Oladeji, O. S., Inyinbor, A. A., & Egharevba, G. O. (2020). Promising Natural Products in Crop Protection and Food Preservation: Basis, Advances, and Future Prospects. International Journal of Agronomy. Hindawi Limited. https://doi.org/10.1155/2020/8840046
ON, H., PC, G., & EA, C. (2018). Toxic Effect of Dichlorvos-Pesticide on Lipid Peroxidation, Superoxide Dismutase and Catalase of Clarias gariepinus. Journal of Fisheries & Livestock Production, 06(03). https://doi.org/10.4172/2332-2608.1000276
Özkara, A., Akyil, D., & Konuk, M. (2016). Pesticides, Environmental Pollution, and Health. In Environmental Health Risk – Hazardous Factors to Living Species. InTech. https://doi.org/10.5772/63094
Pontes, F. J. S., Maia, R. T., Lima, M. C. P., Ayres, C. F. J., & Soares, T. A. (2016). The role of the conformational dynamics of glutathione S-transferase epsilon class on insecticide resistance in Anopheles gambiae. Journal of the Brazilian Chemical Society, 27(9), 1602–1615. https://doi.org/10.5935/0103-5053.20160040
R., D., S., M., S.K., B., & R.K., M. (2017). An observational study of 11 cases of Cartap poisoning-a rare poisoning. Asian Journal of Pharmaceutical and Clinical Research, 10(11), 366–369. Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L619212341
Rahimikia, E. (2017). Analysis of antioxidants and serum biochemical responses in goldfish under nickel exposure by sub-chronic test. Journal of Applied Animal Research, 45(1), 320–325. https://doi.org/10.1080/09712119.2016.1190732
Raibeemol, K. P., & Chitra, K. C. (2018). Effects of Chlorpyrifos as Inducer for Oxidative Stress in Liver, Kidney and Spleen of Freshwater Fish, Pseudetroplus maculatus (Bloch, 1795). Journal of Toxicology, 8(1), 20–29. Retrieved from www.stmjournals.com
Reza, A. H. M. M., Rakhi, S. F., Hossen, M. S., & Hossain, Z. (2017). Organ specific histopathology and brain acetylcholinesterase inhibition in rohu, Labeo rohita and silver barb, Barbonymus gonionotus: Effects of three widely used organophosphate pesticides. Turkish Journal of Fisheries and Aquatic Sciences, 17(4), 821–832. https://doi.org/10.4194/1303-2712-v17_4_18
Sah, A. K., Vijaysimha, & Mahamood, M. (2018). The tulsi, queen of green medicines: Biochemistry and pathophysiology. A review. International Journal of Pharmaceutical Sciences Review and Research, 50(2), 106–114.
Saravanan, M., Prabhu Kumar, K., & Ramesh, M. (2011). Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pesticide Biochemistry and Physiology, 100(3), 206–211. https://doi.org/10.1016/j.pestbp.2011.04.002
Sethi, J., Sood, S., Seth, S., & Talwar, A. (2004). Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian Journal of Clinical Biochemistry, 19(2), 152–155. https://doi.org/10.1007/BF02894276
Smita, K. (2018). Evaluation of α-glucosidase inhibitory potential of methanolic leaf extract of Ocimum canum. International Journal of Pharmacy and Pharmaceutical Sciences, 10(1), 126. https://doi.org/10.22159/ijpps.2018v10i1.22268
Stoyanova, S., Georgieva, E., Velcheva, I., Iliev, I., Vasileva, T., Bivolarski, V., … Yancheva, V. (2020). Multi-biomarker assessment in common carp (Cyprinus carpio, Linnaeus 1758) liver after acute chlorpyrifos exposure. Water (Switzerland), 12(6). https://doi.org/10.3390/w12061837
Tan, B. L., Norhaizan, M. E., Liew, W. P. P., & Rahman, H. S. (2018, October 16). Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Frontiers in Pharmacology. Frontiers Media S.A. https://doi.org/10.3389/fphar.2018.01162
Tsagkaris, A. S., Uttl, L., Pulkrabova, J., & Hajslova, J. (2020). Screening of carbamate and organophosphate pesticides in food matrices using an affordable and simple spectrophotometric acetylcholinesterase assay. Applied Sciences (Switzerland), 10(2). https://doi.org/10.3390/app10020565
Upadhyay, R. K. (2017, January 1). Tulsi: A holy plant with high medicinal and therapeutic value. International Journal of Green Pharmacy. BRNSS Publication Hub.
Vani, G., Veeraiah, K., Vijaya Kumar, M., Parveen, S. K., & Prasad Rao, G. D. V. (2020). Biochemical changes induced by Cartap hydrochloride (50% SP), carbamate insecticide in freshwater fish Cirrhinus mrigala (Hamilton, 1822). Nature Environment and Pollution Technology, 19(5), 1821–1829. https://doi.org/10.46488/NEPT.2020.v19i05.005
Vivek, C., Veeraiah, K., Padmavathi, P., Rao, H. D., & Bramhachari, P. V. (2016). Acute toxicity and residue analysis of cartap hydrochloride pesticide: Toxicological implications on the fingerlings of fresh water fish Labeo rohita. Biocatalysis and Agricultural Biotechnology, 7, 193–201. https://doi.org/10.1016/j.bcab.2016.06.005
Yang, C., Lim, W., & Song, G. (2020, August 1). Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comparative Biochemistry and Physiology Part – C: Toxicology and Pharmacology. Elsevier Inc. https://doi.org/10.1016/j.cbpc.2020.108758
Zhang, Q., Zhang, Y., Du, J., & Zhao, M. (2017). Environmentally relevant levels of Λ-cyhalothrin, fenvalerate, and permethrin cause developmental toxicity and disrupt endocrine system in zebrafish (Danio rerio) embryo. Chemosphere, 185, 1173–1180. https://doi.org/10.1016/j.chemosphere.2017.07.091
Zhang, Q., Zhu, L., Wang, J., Xie, H., Wang, J., Han, Y., & Yang, J. (2013). Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environmental Science and Pollution Research, 20(1), 201–208. https://doi.org/10.1007/s11356-012-0962-5


