1Department of Biotechnology, School of Sciences , Jain University, #18/3, 9th
Main, 3rd Block, Jayanagar, Bangalore 560011, Karnataka, India.
Corresponding author email: kn.varalakshmi@jainuniversity.ac.in
Article Publishing History
Received: 05/07/2020
Accepted After Revision: 15/09/2020
Marine algae or seaweeds serve as excellent resources of bioactive secondary metabolites with wide range of therapeutic applications and multiple biological activities. Despite the growing worth of algae as a source of pharmacological compounds, the biotechnological or anticancer application of marine algae are still under-exploited. Hence the present study is aimed at exploring the anti proliferative activity of the marine macroalga, Gelediella acerosa against HeLa,HepG2 and MCF-7 cancer cell lines and to identify the bioactive compound. The secondary metabolites of G. acerosa were extracted using methanol, the extract was purified by TLC. The bioactive fraction was selected through bioassay guide fractionation and the cytotoxic, apoptogenic potential of this fraction was analysed by different in vitro assays such as MTT assay, LDH assay, Trypan blue dye exclusion, DNA fragmentation, caspase activity and cell cycle analysis by flow cytometry. The characterization of the bioactive fraction was performed through GC MS analysis. The results of MTT and trypan blue assays indicated significant cytotoxicities to the GF7 treated HeLa, HepG2 and MCF-7 cancer cells and at the same time demonstrated non-toxicity to normal human lymphocytes at 50µg/mL concentration.
The mechanism of action of this fraction on the cancer cells was observed as apoptosis induction as indicated by significantly elevated caspase activity, decreased cell counts, DNA fragmentation pattern and elevated LDH enzyme activities. Cell cycle analysis showed majority of cells accumulating in the sub-G1 phase that further confirmed apoptosis induction by the algal fraction. GC-MS analysis indicated the presence of hexadecanoic acid, previously documented for anticancer activity, that might be responsible for its bioactivity. It can be concluded that this algal bioactive fraction (GF7) has significant anti-cancer potential at low concentrations and shows promise for future in-vivo studies that might lead towards a safer anti cancer compound.
Anti-Cancer, Apoptosis, Cell Cycle, Gelidiella Acerosa, Sub-G1
Parveen S, Varalakshmi K.N. Accumulation of Cells in Sub-G1 Phase and Apoptosis Induction by A Bioactive Fraction from the Seaweed Gelidiella acerosa. Biosc.Biotech.Res.Comm. 2020;13(3).
Parveen S, Varalakshmi K.N. Accumulation of Cells in Sub-G1 Phase and Apoptosis Induction by A Bioactive Fraction from the Seaweed Gelidiella acerosa. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/30XPcrJ
Copyright © Parveen and Varalakshmi This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cancer is one of the major challenging diseases that leads to the death of several million people globally in an annual basis (Aggarwal et al., 2009). The fundamental characteristic of all types of cancers is uncontrolled cell division (Tay et al 2019). With most of the existing treatment modalities ineffective in completely curing the disease, there is a need to search for ideal anti-cancer agents from natural resources. It has been reported that uncontrolled symmetric cell division is the major factor that contributes to cancer. Hence it is quite natural for scientists to aim anticancer drugs that control cell cycle machineries and that induce apoptosis (Bai et al., 2017, Ashraf 2020).
Marine organisms with a wide range of bioactive secondary metabolites of pharmaceutical significance have the potential towards the development of anti cancer agents (Newman and Gordon, 2016). Marine algae comprise one of the rich sources of a wide range of secondary metabolites. The protective effect of edible seaweeds has been established against mammary, skin and intestinal carcinogenesis (Yuan and Walsh 2006). Marine algae have been used as food and also as traditional medicine in the eastern hemisphere (Kilinc et al., 2013). The red alga, Gelidiella acerosa is abundantly found in the coastal area of South India, especially in inter tidal region of Gulf of Mannar (Taskin et al., 2007; Bernecker et al., 2009). G. acerosa is mainly used for agar production and also has many phytochemical constituents having biological activities including anti cancer activities (Duraikannu et al., 2014; Begum et al., 2018).
Though anticancer activity was reported for G. acerosa, previous studies (both in-vitro and in-vivo) were carried out using crude extracts of this promising algal species. Hence we felt the necessity to purify and characterize the bioactive compounds from this red alga and aimed to study the mechanism of action of its bioactive components on cell cycle stages of cancer cell lines in the present study.
MATERIAL AND METHODS
Preparation of algal extract and extraction of metabolites: Seaweed was collected from the Mandapam camp at Rameshwaram, Tamilnadu in India. The collected seaweed was identified as Gelidiella acerosa (Figure 1) and authenticated by Dr.Eswaran, Principle scientist at marine algal research station, Rameshwaram.G. acerosa was washed and dried for 7 days under the shade. The dried samplewas powdered with the help of amixer grinder and themetaboliteswere extracted in a soxhlet apparatus using methanol as the solvent. The extract was further concentrated in a rotary evaporator (IKA, Germany) at 40º C.
The methanol extract of G.acerosa was initially screened for cytotoxicity to cancer cell lines (HepG2, HeLa and MCF-7) by MTT cell viability assay.The methanol extract was further fractionated by TLC sheets using different solvent combinations.Through bioassay guided fractionation, the bioactive fraction was chosen for further cytotoxicity assays.
Figure 1: Macroscopic view of Gelidiellaacerosa
MTT Cell viability assay: MTT[3-(4,5-dimethythiazol2-yl)-2,5-diphenyl tetrazolium bromide] assay is a colorimetric assay to determined the viability of the cells treated with the samples (Mosmann, 1983). The percentage viability of the cells was calculated as follows:
Percentage viability (%) = O.D 540 of treated / O.D 540 of control × 100.
Trypan Blue assay:Trypan blue dye exclusion test was carried out to determine the concentration of viable and dead cells in a cell suspension (Strober, 1997).Cells were treated with 50µg/ml of the sample for 48 hrs. The cellconcentration was determined by counting the number of viable cells in the treated group with the help of a hemocytometer.
LDH cytotoxicity assay:The extent of damage in the treated cells was analysed by LDH cytotoxicity assay in the treated cells as per the protocol given in the kit manual (Weyermann et al., 2005).
Caspase -3,7 and 10 activities:The activity of caspase enzyme in the sample treated cancer cells was measured using CasPASE(tm) Apoptosis Colorimetric Assay kit (G Biosciences Ltd, USA),as per the instructions given in the manual of the kit manufacturer.
DNA fragmentation assay:DNA fragmentation is a semi quantitative method to analyse the fragmentation of DNA in the treated group in which the cells were harvested, DNA was extracted and loaded to 0.8% agarose gel for electrophoresis (Shidoji and Ogawa, 2004).
Flow cytometry analysis with Propidium Iodide staining:Flow cytometry analysis of the sample treated and untreated cancer cells was performed to analysethe distribution of DNA at different stages of the cellcycle.After 48 hrs of sample treatment, the cells were harvested and the cell cycle was analysed as per the standard methodology (Pazarowski and Darzynkiewick, 2004).
GC-MS analysis:The GF7 fraction of G. acerosa was subjectedto analysis through GC-MS method at the facility of Central Silk Technological Research Institute, Bangalore. The resulting mass spectral peaks of unknown compounds were analyzed and compared with the database of anti-cancer compounds so as to identify the bioactive component.
RESULTS AND DISCUSSION
Drug discovery from natural sources such as marine algae is an important area of recent research in cancer Biology. Many marine algae were found to produce structurally diverse secondary metabolites of therapeutic significance.In the current study, we mainly focussed toevaluate the anti cancer potential of the red alga G. acerosa towards in-vitro cancer cell lines.
The methanol extract of G.acerosa was found to have anti proliferative activity to all the tested cancer cell lines at 24,48,72 and 96 h of treatment. The maximum inhibition of cell proliferation was observed at the higher concentration of 100µg/ml. The viability was 46.82% for HepG2, 40.38% for MCF-7 cells and 36.59% for HeLa cells after 96 h of treatment with 100µg/ml of the methanol extract of G. acerosa (Table 1).
Table 1. Effect of methanol extract of G. acerosa to various cancer cell lines at different concentrations.
| Concentration (µg/ml) | Viability (%) | |||||
| HeLa | ||||||
| Control | Treated | |||||
| 24 h | 48 h | 72 h | 96 h | |||
| 1 | 100 | 100 | 83.31 | 71.65 | 49.97 | |
| 10 | 100 | 100 | 82.61 | 62.77 | 68.51 | |
| 50 | 100 | 100 | 76.63 | 53.40 | 37.72 | |
| 100 | 100 | 98.1 | 66.20 | 51.82 | 36.59 | |
| HepG2 | ||||||
| 1 | 100 | 100 | 100 | 80.63 | 72.72 | |
| 10 | 100 | 100 | 82.37 | 75.25 | 67.58 | |
| 50 | 100 | 89.6 | 81.55 | 62.96 | 53.58 | |
| 100 | 100 | 73.7 | 73.77 | 57.74 | 46.82 | |
| MCF-7 | ||||||
| 1 | 100 | 99.48 | 97.1 | 92.13 | 80.87 | |
| 10 | 100 | 79.23 | 73.3 | 70.48 | 58.90 | |
| 50 | 100 | 72.43 | 73.0 | 69.6 | 46.67 | |
| 100 | 100 | 66.66 | 55.1 | 54.8 | 40.38 | |
The methanol extract of G. acerosa was partially purified by thin layer chromatography using the solvent combination ofAcetonitrile : chloroform: dichloromethane: Toulene (1:2:2:1). Seven different fractions were observed under UV and visible lights. When each of the fractions were tested by MTT assay,the 7th fraction (GF7) demonstrated maximum cytotoxicity thanthe other fractions (results not shown). When the effect of GF7 was analysedon different cancer cell lines, an increase in the treatmentconcentration of GF7 (from 1 to 50 µg/ml) resulting in a decreased viability of cells was seen. The cell viability at 50µg/ml was 36.5 % for HeLa, 46.8% for HepG2 and 40.3% for MCF-7 cells after 96h treatment (Figure 2). The IC50 value of GF7 was calculated as 39 µg/ml for HeLa, 27µg/ml for HepG2 and 37µg/ml for MCF-7cells for 96h of treatment.
Figure 2: Percentage cell viability of the cancer cells treated with GF7 at different treatment periods of 24, 48 and 96 hrs. * represents significance at p>0.05,** represents significance at p>0.01.
In a previous report (Lakmal et al., 2014), where the anticancer activity of six different seaweeds were analysed, G. acerosa had moderate inhibition against HL-60 cells and had no cytotoxicity against mouse melanoma (B16F10) and human lung carcinoma (A549) cells. As compared to this report, the present study shows promising anti-cancer activity of G. acerosa.
When the effect of GF7 on HepG2, HeLa and MCF-7 cell concentration was examined by trypan blue assay,it wasclearthat all the cell lines treated with the bioactive fraction GF7 had a decreased viable cell count as compared tothe control cells (Table 2). There were 3.29×106 cells/ml in HeLa, 3.2 X 106cells/ml in MCF-7 and 3.57 x 106 cells/ml in HepG2 cells after 48h of treatment with the viability ranging between 35.0-38.79%. No cytotoxicity was observed to the treated normal lymphocytes.
Table 2. Determination of cell count and cell viability by Trypan blue method
| Cell lines | Untreated Control | GF7 Treated | ||
| Viable Cell count
(1×106 cells/ml) |
Viability (%) | Viable Cell count (1×106cells/ml) | Viability (%) | |
| HeLa | 8.56 | 99.76 | 3.29 | 38.79 |
| MCF-7 | 9.93 | 96.59 | 3.62 | 35.01 |
| HepG2 | 9.81 | 98.29 | 3.57 | 35.87 |
| Normal Lymphocytes | 1.51 | 98.69 | 0.97 | 97.53 |
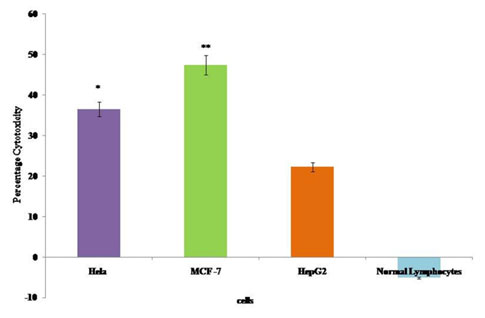
The cytotoxic effect of the bioactive fraction GF7 to the cancer cells was assessed by LDH assay. Changes in the membrane integrity or membrane damage cause the release of LDH enzyme into extracellular media. The cytotoxicity caused by GF7 fraction was 36.47% on HeLa, 47.38% on MCF-7 and 22.25% on HepG2 cells as compared to the control cells. No cytotoxic effect was observed tonormal human lymphocytes by GF7 treatment (Figure 3).
Figure 3: LDH cytotoxicity of the cancer cells treated with GF7.
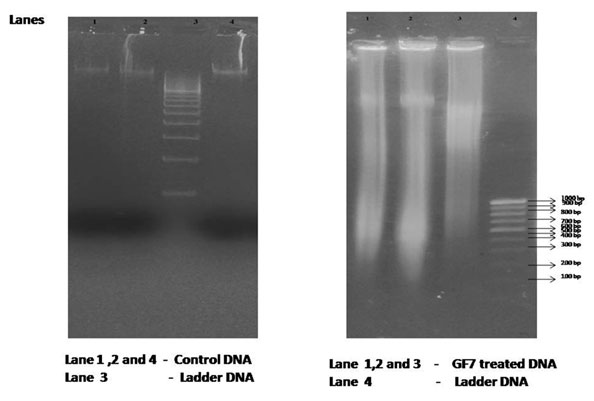
The hallmark of apoptosis is the degradation of DNAinto fragments by endogenous DNAses. The mechanism of cell deathcaused by GF7 is determined by DNA fragmentation analysis (Saraste and Pulkki, 2000; Elmore, 2007).Based on the results of this analysis, we could seeshearing of DNA in the treated cancer cells as compared to the control cells, where the DNAwas intact and a single band was visible (Figure 4).
Figure 4: DNA fragmentation analysis of cells treated with fraction GF7
Caspases are a family of aspertate- specific cysteine proteases and detection of activation of caspase activity is avalid method for assessing apoptosis ( Chang and Yang, 2000).In our study, cells treated with GF7 had significantly higher caspase activity (Figure 5). The percentage increase in caspase activity of HepG2, HeLa and MCF-7 cells were 67.20%,16.02% and 7.9% respectively.
Figure 5: Caspase activity in cancer cells treated with 50µg/ml of fraction GF7.* represent significant difference (p>0.05) between treated and untreated cells.
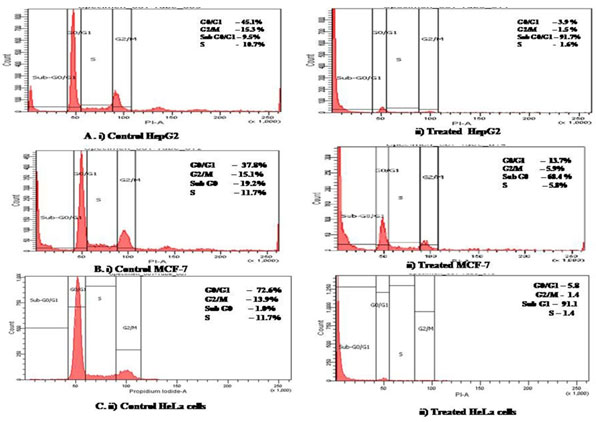
When cell cycle stages were analysed by flow cytometry, we observed that GF7 treated cancer cellshad increased number of dead cells which accumulated in the sub G1 phase of the cell cycle (Figure 6).Sub G1 indicates apoptotic cells. The concentration of sub-G1 phase was 81.7% in HepG2, 68.4% in MCF-7and 80.4% in HeLa cells.
Figure 6: Flow cytometry analysis of cell cycle stages by propidium iodide staining in cancer cells untreated and treated with GF7.
As the bioactive fraction GF7 from G. acerosa showed significant apoptogenic potential towards cancer cells, this fraction was subjected to GC-MS method for characterization. From the results, we found hexadecanoic acid in the major peak at RT 18.3 min (Figure 7).
Figure 7: GC-MS analysis of fraction GF7. A. Gas Chromatogram and B. mass spectrum of fraction GF7 along with the Structure of Hexadecanoic acid
Apoptosis induction is an important strategy to control and manage cancer. Cell cycle arrest at key points such as G1, S, G2 and G2/M drives the cells through apoptosis. Many synthetic or natural anticancer compounds lead to cell cycle arrest and apoptosis as reported by many researchers (Alabsi et al., 2013; Wafa et al., 2020). In the current study, we could establish the anticancer potential of the methanol extract of the marine alga G. acerosa, to cervical, breast and liver cancer cell lines at lower concentrations of 25 and 50µg/ml when compared to earlier reports (Murugan and Iyer, 2013; Lakmal et al., 2014; Duraikannu et al., 2014; Begum et al., 2018).
In a previous study, the methanol extract of G. acerosa has shown cytotoxicity to HL60 cancer cell line at higher concentrations of 100 and 200µg/ml, where the IC50 value was reported as 104.4 µg/ml (Lakmal et al., 2014). In another much recent study the IC50 value of G. acerosa against lung cancer cells was reported as 1.5 mg/ml (Begum et al., 2018). As compared to these earlier study reports, the crude methanol extract of G. acerosa in our present study we got higher cytotoxicity to all the tested cancer cell lines with IC50 values <50 µg/ml for 96h of treatment duration and after purification by TLC the IC50 value of the bioactive GF7 fraction much greater cytotoxicity with IC50 values further decreasing, with a range of 27µg/ml -39 µg/ml towards HeLa, MCF-7 & HepG2 cancer cells.
The bioactive compounds which are present in crude extracts at very low concentrations get concentrated during the purification process and that could be the reason for higher specificity and low IC50 value of G. Acerosa purified fraction in the current study. There are reports from many other red algae having cytotoxicity to human cancer cell lines (Harada and Kamei, 1997; El, Baroty et al., 2007). In a previous study, G. acerosa was reported to reduce cancer cell growth and tumor weight in mice at 200mg/kg body weight (Duraikannu et al., 2014). In another study G. acerosa crude extract was reported as to have cytotoxicity to cancer cells and they also reported that the presence of polyphenols and flavonoids in the ethyl acetate extract of G. acerosa (GAE) being is the reason for inhibition of lung cancer cell proliferation at 1.5 mg/mL (Begum et al., 2018).
On the contrary, in our study we report the presence of Hexadecanoic acid in the bioactive fraction of G. acerosa as per the results of GC-MS analysis. Hexadecanoic acid is a well documented for anticancer potential and is reported from some other marine algae also (Parveen and Nadumane 2020). To the best of our knowledge, we are reporting its presence for the first time from G. acerosa. We also found that the basis for the anticancer mechanism of G. acerosa is cell cycle arrest, accumulation of cells in subG1 phase leading to the apoptosis of treated cancer cells. In our study apoptosis induction was clearly seen in DNA fragmentation, increased caspase activity, cell cycle stages, LDH cytotoxicity and highly decreased cancer cell counts due to G. acerosa treatment.
Though there are previous studies reporting the in-vitro and in-vivo cytotoxicity of the crude extracts of G. acerosa, no attempts were made to purify the active component involved in the reported activity. To the best of our knowledge,this study is the first attempt to analyse the mechanism of anti cancer action of TLC purified fraction of G. acerosa and characterizing the bioactive compound. Through the results of the present study, we report the presence of hexadecanoic acid in the bioactive fraction and assume it to be the reason for the observed anti cancer activity of G. acerosa. Fatty acids comprise one of the predominant phytochemical constituents in marine macroalgae and reported to have many biological activities (Hema et al., 2011). Palmitic acid (n-hexadecanoic acid) is documented to have anticancer potential with apoptosis inducing ability in human cancer cell lines (Yoo et al., 2007; Mericli et al., 2017; Parveen and Nadumane 2020). Our results too are validating these earlier reports.
CONCLUSION
Through the present work, it can be concluded that G. acerosa has cytotoxic activity against human cancer cell lines along with no toxicity to normal human lymphocytes. Presence of Hexadecanoic acid in the bioactive fraction GF7, might be the reason for the anti cancer activity of G. acerosa. Further studies need to be carried out for the development of an efficient drug against cancer from this promising Marine red alga.
ACKNOWLEDGEMENTS
The authors are grateful to Jain (Deemed-to-be-university) for providing the fellowship and for the infrastructural facilities to carry out the work. The authors are thankful to Dr. K. Eswaran, Principal scientist in Marine Algal Research station for the identification of seaweed for the work. The authors are grateful to Mr. M.A. Joseph, Scientist at Central Silk Technological Research Institute, Bengaluru, for providing GC-MS facility and for analysis of the samples for our work in his institute.
Conflict of Interest: The authors declare that there are no conflicts of interest.
REFERENCES
Aggarwal, B.B.,Danda, D., Gupta, S. and Gehlot, P. (2009). Models for prevention and treatment of cancer: Problems vs. promises. Biochemical pharmacology, 78(9):1083–1094
Ahn C.B., Jeon Y.J., Kang D.S., Shin T.S. & Jung B.M. (2004). Free radical scavenging activity of enzymatic extracts from a brown seaweed Scytosiphon lomentaria by electron spin resonance spectrometry. Food Research International, 37: 253 − 258.
Alabsi AM, Ali R, Ali AM, Al-Dubai SA, Harun H, Abu Kasim NH, Alsalahi A. (2012). Apoptosis induction, cell cycle arrest and in vitro anticancer activity of gonothalamin in a cancer cell lines. Asian Pac J Cancer Prev.; 13(10):5131-5136.
Aneiros A. & Garateix A. (2004). Bioactive peptides from marine sources: pharmacological properties and isolation procedures. Journal of Chromatography B 803: 41 − 53.
Begum, F. M. S., Chitra, K., Joseph, B., Sundararajan, R., & Hemalatha, S. (2018). Gelidiella acerosa inhibits lung cancer proliferation. BMC complementary and alternative medicine, 18.
Ashraf M A (2020). Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s
Bounty. BioMed Research International. 2020, Article ID 8602879, 7 pages.
Bai, J. Li, Y. and Zhang G (2017). Cell cycle regulation and anticancer drug discovery, Cancer
Biology & Medicine, 14: 348.
Bernecker, A., In Whertmann, I.S. and Cortes, J. (2009). Marine benthic algae, Marine Biodiversity of Costa Rica, Central America Springer, 538: 109-118
Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. (2014). Marine natural products. Nat Prod Rep. 31(2):160.
Boopathy, S.N. and Kathiresan, K (2010). Anticancer drugs from marine flora: an overview, Journal of oncology. 1-18.
Chang, H.Y. and Yang, X. (2000). Proteases for cell suicide: functions and regulation of caspases, Microbiology and Molecular Biology Reviews. 64(4): 821-46.
Coppejans E., Leliaert F., Dargent O., Guneseakra R. & Clerck O.D. (2009). Sri Lankan seaweeds-methodologies and field guide to the dominant species. Abc Taxa, 6: 265.
Devi K.P., Suganthy N., Kesika P., Karutha Pandian S. (2008). Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complementary Alternative Medicine. 8:38.
Dimmick, I. (2011). Multiple flow cytometry applications: use of DAPI. Biomedical Scientist, 55(8), 541.
Duraikannu, K., Rani K.S., Anithajothi, R., Umagowsalya G, Ramakritinan CM. (2014). In-vivo anticancer activity of red algae (Gelidiela acerosa and Acanthophora spicifera). International Journal of Pharmaceutical Sciences and Research. 5(8): 3347-52.
El-Baroty, G.S., Moussa, M.Y., Shallan, M.A., Ali, M.A. et al. (2007). Contribution to the aroma, biological activities, minerals, protein, pigments and lipid contents of the red alga: Aspara gopsistaxiformis (Delile) Trevisan.Journal of applied science Research 3(12): 1825-1834.
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicologic pathology, 35(4), 495-516
Harada, H. and Kamei, Y (1997). Selective cytotoxicity of marine algae extracts to several human leukemic cell lines, Cytotechnology. 25(1-3), 213
Hema R., Kumaravel S and Alagusundaram K (2011). GC/MS Determination of Bioactive Components of Murraya koenigii. Journal of American Science. 7(1):80-83
Jayaseelan E.C., Kothai S., Kavith R., Tharmila S. & Thavaranjit A.C. (2012). Antibacterial activity of some selected algae present in the coastal lines of Jaffna Peninsula. International Journal of Pharmaceutical and Biological Archives. 3: 352 − 356.
Kılınç., Berna., Semra C., Gamze T. et al.(2013).”Seaweeds for food and industrial applications,” In Food industry. IntechOpen. 735-748
Lakmal, H.H.C., Samarakoon, K.W., Lee, W.W. et al. (2014). Anticancer and antioxidant effects of selected Sri Lankan marine algae. Journal of the National Science Foundation of Sri Lanka, 42(4): 315–23.
Mericli, F., Becer, E., Kabaday, H., Hanoglu, A. and Hanoglu, D.Y. et al., (2017). Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunusdulcis seed oil. Pharmaceutical biology. 55(1): 1239-1248.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 65(1-2): 55-63
Murugan, K. and Iyer, V.V. (2013).Differential growth inhibition of cancer cell lines and antioxidant activity of extracts of red, brown, and green marine algae. In Vitro Cellular&Developmental Biology-Animal. 49(5): 324–34
Newman, D.J. and Gordon M.C. (2016). Drugs and drug candidates from marine sources: An assessment of the current “state of play”. Planta medica. 82(09/10): 775–789
Ngo D.H., Wijesekara I., Vo T.S., Ta Q.V. & Kim S.K. (2011). Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Research International. 44: 523 – 529
Parveen S and Nadumane VK (2020). Anti-angiogenesis and apoptogenic potential of the brown marine alga, Chnoospora minima. Future Journal of Pharmaceutical Sciences, 6:19.
Pazarowski, P. and Darzynkiewicz, Z. (2004). Analysis of cell cycle by flow cytometry.In Checkpoint controls and cancer. 281: 301-11
Samarakoon K.W., Nam O.K., Ko J.Y., Lee J.H., Kang M.C., Kim D., Lee B.J., Lee J.S. & Jeon Y.J. (2013). Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochoropsis oculata) protein hydrolysate. Journal of Applied Phycology. 25: 1595 − 1606.
Saraste, A. and Pulkki, K. (2000).Morphologic and biochemical hallmarks of apoptosis.Cardiovascular research. 45(3): 528-37
Shidoji, Y and Ogawa, H. (2004) Natural occurrence of cancer-preventive geranylgeranoic acid in medicinal herbs. Journal of lipid research. 45(6): 1092-1103
Strober W.(1997) Trypan blue exclusion test of cell viability. Current protocols in immunology, 21 (1):, A-3B
Taskin, E., Ozturk, M., Taskin, E. and Kurt, O. (2007). Antibacterial activities of some marine algae from the Aegean Sea (Turkey). African journal of Biotechnology, 6(24): 2746- 2751
Tay K C., Tan LTH., Chan C K. et al. (2019). Formononetin: a review of its anticancer potentials and mechanisms. Frontiers in Pharmacology, 10.
Wafa OMA, Daabees HMG, Badawi WA. (2020). 2-Anilinopyrimidine derivatives: Design, synthesis, in vitro anti-proliferative activity, EGFR and ARO inhibitory activity, cell cycle analysis and molecular docking study. Bioorganic Chemistry. 99:103798.
Weyermann, J., Lochmann, D. and Zimmer, A. (2005). A practical note on the use of cytotoxicity assays.International journal of pharmaceutics, 288(2): 369- 376
Yoo, Y.C., Shin, B.H., Hong, J.H., Lee, J., et al. (2007). Isolation of fatty acids with anticancer activity from Protaetia brevitarsis Larva. Archives of pharmacal research. 30(3): 361-365
Yuan YV, Walsh NA (2006). Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food and Chemical Toxicology. 44(7):1144-1150
Yubin J, Guangmei Z. Pharmacological Action and Application of Available Antitumor Composition of Traditional Chinese Medicine. Heilongjiang, China: Heilongjiang Science and Technology Press; 1998.