1Department of Veterinary Biochemistry, College of Veterinary Science and Animal Husbandry, U.P. Pt. Deen Dayal Upadhyaya
Pashu-Chikitsa Vigyan Vishwavidyalaya Evam Go Anusandhan Sansthan (DUVASU), Mathura, 281 001, Uttar Pradesh, India
2All India Institute of Medical Sciences (AIIMS), Rishikesh-249 203, Uttarakhand, India
Corresponding author email: ashishvet77@gmail.com
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 26/09/2020
Antimicrobial peptides play an important role in host defense and they are nearly present in all forms of life. Domestic goat (Capra hircus) also known as poor’s man cow is a backbone to lower income group people of India. Goats are reared generally for meat and milk purposes. Goat has also been found to express different types of antimicrobial peptides like defensins, cathelicidins, having broad spectrum of antimicrobial activity against Gram-positive and Gram-negative bacteria, and fungi. Some of them may have cytotoxic effects also. These antimicrobial peptides may also act an immunomodulators. This review briefly describes antimicrobial peptides identified from goat and their potential role in host defense.
Antimicrobial Peptides, Capra hircus, Defensins, Cathelicidins, S100A8, Hepcidin.
Sharma A, Kumar A, Nigam R, Pandey V, Singh P. A Minireview on Antimicrobial Peptides of Goats and their Role in Host Defense. Biosc.Biotech.Res.Comm. 2020;13(3).
Sharma A, Kumar A, Nigam R, Pandey V, Singh P. A Minireview on Antimicrobial Peptides of Goats and their Role in Host Defense. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2QVPfQk
Copyright © Sharma et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Goat is one of the oldest domesticated animals, popularly known as mortgage lifters of India along with sheep. India occupies second position in terms of goat population and first position in goat milk production. Goat meat known as Chevon, is most preferred and widely consumed meat in the country and constitutes about 37% of total meat production. Goat milk possesses many advantages over cow milk as a nutritional source for infants and children (Kumar and Sharma, 2016). Since ages Goats have been poor people’s most reliable livelihood resource. India has 34 registered breeds of Goat (Hegde, 2020).
Goats are resistant to many diseases and they have ability to survive in harsher conditions compared to other ruminants. Antimicrobial Peptides (AMPs) are evolutionary conserved in the genome and produced by all life forms, from prokaryotes to humans (Hancock and Diamond, 2000). In animals, AMPs are believed to be the first line of the innate immune defense against bacteria, fungi and viruses (Zasloff, 2002). They are widely distributed in animal tissues and cells that are exposed to invading organisms. The first mammalian peptides, MCP-1&2 were isolated from rabbit macrophages (Selsted et al., 1983). AMPs are produced by polymorphonuclear leukocytes, macrophages and lymphocytes of the immune system (Radek and Gallo, 2007) and by all epithelial cells in response to the direct contact with microbes. These peptides exhibit direct anti-microbial activity as well as chemotactic and regulatory functions and plays an important role in immunity.
At least five genes present in goat genome have been identified which encodes for these antimicrobial peptides (Zanetti, 2005). The antimicrobial peptides are nowadays used as a medicated feed additive in the rations of ruminants, swine and poultry. A combination of recombinant porcine β-defensin-1 and a fly antibacterial peptide in a ratio of 1:1 was used as a medicated feed additive for juvenile goats leading to increased body weight, average daily weight gain, enzymatic activity, influence on ruminal fermentation function and higher rumen microorganism diversity indices (Liu et al., 2017). These peptides have been described to be effective against many Gram-negative and Gram-positive bacteria, fungi, protozoa, viruses as well as cancer cells. Bioactive peptides from goat milk casein hydrolysates ameliorated insulin resistance in HepG2 cells that had been treated with high glucose (Gong et al., 2020).
Antibacterial activity of goat urinary cationic antimicrobial proteins against bacterial strains of Staphylococcus aureus and E. coli has been demonstrated (Tomar et al., 2018). Researchers across the globe have been able to identify antimicrobial peptides form the native goat which comprise mainly the Defensins and Cathelicidins. Antimicrobial peptides from goats and its source tissue has been presented in Table-1. The three dimensional structure of goat antimicrobial peptides has been depicted in Figure-1. Antimicrobial peptides have been shown to have immunomodulatory properties that includes gene expression, chemotaxis, wound healing properties and cytokine release. These peptides suppress the toll like receptors (TRL) signalling and tumor necrosis factor-α (Haversen et al., 2002; Davidson et al., 2004).
The antibacterial cationic peptides are at an early stage of drug development. However, the development of AMPs as potential therapeutics is hindered by several challenges like low specificity, high manufacturing cost, and potential toxicity to animal cells (Bahar and Ren, 2013). These peptides also have least ability to develop resistance due to the ability of these peptides (AMPs) for attacking multiple low-affinity targets rather than one defined, high-affinity target, characteristic for conventional antibiotics (Mahlapuu et al., 2016). Many statistical and computational algorithms like support vector machines (SVM), hidden Markov model, artificial neural networks (ANN) with cheminformatics approaches is being used for the development of novel antimicrobial peptides (Divyashree et al., 2020).
Various in-silico approach is being tried for designing novel peptides (Farcas et al., 2020). Development of antimicrobial peptides to use as a dietary supplement for human for therapeutic purposes against pathogens has been described (Bakare et al., 2020). The use of antimicrobial peptides in the age of resistance provides immense opportunities in dealing with the multidrug-resistant pathogens (Magana et al., 2020). This review has briefly summarized considering the role of caprine antimicrobial peptides in host defense.
Defensins: Defensins are small (29-45 amino acid residues) cationic antimicrobial peptides with β-sheet structures that are stabilized by three intramolecular disulfide bonds (Lehrer and Ganz, 1996). Three different types of defensins namely α-, β- and θ- have been identified till date, most common being the β-defensins. θ–defensin have been isolated only in rhesus monkey leukocytes (Tang, 1999). Defensins has been isolated from various species and from various tissue and secretions. Two novel β-defensin GBD-1 and GBD-2, 64 amino acids long, were identified in the respiratory (GeneBankY17679), and digestive tissues (AJ009877) from a goat, respectively (Zhao et al., 1999). These peptides were identical in 96.8% of their bases and 88.2% of their amino acids.
Goat beta defensin-1 (GBD-1) was expressed principally in the tongue and respiratory tract, whereas GBD-2 was expressed throughout the intestine. Cationic peptides were isolated from goat tongue (Anbu, More and Kumar, 2003), demonstrating their germicidal activity against both Gram-positive and Gram-negative bacteria. Transcripts of GBD-1 and GBD-2 were identified in kidneys, trachea, tongue epithelium, spinal cord, and in mammary gland of non-lactating goats (Bagnicka et al., 2005). GBD-1 was also expressed in the reproductive tract (vaginal, uterus and ovarian tissue) of black goats (Xiaoyan and Wu, 2015). The mRNA sequence of a gene encoding caprine lingual antimicrobial peptide (LAP) was cloned and characterized (Sharma et al., 2010).
LAP was isolated from goat tongue epithelium. At nucleotide level goat LAP showed 99.5%, 99.4% similarity when compared with GBD-1, Goat EBD and GBD-2, respectively, whereas at amino acid level Goat LAP showed 98.5%, 87.7% homology with GBD-1 and goat EBD, respectively. Goat LAP is evolutionary closer to GBD-1. LAP is 18 amino acids larger than the GBD-1, while goat enteric β-defensin (EBD) shows similar number of amino acids as in GBD-1. Caprine enteric β-defensin (EBD) mRNA was cloned and characterized form goat ileum (Kumar et al., 2010). Goat EBD showed 97.4% and 95.4% homology with GBD-2 and GBD-1, respectively at nucleotide level.
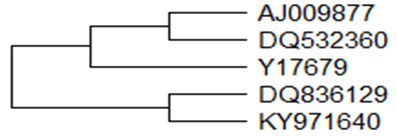
The amino acids sequence of goat EBD has four and seven substitutions when compared with GBD-2 and GBD-1, respectively. Defensin gene of Assam Hill goat was cloned and characterized (Bharalii et al., 2018) and found to be 64 amino acids long as in case of GBD-1 and EBD. The phylogenetic relationships of different beta defensin from goat has been portrayed in Figure-2 showing the evolutionary relationship of goat β–defensin nucleotides. The concentration of beta defensin-1 was determined in semen pellet and seminal plasma of Indian goat breed namely Barbari, Jamunapari and Jakhrana (Ranjan et al., 2019).
In mammals, β-defensin are mainly expressed and secreted in the epididymis resulting in their detection on the plasma membrane of sperm (Yudin et al., 2005). β‐defensin 1 gene has been used as a molecular marker for selection of goats regarding the susceptibility to nematodes and haemoprotozoans infections (Maia et al., 2019). Beta defensin-2 significantly augments the mRNA and protein expression of Toll-like receptors (TLRs) and retinoic acid-inducible gene-I-like receptor (RLR) essential for the detection of viral molecules in mature tissue rat peritoneal mast cells (Agier et al., 2020). Beta defensin-2 from swine has been investigated for its antiviral efficacy against the pseudorabies virus (PRV), causing Aujeszky’s disease (Huang et al., 2020).
Concentrations of beta‐defensin (GBD-1), cathelicidin (CATH-2, CATH-7), lactoferrin, and S100A7 were determined in goat milk after being fed with colostrum whey using ELISA and it was found that it has a significant effect on their expression and secretion in milk (Isobe et al., 2020). Though defensin plays an important role in host defense against pathogens, recent evidence suggests, that they can also be pathogenic under certain biological conditions by promoting viral and bacterial infections (Xu and Lu, 2020).
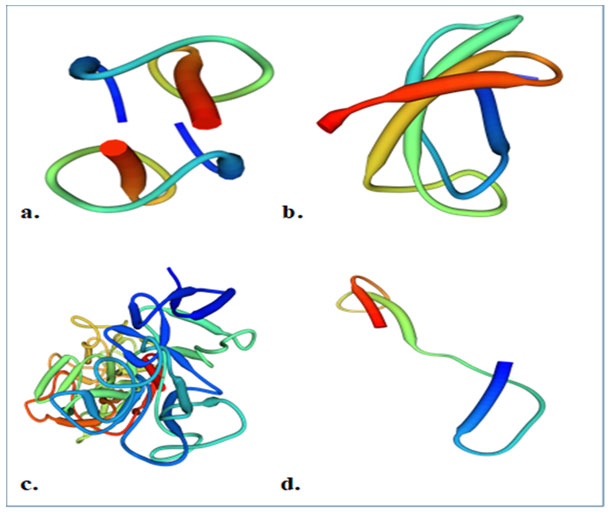
Figure 1: Structure of caprine antimicrobial peptides; a. GBD-1 b. Cath-2 (ChBac5) c. Lactoferrin d. Myeloid cathelicidin (Source: Uniprot)
Figure 2: Phylogenetic relationships of different goat β–defensin. A bootstrapped (1000 trials) neighbour-joining phylogenetic tree showing the evolutionary relationship of goat β–defensin nucleotides.
Cathelicidin: Cathelicidins are small, cationic, antimicrobial peptides present in human and other vertebrates. These are proteolytically activated peptides and are part of the innate immune system having a broad spectrum of antimicrobial activity against bacteria, viruses and fungi (Kosciuczuk et al., 2012). It may be used as an adjuvant for vaccine as well as in anticancer therapy by modulating TLR-activation and inflammation (Scheenstra et al., 2020). Cathelicidins are synthesized as prepropeptide, containing a signal peptide, cathelin, and C-terminal mature peptides with antimicrobial properties (Zanetti, Gennaro and Romeo, 1995).
The mRNA sequence of a gene encoding Bac7.5 and MAP34-A was cloned and characterized from goat (Zhao et al., 1999a). A proline-rich antimicrobial peptide of cathelicidin class was purified from elastase-treated extracts of goat leukocytes namely ChBac5 (Shamova et al., 1999). Ch derives its name from Capra hircus. It’s a 43 amino acid long peptide with a molecular mass of 5.16kDa. ChBac5 (GeneBank Y18873) was homologous to OaBac5a and bovine Bac5. ChBac5 exhibited potent, broad-spectrum antimicrobial activity against E. coli, P. aeruginosa, B. subtilis, C. albicans under low salt concentration. ChBac5 peptides are highly conserved in ruminants and contribute significantly to their innate host defense mechanisms. Another proline-rich peptide (ChBac3.4, 26 amino acid long) was isolated from leukocytes of the goat (Shamova et al., 2009). ChBac3.4 had over 50% sequence identity to the ChBac5 peptides found in the leukocytes of goats, sheep and cattle. ChBac3.4 exhibited broad spectrum antimicrobial activity and also has cytotoxic potential. Mini-ChBac7.5Nα and mini-ChBac7.5Nβ (average molecular masses of 2.89kDa and 2.7kDa) was isolated from neutrophils of the domestic goat (Shamova et al., 2016).
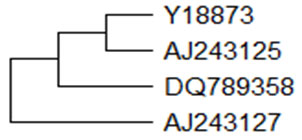
These peptides exhibit significant antimicrobial activity against Gram-negative bacteria. These truncated AMPs may play a crucial role in host defense reactions. The mRNA sequence of a gene encoding myeloid cathelicidin (11.32kDa) was cloned and characterized. This Capra hircus cathelicidin (cath) mRNA, partial cds encodes for 99 amino acids and has been isolated from the bone marrow cells of Indian domestic goat (Sharma et al., 2008). Goat myeloid cathelicidin showed 85.9% similarity with ChBac7.5 at nucleotide level. Phylogenetically goat myeloid cathelicidin is closely related to ChBac7.5 than other cathelicidins. The predicted peptide of cathelicidin isolated from bone marrow of Assam hill goat is composed of 137 amino acids (Bharali et al., 2019) and is phylogenetically closer to Yunnan goat cathelicidin (CATH2). The phylogenetic relationships of different goat cathelicidin has been depicted in Figure-3 using neighbour-joining method. ChMAP-28, a cathelicidin antimicrobial peptides from goat (Capra hircus) leucocytes having α–helical structure and a molecular mass of 3kDa have been identified, ChMAP-28 has potent anticancer activity (Emelianova et al., 2018).
In another study it was found that the regulation of cathelicidin bovine myeloid antimicrobial peptide (BMAP-28), in the inflammatory response against alpha-herpes viruses is dependent on the stage of virus infection in the bovine nervous system (Burucua et al., 2020). Five cathelicidin mRNAs (Cath-1, 2, 3, 6 & 7) were expressed in deep region of the mammary gland in healthy goats, however, cathelicidin-7 was not expressed in the teat and cathelicidin-2 is expressed in polymorphonuclear cells in the mammary gland and is secreted into milk in goat (Zhang et al., 2014). Goat cathelicidin‐2, an antimicrobial peptide, localizes in leukocytes and is present in milk even without lipopolysaccharide stimulation (Srisaikham et al., 2016). Goat cathelicidin-2 has broad-spectrum of activity in-vitro against Gram-negative bacteria such as E.coli (Shamova et al., 1999). Cathelicidin-1 was also detected in the raw bovine colostrum using LC-MS/MS (Chatterton et al., 2020). The role of cathelicidin as a biomarker in the late lactation period in goats has been demonstrated in relation to mammary gland infection (Puggioni et al., 2020).
Figure 3: Phylogenetic relationships of different goat Cathelicidin. A bootstrapped (1000 trials) neighbour-joining(Saitou and Nei, 1987) phylogenetic tree showing the evolutionary relationship of goat cathelicidin nucleotides
Lactoferricin / Lactoferrin: Lactoferrin is an important antimicrobial component of milk and it protect the infants from infectious diseases (Reiter, 1978). Lactoferrin also helps in modulation of the inflammatory response, activation of the immune system, and control of myelopoiesis (Brock, 1995). Lactoferrin exert its antimicrobial action by depriving bacteria of the Iron (Arnold, Cole and McGhee, 1977). The presence of antimicrobial domains near the N-terminus of lactoferrin was first reported by (Bellamy et al., 1992) and they named the isolated peptides lactoferricin. Lactoferricin have a broad-spectrum antibacterial property against Gram-positive and Gram-negative bacteria, and fungi. Lactoferrin of goat milk upon pepsin digestion releases a potent antimicrobial peptide called Lactoferricin. This derived peptide is 16 amino acid long and it corresponds to the sequence of residues 20 and 35 in the N lobe of Korean Native (KN) goat Lactoferrin.
The sequence of the antimicrobial peptide from KN goat lactoferrin showed 75% and 44% similarity with the sequences of the regions between the two cysteine residues of bovine and human lactoferricin, respectively (Kimura et al., 2000). Lactoferrin in goat milk has been confirmed for its role in increasing the activity of natural killer (NK) cells, and increasing the phagocytic activity of phagocytes (Kanwar et al., 2015). Bioactive peptides released during the fermentation of goat milk exhibits antimicrobial activity inhibiting the growth of E. coli, Salmonella, Micrococcus luteus and Proteus mirabilis (Biadała et al., 2020).
It has also been established that the casein phosphopeptides present in goat milk can help in increasing the level of IgA in stool, suggesting a positive effect on mucosal immunity (Kao et al., 2020). Goat milk whey hydrolysate, particularly lactoferrin has been established to possess antifungal activity against at least ten toxigenic fungi from the genus Penicillium (Luz et al., 2020).
Hepcidin: Hepcidin (Park et al., 2001) is a cysteine-rich antimicrobial peptide isolated for the first time from human urine and named it hepcidin because of its origin in the liver and its antimicrobial properties. Hepcidin plays a crucial role in regulating iron homeostasis. The role of feeding of fermented goat milk on the expression of hepcidin antimicrobial peptides (HAMP) has been studied and it was found that HAMP mRNA expression was lower in control and anaemic animals fed fermented goat milk with normal iron and also in control and anaemic animals fed fermented goat milk with high Fe content (Moreno-Fernandez et al., 2020). Hepcidins exhibited antifungal activity against Candida albicans, Aspergillus fumigatus, and Aspergillus niger and antibacterial activity against Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and group B. streptococcus. Capra hircus hepcidin antimicrobial peptide (HAMP), mRNA (GeneBank XM013971234) was predicted by automated computational analysis.
Table 1. Expression of antimicrobial peptides in different parts of goat
| Peptide
or gene name |
Tissue(s) Localization | Sources | |
| Defensin | GBD-1 | Milk somatic cells, tongue, trachea, bronchi, lungs, vaginal, uterus and ovarian tissue, Semen | Zhao et al., 1999 Bagnicka et al., 2005
Xiaoyan et al., 2015 Ranjan et al., 2019 |
| GBD-2 | Kidneys, trachea, tongue epithelium, spinal cord, mammary gland, stomach, jejunum, ileum, large intestine, rectum | Zhao et al., 1999 Bagnicka et al., 2005 | |
| LAP | Tongue epithelium | Sharma et al., 2006
Bharali et al., 2017 |
|
| EBD | Ileum | Kumar et al., 2010 | |
| Cationic antimicrobial peptides | Urine | Tomar et al., 2018 | |
| Cathelicidin | ChBac5, ChBac3.4 | Leukocytes | Shamova et al., 1999
Shamova et al; 2009 |
| ChBac7.5Nα ChBac7.5Nβ | Neutrophils | Shamova et al; 2016 | |
| Myeloid Cathelicidin | Bone marrow | Sharma et al., 2010 | |
| ChMAP-28 | Leucocytes | Emelianova et al., 2018 | |
| Cath-1,2 | Polymorphonuclear cells of the mammary gland, Milk | Zhang et al., 2014 | |
| Cath-7 | Leucocytes | (Nishikawa et al., 2018) | |
| Lactoferrin | Lactoferricin | Milk | Kimura et al., 2000 |
| S1008 | Milk | Purba et al., 2019
Isobe et al., 2020 |
|
S100A8: S100A8 is a calcium- and zinc-binding protein which plays a prominent role in the regulation of inflammatory processes and immune response. It can induce neutrophil chemotaxis and adhesion. The expression and localization of antimicrobial peptide S100A8 was established in the mammary gland parenchyma, teat, blood leukocytes, and milk somatic cells of goat (Purba et al., 2019). S100A8 protein is one of the important biomarkers in polycystic ovary syndrome (Manibalan et al., 2020).
CONCLUSIONS and FUTURE PROSPECTS of ANTIMICROBIAL PEPTIDES
Antibiotic resistance is a big global problem. To thwart this problem, we need to develop new generation of antibiotics and the antimicrobial peptides best fit into this category. These peptides are produced in animals as part of their innate immune response. Antimicrobial peptides have unique ability to be used in conditions like chronic inflammation, wound healing, infectious diseases and multidrug-resistant pathogens. The immunomodulatory activities of these antimicrobial peptides can be exploited in future for the development of vaccines as well as a therapy against cancer and other autoimmune diseases. However, we have to be careful while exploiting these peptides as it may lead to the disturbances in animal’s innate defense if the pathogen develops resistance to these antimicrobial peptides.
REFERENCES
Agier J, Brzezińska-Błaszczyk E, Różalska S, Wiktorska M, Wawrocki S and Żelechowska P (2020) β-defensin strengthens antimicrobial peritoneal mast cell response Journal of Immunology Research Vol 2020 p e5230172 doi: https://doi.org/10.1155/2020/5230172
Anbu KA, More T and Kumar A (2003) Isolation and characterisation of cationic antibacterial proteins and peptides from goat tongue epithelium Indian J Anim Sci Vol 73 No 12 pp 1307–1311
Arnold RR, Cole MF and McGhee JR (977) A bactericidal effect for human lactoferrin Science Vol 197 No 4300 pp 263–265 doi: 10.1126/science.327545
Bagnicka E, Flisikowski K, Strzałkowska N, Krzyżewski J, Prusak B, Sakowski T and Zwierzchowski L (2005) Expression level of goat β-defensin genes in different goat tissues and in somatic cells of goat milk-preliminary study Proceedings of the XI Baltic Animal Breeding and Genetics Conference Palanga Lithuania pp 144–146
Bahar A and Ren D (2013) Antimicrobial peptides Pharmaceuticals Vol 6 No 12 pp 1543–1575 doi: 10.3390/ph6121543
Bakare OO, Fadaka AO, Klein A and Pretorius A (2020) Dietary effects of antimicrobial peptides in therapeutics All Life Vol 13 No 1 pp 78–91 doi: 10.1080/26895293.2020.1726826
Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K and Tomita M (1992) Identification of the bactericidal domain of lactoferrin Biochimica et Biophysica Acta (BBA) -Protein Structure and Molecular Enzymology Vol 1121 No 1–2 pp 130–136 doi: 10.1016/0167-4838(92)90346-F
Bharali K, Kalita D, Yadav S, Saikia D and Devi B (2019) Molecular characterization of cathelicidin gene in Assam hill goat International Journal of Chemical Studies Vol 7 No 1 pp 1026–1030
Bharalii K, Kalita D, Bora P, Saikia D, Devi B and Das L (2018) Characterization of defensin gene of Asom hill goat and in-silico designing of novel antimicrobial peptides The Indian Journal of Animal Sciences Vol 88 No 5 pp 530–533
Biadała A, Szablewski T, Lasik-Kurdyś M and Cegielska-Radziejewska R (2020) Antimicrobial activity of goat’s milk fermented by single strain of kefir grain microflora European Food Research and Technology Vol 246 No 6 pp 1231–1239 doi: 10.1007/s00217-020-03483-2
Brock J (1995) Lactoferrin: a multifunctional immunoregulatory protein? Immunology Today Vol 16 No 9 pp 417–419 doi: 10.1016/0167-5699(95)80016-6
Burucúa MM, Pérez SE, Odeón AC, Cobo ER, Quintana S and Marin MS (2020) Cathelicidin bovine myeloid antimicrobial peptide (BMAP) 28 is involved in the inflammatory response against alpha-herpesviruses in the bovine nervous system Molecular Immunology Vol 122 pp 148–155 doi: 10.1016/j.molimm.2020.04.017
Chatterton DEW, Aagaard S, Hesselballe Hansen T, Nguyen DN, De Gobba C, Lametsch R and Sangild PT (2020) Bioactive proteins in bovine colostrum and effects of heating drying and irradiation Food & Function Vol 11 No 3 pp 2309–2327 doi: 10.1039/C9FO02998B
Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, Hancock REW and Speert DP (2004) The Cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization The Journal of Immunology Vol 172 No 2 pp 1146–1156 doi: 10.4049/jimmunol.172.2.1146
Divyashree M, Mani MK, Reddy D, Kumavath R, Ghosh P, Azevedo V and Barh, D (2020) Clinical applications of antimicrobial peptides (AMPs): Where do we stand now? Protein & Peptide Letters Vol 27 No 2 pp 120–134 doi: 10.2174/0929866526666190925152957
Emelianova AA, Kuzmin DV, Panteleev PV, Sorokin M, Buzdin AA and Ovchinnikova TV (2018) Anticancer activity of the goat antimicrobial peptide ChMAP-28 Frontiers in Pharmacology Vol 9 No 1501 doi: 10.3389/fphar.2018.01501
Farcas A, Iarinca LB, Floare C and Janosi L (2020) Design of novel antimicrobial peptides in a multi-stage in-silico approach Biophysical Journal Volume 118 No 3 p 384a doi: 10.1016/j.bpj.2019.11.2190
Gong H, Gao J, Wang Y, Luo QW, Guo KR, Ren FZ and Mao XY (2020) Identification of novel peptides from goat milk casein that ameliorate high-glucose-induced insulin resistance in HepG2 cells Journal of Dairy Science Vol 103 No 6 pp 4907–4918 doi: 10.3168/jds.2019-17513
Hancock REW and Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences Trends in Microbiology Vol 8 No 9 pp 402–410 doi: 10.1016/S0966-842X(00)01823-0
Haversen L, Ohlsson BG, Hahn-Zoric M, Hanson LÅ and Mattsby-Baltzer I (2002) Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB Cellular Immunology Vol 220 No 2 pp 83–95 doi: 10.1016/S0008-8749(03)00006-6
Hegde NG (2020) Goat development: an opportunity to strengthen rural economy in Asia and Africa Asian Journal of Research in Animal and Veterinary Sciences Vol 5 No 4 pp 30–47
Huang J, Qi Y, Wang A, Huang C, Liu X, Yang X, Li L and Zhou R (2020) Porcine β-defensin-2 inhibits proliferation of pseudorabies virus in-vitro and in transgenic mice Virology Journal Vol 17 No 1 p 18 doi: 10.1186/s12985-020-1288-4
Isobe N, Matsukawa S, Kubo K, Ueno K, Sugino T, Nii T and Yoshimura Y (2020) Effects of oral administration of colostrum whey in peripartum goat on antimicrobial peptides in postpartum milk Animal Science Journal Vol 91 pp: e13365 doi: 10.1111/asj.13365
Kanwar J, Roy K, Patel Y, Zhou SF, Singh M, Singh D, Nasir M, Sehgal R, Sehgal A, Singh R, Garg S and Kanwar R (2015) Multifunctional iron bound lactoferrin and nanomedicinal approaches to enhance its bioactive functions Molecules Vol 20 No 6 pp 9703–9731 doi: 10.3390/molecules20069703
Kao HF, Wang YC, Tseng HY, Wu LSH, Tsai HJ, Hsieh MH, Chen PC, Kuo WS, Liu LF, Liu ZG and Wang JY (2020) Goat milk consumption enhances innate and adaptive immunities and alleviates allergen-induced airway inflammation in offspring mice Frontiers in Immunology Vol 11 p 184 doi: 10.3389/fimmu.2020.00184
Kimura M, Nam MS, Ohkouchi Y, Kumura H, Shimazaki K and Yu DY (2000) Antimicrobial peptide of Korean native goat lactoferrin and identification of the part essential for this activity Biochemical and Biophysical Research Communications Vol 268 No 2 pp 333–336 doi: 10.1006/bbrc.2000.2141
Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L and Bagnicka E (2012) Cathelicidins: family of antimicrobial peptides, A review Molecular Biology Reports Vol 39 No 12 pp 10957–10970 doi: 10.1007/s11033-012-1997-x
Kumar A and Sharma A (2016) Nutritional and medicinal superiority of goat milk over cow milk in infants International Journal of Pediatric Nursing Vol 2 No 1 pp 39–43 doi: 10.21088/ijpen.2454.9126.2116.5
Kumar A, Sharma A, Kumar A and Dev K (2010) Cloning and characterization of goat enteric β-defensin cDNA Indian Journal of Veterinary Research Vol 19 No 1 pp 1–7
Lehrer RI and Ganz T (1996) Endogenous vertebrate antibiotics, Defensins protegrins and other cysteine-rich antimicrobial peptides Annals of the New York Academy of Sciences 797 pp 228–239 doi: 10.1111/j.1749-6632.1996.tb52963.x
Liu Q, Yao S, Chen Y, Gao S, Yang Y, Deng J, Ren Z, Shen L, Cui H, Hu Y, Ma X and Yu S (2017) Use of antimicrobial peptides as a feed additive for juvenile goats Scientific Reports Vol 7 No 1 pp 12254 doi: 10.1038/s41598-017-12394-4
Luz C, Izzo L, Ritieni A, Mañes J and Meca G (2020) Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread LWT: Food Science and Technology Vol 118 p 108717 doi: 10.1016/j.lwt.2019.108717
Magana M, Pushpanathan M, Santos AL, Leanse L, Fernandez M, Ioannidis A, Giulianotti MA, Apidianakis Y, Bradfute S, Ferguson AL, Cherkasov A, Seleem MN, Pinilla C, de la Fuente-Nunez C, Lazaridis T, Dai T, Houghten RA, Hancock REW and Tegos GP (2020) The value of antimicrobial peptides in the age of resistance The Lancet Infectious Diseases p S1473309920303273 doi: 10.1016/S1473-3099(20)30327-3
Mahlapuu M, Håkansson J, Ringstad L and Björn C (2016) Antimicrobial Peptides: an emerging category of therapeutic agents Frontiers in Cellular and Infection Microbiology Vol 6 No 194 doi: 10.3389/fcimb.2016.00194
Maia FSP, Campelo JEG, Sarmento JLR, Silva CS, Marques JRF, Alves FAS, Guimarães RC and Filho ES (2019) Association of polymorphisms of the β‐defensin 1 gene with nematode and protozoan infection traits in goat Parasite Immunology Vol 41 No 4 p e12613 doi: 10.1111/pim.12613
Manibalan S, Shobana A, Kiruthika M, Achary A, Swathi M, Venkatalakshmi R, Thirukumaran K, Suhasini K and Roopathy S (2020) Protein network studies on PCOS biomarkers with S100A8 druggability assessment and RNA aptamer designing to control its cyst migration effect Frontiers in Bioengineering and Biotechnology Vol 8 No 328 doi: 10.3389/fbioe.2020.00328
Moreno-Fernandez J, Alférez MJM, López-Aliaga I and Díaz-Castro J (2020) Role of fermented goat milk on liver gene and protein profiles related to iron metabolism during anemia recovery Nutrients Vol 12 No 5 pp 1336 doi: 10.3390/nu12051336
Nishikawa M, Nii T, Yoshimura Y and Isobe N (2018) Investigation of the binding of goat cathelicidin-7 to lipopolysaccharide and leucocidal suppression of pro-inflammatory cytokines Small Ruminant Research Vol 168 pp 101–106 doi: 10.1016/j.smallrumres.2018.10.006
Park CH, Valore EV, Waring AJ and Ganz T (2001) Hepcidin a urinary antimicrobial peptide synthesized in the liver Journal of Biological Chemistry Vol 276 No 11 pp 7806–7810 doi: 10.1074/jbc.M008922200
Puggioni GMG, Tedde V, Uzzau S, Dore S. Liciardi M, Cannas EA, Pollera C, Moroni P, Bronzo V and Addis, MF (2020) Relationship of late lactation milk somatic cell count and cathelicidin with intramammary infection in small ruminants Pathogens Vol 9 No 1 doi: 10.3390/pathogens9010037
Purba FY, Nii T, Yoshimura Y and Isobe N (2019) Short communication: production of antimicrobial peptide S100A8 in the goat mammary gland and effect of intramammary infusion of lipopolysaccharide on S100A8 concentration in milk Journal of Dairy Science Vol 102 No 5 pp 4674–4681 doi: 10.3168/jds.2018-15396
Radek K and Gallo R (2007) Antimicrobial peptides: natural effectors of the innate immune system Seminars in Immunopathology Vol 29 No 1 pp 27–43
Ranjan R, Singh SP, Gururaj K, Jindal SK and Chauhan MS (2019) Status of beta defensin-1 in Indian goat breeds Indian Journal of Animal Sciences Vol 89 No 10 pp 1078–1081
Reiter B (1978) Review of nonspecific antimicrobial factors in colostrum Ann Rech Vet Vol 9 pp 205–224
Saitou N and Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees Molecular Biology and Evolution Vol 4 No 4 pp 406–425 doi: 10.1093/oxfordjournals.molbev.a040454
Scheenstra MR, Van Harten RM, Veldhuizen EJA, Haagsman HP and Coorens M (2020) Cathelicidins modulate TLR-activation and inflammation Frontiers in Immunology Vol 11 doi: 10.3389/fimmu.2020.01137
Selsted ME, Brown DM, DeLange RJ and Lehrer RI (1983) Primary structures of MCP-1 and MCP-2 natural peptide antibiotics of rabbit lung macrophages The Journal of Biological Chemistry Vol 258 No 23 pp 14485–14489
Shamova O, Brogden KA, Zhao C, Nguyen T, Kokryakov VN and Lehrer RI (1999) Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes Infection and Immunity Vol 67 No 8 pp 4106–4111
Shamova O, Orlov D, Stegemann C, Czihal P, Hoffmann R, Brogden K, Kolodkin N, Sakuta G, Tossi A, Sahl HG, Kokryakov V and Lehrer RI (2009) ChBac3.4: A novel proline-rich antimicrobial peptide from goat leukocytes International Journal of Peptide Research and Therapeutics Vol 15 No 2 pp 107–119 doi:10.1007/s10989-009-9170-7
Shamova OV, Orlov DS, Zharkova MS, Balandin SV, Yamschikova EV, Knappe D, Hoffmann R, Kokryakov VN and Ovchinnikova TV (2016) Minibactenecins ChBac7.Nα and ChBac7.Nβ -antimicrobial peptides from leukocytes of the goat Capra hircus Acta Naturae Vol 8 No 3 pp 136–146
Sharma A, Kumar A, Kumar A and Dev K (2008) Cloning and characterization of Goat cathelicidin cDNA The Indian Journal of Veterinary Research Vol 17 No 1 pp 13–20
Sharma A, Kumar A, Kumar A, Dev K (2010) Characterization of goat lingual antimicrobial peptide cDNA Journal of Immunology and Immunopathology Vol 12 No 1 pp 46–51
Srisaikham S, Suksombat W, Yoshimura Y and Isobe N (2016) Goat cathelicidin-2 is secreted by blood leukocytes regardless of lipopolysaccharide stimulation:goat leukocytes secrete cathelicidin-2 Animal Science Journal Vol 87 No 3 pp 423–427 doi: 10.1111/asj.12438
Tang Y (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins Science Vol 286 No 5439 pp 498–502 doi: 10.1126/science.286.5439.498
Tomar V, Nigam R, Pandey V, Singh AP, Roy D, Sharma A, Singh P and Pal A (2018) Evaluation of in-vitro anti-microbial activity of goat urine peptides Journal of Animal Research Vol 8 No 1 pp 33-37 DOI: 10.30954/2277-940X.2018.00150.06
Xiaoyan S and Wu S (2015) RT-PCR detection of relative expression of β1-defensin mRNA in reproductive tract of black goat Journal of Sichuan Institute of Animal Husbandry and Veterinary Medicine Vol 9 pp 27–29
Xu D and Lu W (2020) Defensins: A double-edged sword in host immunity Frontiers in Immunology Vol 11 doi:10.3389/fimmu.2020.00764
Yudin AI, Generao SE, Tollner TL, Treece CA, Overstreet JW and Cherr GN (2005) Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies Biology of Reproduction Vol 73 No 6 pp 1243–1252 doi:10.1095/biolreprod.105.042432
Zanetti M (2005) The role of cathelicidins in the innate host defenses of mammals Current Issues in Molecular Biology Vol 7 No 2 pp 179–196
Zanetti M, Gennaro R and Romeo D, (1995) Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain FEBS letters Vol 374 No 1 pp 1–5 doi: 10.1016/0014-5793(95)01050-o
Zasloff M (2002) Antimicrobial peptides of multicellular organisms Nature Vol 415 No 6870 pp 389–395 doi:10.1038/415389a
Zhang, GW, Lai SJ, Yoshimura Y and Isobe N (2014) Expression of cathelicidins mRNA in the goat mammary gland and effect of the intramammary infusion of lipopolysaccharide on milk cathelicidin-2 concentration Veterinary Microbiology Vol 170 No 1–2 pp 125–134 doi: 10.1016/j.vetmic.2014.01.029
Zhao C, Nguyen T, Liu L, Shamova O, Brogden K and Lehrer RI (1999) Differential expression of caprine beta-defensins in digestive and respiratory tissues Infection and Immunity Vol 67 No 11 pp 6221–6224