School of Biotechnology, Devi Ahilya University, Khandwa Rd., Indore-452001, India
Corresponding author Email: singhgauravbiotech@gmail.com
Article Publishing History
Received: 12/01/2019
Accepted After Revision: 02/03/2019
Soybean (Glycine max) and common bean (Phaseolus vulgaris) are the two important of the leguminous family, Phaseoleae. Synteny gives a framework in which preservation of genes and gene order is determine between genomes of various species. The syntenic relationship between G. max and P. vulgaris is important to determine the potential for comparative genomic analysis. Here, synteny analysis has been performed between G. max and P. vulgaris by using the tool ‘SATSUMA’. Genome data of these two legume species were retrieved from NCBI database, gene synteny alignment was then performed and comparatively analysed. Result was visualized by developing custom script in BioPython software version 3.7. The soybean chromosome 13 was aligned with whole genome of common bean as it contains genes which code for nucleotide binding site and leucine rich repeats (NBS-LRR) protein. The NBS-LRR genes play a major role in defense against pathogens. On alignment, a set of genes linked with disease resistant proteins (NBS-LRR) in G. max showed synteny with the different chromosomes of P. vulgaris. The data supported the theory that in legumes, genes are highly conserved, as extensive regions of synteny exist between these two species. The present study will be helpful to use both genomic resources as well as genetic data for crucial agronomic traits for the improvement of these two species.
Disease Resistance Genes, Glycine Max, Nbs-Lrr, Phaseolus Vulgaris, Synteny
Singh G, Kumar A. Synteny Analysis of Glycine Max and Phaseolus Vulgaris Revealing Conserved Regions of NBS-LRR Coding Genes. Biosc.Biotech.Res.Comm. 2019;12 (1).
Singh G, Kumar A. Synteny Analysis of Glycine Max and Phaseolus Vulgaris Revealing Conserved Regions of NBS-LRR Coding Genes. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2MhxhIi
Copyright © Singh and Kumar, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Synteny word came from greek where ‘syn’ means together and ‘taenia’ means ribbon. When gene order is conserved between the organism is commonly refered as Synteny. It is of two different type’s viz. macrosynteny when many genes or large chromosomal segments of different organisms are syntenic; and microsynteny when only few genes are conserved in different species (Zhu et al., 2005 and Passoupathy, 2016). Relationship between plants, pathogens and pests have been currently discussed in different models (Andolfo and Ercol-ano, 2015). This models mainly include proteins which are coded by clustered disease-resistance (R) genes in plant genomes (Hulbert et. al., 2001). In earlier study, the R gene-encoded proteins were divided into eight major group on the basis of amino acid motif organization and localization in the cell (Gururani et. al., 2012). Among
these group two main R gene proteins are (CC) NBS-LRR or CNL proteins and TNL proteins. NBS-LRR genes have been grouped into three classes (TNL, CNL and R NLs) (Shao et. al., 2016 Neupane et. al., 2018).
The legumes are extremely diverse and can be distributed into 3 subfamilies viz. Caesalpinioideae, Mimosoideae and Papilionoideae (Doyle and Luckow, 2003). The subfamily, Papilionoideae consists of almost all commercially important legumes like Arachis hypogaea (peanut), Cicer arietinum (chick pea), Glycine max (soybean), Phaseolus vulgaris (common bean), Medicago sativa (alfalfa), Vigna radiata (mungbean), Lens culinaris (lentil) and Pisum sativum (pea). Although these crop legumes have close phylogenetic relationship but differ extremely in their chromosome number, genome size and ploidy level (Table 1). However, previous studies on comparative genetic mapping suggested that the Papilionoideae subfamily members showed broad genome conservation (Weeden et al., 1992; Zhu et al., 2005). Young et al (2003) showed that legumes form a systematic taxonomic group with ubiquitous and prevailing macro- and micro- synteny. There are reports indicating that closely related species contain genomes having various genes with same map positions (Bennetzen, 2000; Devos and Gale, 2000; Paterson et al., 2000; Schmidt, 2000; Gualtieri et al., 2002).
The two most important members of legume family Phaseoleae are soybean and common bean. These two species are commercially valuable legumes, common bean as a nutritional crop for poor population and soybean for its various human and animal uses (Mc Clean et al., 2010). Common bean has been diverged from soybean 19 million years ago. Considering syntenic relationship between two species is crucial to determine the potential for comparative genomic analysis. Whole genome duplication is one of the most salient characteristics of the soybean genome (Cannon and Shoemaker, 2012).Earlier, in plants, synteny analyses were aimed only on the species belonging to the families Poaceae and Brassicaceae. In recent time, such studies are being extended on other plants especially legumes (Gualtieri et al., 2002). It has been shown that sequence based tools help to study the evolution, organization and syntenic relationships of genomes. Macro- and micro- synteny of various species can be compared using linkage maps (Mc Connell et al., 2010). The studies indicated that level of synteny is found high in closely related species, and it decreases with growing phylogenetic distance (Choi et al., 2004). Recently, a linkage map for Apios Americana (a tuberous perennial legume in phaseolae tribe) has been reported and it showed synteny with selected warm-seasoned legumes. It also revealed a translocation event in Glycine max and common bean against Apios and Vigna species (Singh et al., 2018).
The RFLP mapping (Shoemaker et al., 1996) and EST Ks analysis have provided substantial evidences that whole genome duplication (WGD) in soybean has occurred about 13 million years ago (Schmutz et al., 2010 and Schlueter et al., 2004). Boutin et al (1995) have reported a number of syntenic linkage blocks between common bean and soybean by exploiting shared RFLP markers. They used using low density RFLP mapping, they showed that these two species share a high degree of homology in sequence, however, synteny has been found over short blocks of genomes. A clear one to two relationship between common bean and soybean genomes was shown by Lee et al (2001). A few synteny analyses have been reported between soybean and common bean. Mc Clean et al., (2010) studied the synteny between soybean and common bean on the basis of 300 gene base loci. Synteny blocks of averaging 32 cM in common bean and 4.9 Mb in soybean were found for all 11 common bean linkage groups which were mapped to all soybean linkage groups. A total 55 syntenic blocks each having 7 loci were observed between the common bean and soybean. By analysing the location of these blocks, it was revealed that each locus of common bean genome mapped to two loci of soybean genome (Mc Clean et al., 2010).
In 2014, Schmutz and co-workers observed considerable synteny between Phaseolus vulgaris and Glycine max, except in pericentromeric regions, where due to genomic expansion in one or both genomes micro- colinearity was much extended and due to that was lesser dense. Conservation of genome macrostructure has been reported between the soybean and other legumes including Phaseolus vulgaris (Lee et al., 2017). Studies showed the correlation between Phaseolus linkage group Pv01 and Glycine max chromosomes Gm06 and Gm04 (Cannon and Shoemaker 2012). It is also reported that Gm5 and Gm8 chromosomes share synteny to common bean Pv2 (Mc Clean et al., 2010). In another study, nearly 63% of common bean unigenes were shown to have homology to soybean (Kalavacharla et al., 2011). Microsynteny analysis was also reported between common bean and soybean where 6 BAC clones were sequenced and analyzed for microsynteny (Yadegari, 2013). Recently the genomes of G.max and P. vulgaris were analyzed for investigating synteny. Syntenic blocks were found between chromosomes Gm03 and Pv10, Gm10 and Pv07 as well as Gm14 and Pv01. A large number of present research make use of whole genome sequences to study R genes in legumes (Anderson 2016, Christie et. al., 2016 and Neupane et. al., 2018).
Earlier many research were done on NBS-LRR genes in Glycine max and other legumes by using bioinformatics approach (Benson 2014, Shao et. al., 2016 and Neupane et. al., 2018). Nepal and Benson (2015) showed the complete evolutionary relationship of the CNL- R genes in soybean and common bean. In the present study, synteny analysis between chromosome 13 of soybean having NBS-LRR enriched sequences and whole genome of common bean have been carried out since common bean has been reported to have NBS-LRR enriched regions which help in disease resistance. The results from our study will be helpful in understanding evolutionary relationships of NBS-LRR genes with potential implication in crop improvement.
Materials and Methods
The gene synteny alignment, and genomic linkage analyses between the chromosome 13 of G. max and whole genome of P. vulgaris was performed by using ‘SATSUMA version 3.1.0 freely available on the website, http://satsuma.sourceforge.net/. All the data in FASTA and GFF files required for genome sequence and annotation of soybean and common bean were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/ genome/). For synteny analysis, only sequence alignments of more than 200 bp and percent identity of more than 85% were considered and rest were filtered out. The results were visualized by developing custom script in Biopython module of BioPython, version 3.7 freely available on the website, http://biopython.org/DIST/ docs/install/Installation.html.
Results and Discussion
Comparison of orthologous regions of soybean and common bean
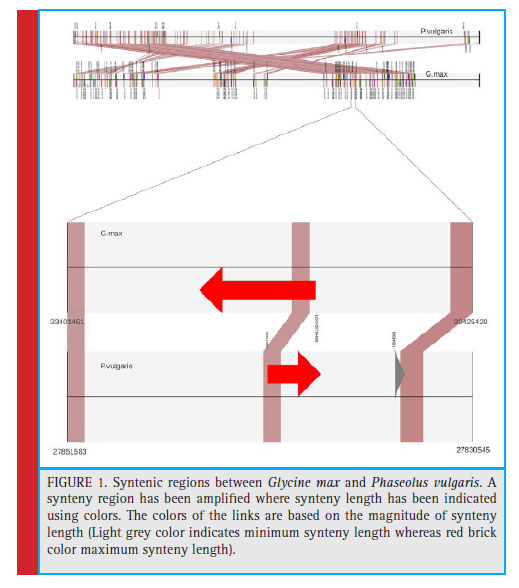
The syntenic analysis between soybean and common bean showed many conserved regions between the two as shown in Fig. 1. Twenty one genes mentioned in Table 2, which were screened on the basis of few parameters of our interest as highest, lowest percent similarity, protein kinase, diseases resistance protein, NBS-LRR proteins represent less than 1/4th of the genes we have obtained after synteny mapping. These genes were mentioned with their gene id, locus tag along with the product formed as shown in table 2. Soybean chromosome 13 region having start from 22449055 bp and up to 22449570 bp showed gene homology with the sequence starting from 31929662 to 31930177 of Phaseolus vulgaris. This genomic region in both the species codes for guanine nucleotide binding protein subunit gamma 3-like isoform X1. The guanine nucleotide binding protein is important for signalling pathway. It is to be noted that the Rpg1b genes in soybean mainly codes for NB and LRR proteins. Various NB-LRR protein coding genes which are present on chromosome 13 of G. max are also showing synteny with P. vulgaris. For instance, F-box/ LRR-repeat protein 4-like (LOC100816140) is located at 27041793 bp to 27042286 bp in Phaseolus vulgaris. A mitogen-activated protein kinase2 (LOC100789734) located on chromosome 13 of Glycine max is showing homology with Phaseolus vulgaris.
A G type lectin S-receptors like serine/threonine-protein kinase coding gene located at chromosome 13 in Glycine max is also present in Phaseolus vulgaris genomic region having starts from 30144768 bp to 30146245 bp. It is having several molecular function like ATP binding, Calmodulin binding and protein serine/threonine kinase activity and few biological functions like protein phosphorylation and recognition of pollen. Glycogen phosphorylase 1-like isoform X1 coding gene which is present at locus LOC100779066 on Glycine max chromosome 13 is present in Phaseolus vulgaris. Uncharacterized ATPdependent helicase C23E6.02-like (LOC100792901) and B3 domain-containing protein Os07g0563300-like both were conserved in these legumes namely Glycine max and Phaseolus vulgaris. Highest synteny was found to be 91.794 % similarity between Glycine max and Phaseolus vulgaris for a region which codes for an uncharacterized protein LOC100775655 in both the legume. Similarly, lowest synteny was found to be 85.049 % similarity at loci LOC102661544 of Glycine max.
Various syntenic regions were found between the two legumes, which codes for different proteins like MAP Kinase kinase2, Guanine nucleotide binding protein, 1-phosphatidylinositol-3-Phosphate 5-kinase and several Serine threonine kinases. All the proteins have different molecular and biological functions like ATP and ADP binding, Kinase activity, Metal and Calcium Ion binding, signal transduction, defense responses respectively.
Different gene functions and their position in soybean and common bean are described with details in Table 3.
In the present study, a different approach has been used for comparison of soybean genome with common bean. Advantage of synteny analysis is based on the concept that the species which are evolutionary related are diverged from their common ancestor and conserved genome synteny can be efficiently interpreted from a well-studied species to another less characterized genomes. Synteny is found to be higher between the closely related species (Lee et. al., 2017) and this concept is confirmed in this study. The outcome from the analysis identifies the best match to the Glycine max genome. Here focus was done only on chromosome 13 of Glycine max. We selected this chromosome because in Glycine max, diseases resistance coding genes are known to be found mostly on this region (Chr. 13). By the comparison of both the legumes sequences we get NB-LRR protein coding genes, which are responsible for disease resistance in Glycine max and Phaseolus vulgaris.
Identifi cation of conserved synteny has noticeable advantage in understanding the legumes genetics. Large region of synteny occurs between Glycine max and Phaseolus vulgaris (Mc Clean et. al., 2010), our results also showed the gene conservation between both the legumes. Studies of the NBS-LRR gene family in legume plants can provide knowledge on the genomic and molecular mechanism that form the basis of gene regulation and protein functions. In this study, various NBS-LRR proteins (as mentioned in table 3 showing different functions of gene products) were found to be conserved in Glycine max and Phaseolus vulgaris. Now a day’s gene cloning approach is advancing to make disease resistance varieties of legumes as in Medicago sativa cloning of a disease resistance gene was done by utilizing Medicago truncatula gene information through synteny (Yang et. at., 2008). In the same way this synteny analysis result will be useful for evolutionary studies that help in long term planning, breeding and developing disease resistance varieties of legumes.
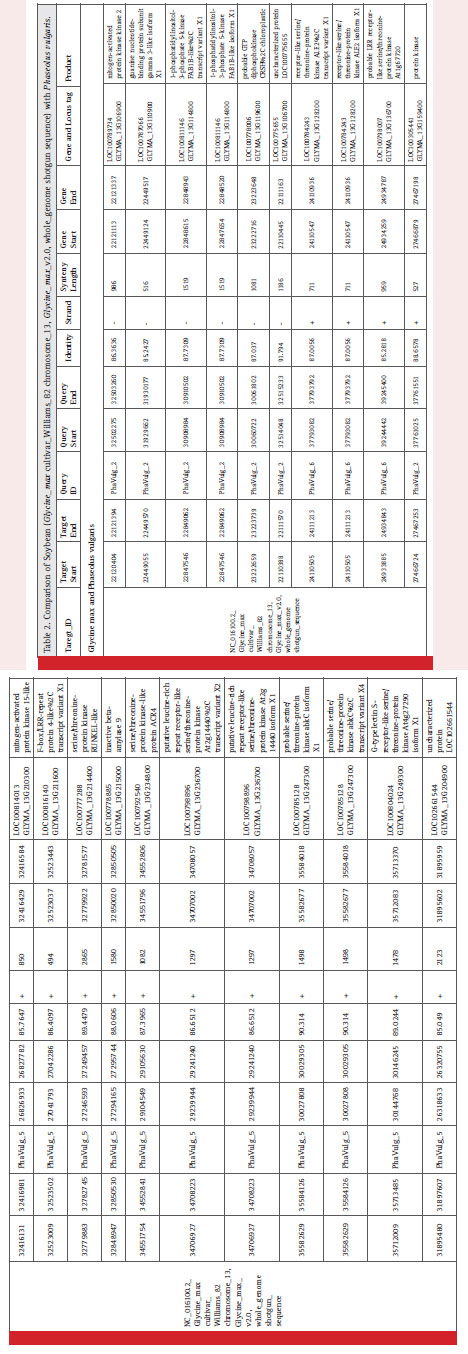 |
Table 2: max cultivar_Williams_82 chromosome_13, Glycine_max_v2.0, whole_genome shotgun sequence) with Phaseolus vulgaris. |
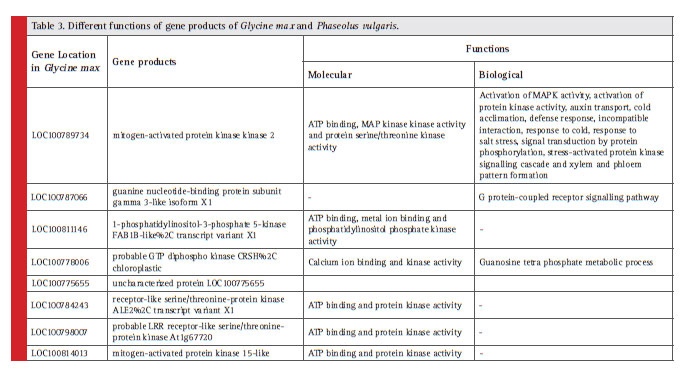 |
Table 3: Different functions of gene products of Glycine max and Phaseolus vulgaris. |
Acknowledgements
Authors acknowledge the facilities of the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (DBT) under the Bioinformatics Sub Centre as well as the golden jubilee research fellowship (GJRF) provided by School of Biotechnology, Devi Ahilya University Indore. M.P. India.
Conflict of Interest
The authors confirm that they have no conflict of interest.
References
Andersen, E. J., Ali, S., Reese, R. N., Yen, Y., Neupane, S., & Nepal, M. P. (2016). Diversity and evolution of disease resistance genes in barley (Hordeum vulgare L.). Evolutionary Bioinformatics, 12, EBO-S38085.
Andolfo, G., & Ercolano, M. R. (2015). Plant innate immunity multicomponent model. Frontiers in plant science, 6, 987.
Bauchan, G. R., & Hossain, M. A. (2001). Distribution and characterization of heterochromatic DNA in the tetraploid African population alfalfa genome. Crop science, 41(6), 1921-1926.
Bennetzen, J. L. (2000). Comparative sequence analysis of plant nuclear genomes: microcolinearity and its many exceptions. The Plant Cell, 12(7), 1021-1029.
Benson, B. V. (2014). Disease resistance genes and their evolutionary history in six plant species. Boutin, S. R., Young, N. D., Olson, T., Yu, Z. H., Vallejos, C. E., & Shoemaker, R. C. (1995). Genome conservation among three legume genera detected with DNA markers. Genome, 38(5),
928-937.
Cannon, S. B., & Shoemaker, R. C. (2012). Evolutionary and comparative analyses of the soybean genome. Breeding science, 61(5), 437-444.
Chen, X., Li, H., Pandey, M. K., Yang, Q., Wang, X., Garg, V., … & Upadhyaya, H. (2016). Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proceedings of the National Academy of Sciences, 113(24), 6785-6790.
Choi, H. K., Mun, J. H., Kim, D. J., Zhu, H., Baek, J. M., Mudge, J., … & Young, N. D. (2004). Estimating genome conservation between crop and model legume species. Proceedings of the National Academy of Sciences, 101(43), 15289-15294.
Christie, N., Tobias, P. A., Naidoo, S., & Külheim, C. (2016). The Eucalyptus grandis NBS-LRR gene family: physical clustering and expression hotspots. Frontiers in plant science, 6, 1238.
Devos, K. M., & Gale, M. D. (2000). Genome relationships: the grass model in current research. The Plant Cell, 12(5), 637-646.
Doyle, J. J., & Luckow, M. A. (2003). The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiology, 131(3), 900-910.
Franssen, S. U., Shrestha, R. P., Bräutigam, A., Bornberg-Bauer, E., & Weber, A. P. (2011). Comprehensive transcriptome analysis of the highly complex Pisum sativum genome using next generation sequencing. BMC genomics, 12(1), 227.
Gualtieri, G., Kulikova, O., Limpens, E., Kim, D. J., Cook, D. R., Bisseling, T., & Geurts, R. (2002). Microsynteny between pea and Medicago truncatula in the SYM2 region. Plant molecular biology, 50(2), 225-235.
Gururani, M. A., Venkatesh, J., Upadhyaya, C. P., Nookaraju, A., Pandey, S. K., & Park, S. W. (2012). Plant disease resistance genes: current status and future directions. Physiological and molecular plant pathology, 78, 51-65.
Hulbert, S. H., Webb, C. A., Smith, S. M., & Sun, Q. (2001). Resistance gene complexes: evolution and utilization. Annual review of phytopathology, 39(1), 285-312.
Kalavacharla, V., Liu, Z., Meyers, B. C., Thimmapuram, J., & Melmaiee, K. (2011). Identifi cation and analysis of common bean (Phaseolus vulgaris L.) transcriptomes by massively parallel pyrosequencing. BMC plant biology, 11(1), 135.
Kang, Y. J., Kim, S. K., Kim, M. Y., Lestari, P., Kim, K. H., Ha, B. K., … & Shim, S. (2014). Genome sequence of mungbean and insights into evolution within Vigna species. Nature communications, 5, ncomms6443.
Lee, C., Yu, D., Choi, H. K., & Kim, R. W. (2017). Reconstruction of a composite comparative map composed of ten legume genomes. Genes & Genomics, 39(1), 111-119.
Lee, J. M., Grant, D., Vallejos, C. E., & Shoemaker, R. C. (2001). Genome organization in dicots. II. Arabidopsis as a’bridging species’ to resolve genome evolution events among legumes. Theoretical and Applied Genetics, 103(5), 765-773.
McClean, P. E., Mamidi, S., McConnell, M., Chikara, S., & Lee, R. (2010). Synteny mapping between common bean and soybean reveals extensive blocks of shared loci. BMC genomics, 11(1), 184.
McConnell, M., Mamidi, S., Lee, R., Chikara, S., Rossi, M.,Papa, R., & McClean, P. (2010). Syntenic relationships among legumes revealed using a gene-based genetic linkage map of common bean (Phaseolus vulgaris L.). Theoretical and Applied Genetics, 121(6), 1103-1116.
Nepal, M. P., & Benson, B. V. (2015). CNL disease resistance genes in soybean and their evolutionary divergence. Evolutionary Bioinformatics, 11, EBO-S21782.
Neupane, S., Ma, Q., Mathew, F. M., Varenhorst, A. J., Andersen, E. J., & Nepal, M. P. (2018). Evolutionary Divergence of TNL Disease-Resistant Proteins in Soybean (Glycine max) and Common Bean (Phaseolus vulgaris). Biochemical genetics, 1-26.
Passoupathy, R. (2016). Synteny with Allied and Model Genomes. 10.1007/978-3-319-47789-3_6.
Paterson, A. H., Bowers, J. E., Burow, M. D., Draye, X., Elsik, C. G., Jiang, C. X., … & Wright, R. J. (2000). Comparative genomics of plant chromosomes. The Plant Cell, 12(9), 1523-1539.
Sato, S., Nakamura, Y., Kaneko, T., Asamizu, E., Kato, T., Nakao, M., & Fujishiro, T. (2008). Genome structure of the legume, Lotus japonicus. DNA research, 15(4), 227-239.
Schlueter, J. A., Dixon, P., Granger, C., & Shoemaker, R. C. (2005). Mining the EST databases to determine evolutionary events in the legumes and grasses. In Genome Exploitation (pp.163-181). Springer, Boston, MA.
Schmidt, R. (2000). Synteny: recent advances and future prospects. Current opinion in plant biology, 3(2), 97-102.
Schmutz, J., Cannon, S. B., Schlueter, J., Ma, J., Mitros, T., Nelson, W., … & Xu, D. (2010). Genome sequence of the palaeopolyploid soybean. Nature, 463(7278), 178.
Schmutz, J., McClean, P. E., Mamidi, S., Wu, G. A., Cannon, S. B., Grimwood, J., … & Torres-Torres, M. (2014). A reference genome for common bean and genome-wide analysis of dual domestications. Nature genetics, 46(7), 707.
Shao, Z. Q., Xue, J. Y., Wu, P., Zhang, Y. M., Wu, Y., Hang, Y. Y., … & Chen, J. Q. (2016). Large-scale analyses of angiosperm nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes reveal three anciently diverged classes with distinct evolutionary patterns. Plant Physiology, pp-01487.
Shoemaker, R. C., Polzin, K., Labate, J., Specht, J., Brummer, E. C., Olson, T., … & Kochert, G. (1996). Genome duplication in soybean (Glycine subgenus soja). Genetics, 144(1), 329-338.
Singh, J., Kalberer, S. R., Belamkar, V., Assefa, T., Nelson, M. N., Farmer, A. D., … & Cannon, S. B. (2018). A transcriptome- SNP-derived linkage map of Apios americana (potato bean) provides insights about genome re-organization and synteny conservation in the phaseoloid legumes. Theoretical and Applied Genetics, 131(2), 333-351.
Varshney, R. K., Song, C., Saxena, R. K., Azam, S., Yu, S., Sharpe, A. G., … & Millan, T. (2013). Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nature biotechnology, 31(3), 240.
Weeden, N. F., Muehlbauer, F. J., & Ladizinsky, G. (1992). Extensive conservation of linkage relationships between pea and lentil genetic maps. Journal of Heredity, 83(2), 123-129.
Yadegari, Z. (2013). Molecular mapping and characterization of phenylpropanoid pathway genes in common bean (Phaseolus vulgaris L.) (Doctoral dissertation).
Young, N. D., Debellé, F., Oldroyd, G. E., Geurts, R., Cannon, S. B., Udvardi, M. K., … & Van de Peer, Y. (2011). The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature, 480(7378), 520.
Young, N. D., Mudge, J., & Ellis, T. N. (2003). Legume genomes: more than peas in a pod. Current opinion in plant biology, 6(2), 199-204.
Zhu, H., Choi, H. K., Cook, D. R., & Shoemaker, R. C. (2005). Bridging model and crop legumes through comparative genomics. Plant physiology, 137(4), 1189-1196.