1Department of Microbiology, School of Life Sciences, Central University of Rajasthan, NH-8, Bandarsindri, Kishangarh, Ajmer-305817, Rajasthan, India
1,2Department of Sports Biosciences, School of Sports Sciences, Central University of Rajasthan, NH-8, Bandarsindri, Tehsil Kishangarh, Dist-Ajmer-305817, Rajasthan, India
Corresponding author Email: csgahan_mbio@curaj.ac.in
Article Publishing History
Received: 08/04/2019
Accepted After Revision: 07/06/2019
The microbial culture as well as the components of the bioleaching medium affects the efficiency of microbial leaching. The present study on mobile phone printed circuit boards (MPPCBs) was conducted to know that whether the addition of energy source is required during the microbial leaching or microorganisms can utilize the iron (Fe) content of the MPPCB. The study was conducted with pure Fe oxidizers in Fe supplemented (9g/L, pure Fe 9K), non-supplemented (0g/L, pure Fe 0K) medium and pure sulfur (S) oxidizers supplemented with 3g/L of elemental sulfur (S0). The copper (Cu) content of the feed material was 26.3% (w/w) by X-Ray Fluorescence (XRF) spectroscopy. The Cu recovery in pure Fe 0K and pure Fe 9K was 100% while with pure S oxidizers it was 39.41%. The acid consumption in pure Fe 0K and pure Fe 9K was 579.65kg/ton and 559.05kg/ton respectively. The bioleaching rate of Cu was 0.128g/L/h, 0.075g/L/h and 0.023g/l/h in order of pure Fe 9K> pure Fe 0K>pure S. Bioleaching with pure Fe oxidizers in 0K medium was found to be efficient and economical in terms of metal recovery and acid consumption. The present study reports that the addition of extra energy source is nevertheless required for bioleaching of PCB. Therefore, this study can be useful in large scale operations where the process cost for the treatment of the waste should be less than the cost of the final product.
Bioleaching, Copper, 9K medium, 0K medium.
Garg H, Nagar N, Dash A, Gahan C. S. Efficiency Assessment of Pure Fe Oxidizing Microorganisms in Iron Supplemented and Non-Supplemented Medium and Pure S Oxidizing Microorganisms for Bioleaching of Mobile Phone Printed Circuit Boards. Biosc.Biotech.Res.Comm. 2019;12(2).
Garg H, Nagar N, Dash A, Gahan C. S. Efficiency Assessment of Pure Fe Oxidizing Microorganisms in Iron Supplemented and Non-Supplemented Medium and Pure S Oxidizing Microorganisms for Bioleaching of Mobile Phone Printed Circuit Boards. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2Zawf2m
Copyright © Garg et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
A significant amount of valuable metals resides in a printed circuit board (PCB) of an electronic device. There are various established technologies for the extraction of metals from PCBs such as mechanical separation, pyrometallurgy, hydrometallurgy, etc. (Shah, Tipre and Dave, 2014). The application of these techniques for treatment of the waste costs more in comparison to the final product. In that respect, bioleaching has been a promising alternative for metal recovery from low-grade ores and the waste (Cui and Zhang, 2008; Mishra and Rhee, 2010; Erüst et al., 2013; Johnson, 2014). The microorganisms which are primarily used for bioleaching belong to genus Acidithiobacillus due to their robust nature and ability to oxidize the inorganic ferrous (Fe2+) and elemental sulfur (S0) (Rawlings, 2005; Arshadi and Mousavi, 2014; L. Wang et al., 2018; Quatrini and Johnson, 2019).
The 100% Cu recovery by cell-free extract of Leptospirillum ferriphilum and Sulfobacillus thermosulfidooxidans from 5g/L of PCB indicates the indirect bioleaching(Wu et al., 2018). However, the regeneration of the reagent (Fe3+) is necessary for the continuation of the bioleaching process at higher pulp densities. Initial pH and Fe2+ ion concentration influence the rate of Cu recovery in the bioleaching medium (Xiang et al., 2010). Few studies suggest that Fe content in PCBs can be utilized by bacteria and addition of extra Fe source leads to jarosite precipitation hence, loss of Fe from the bioleaching system ( Wang et al., 2018). The bioleaching of PCBs is an indirect non-contact mechanism where the significant role of the bacteria is oxidation of ferrous (Fe2+) into ferric (Fe3+), (Silva et al., 2015; Mostafavi et al., 2018). Low pH environment can prevent the jarosite formation; it favors the microbial growth and enhances the Cu recovery ( Wang et al., 2018). The pH values also influence the bioleaching kinetics of Acidithiobacillus ferrooxidans to mediate Cu recovery (Yang et al., 2014). Previous studies on bioleaching emphasize that the recovery and rate of Cu bioleaching from PCBs mainly depends on Fe2+ ion concentration and pH. The parameters such as temperature, pulp density, etc. influence these two factors and consequently affects the bioleaching ( Wang et al., 2018; Arinanda et al., 2019).
The present study aimed to investigate bioleaching efficiency of pure Fe oxidizing microorganisms in two different bioleaching mediums, i.e., with and without Fe supplements. The hypothesis for using medium without external energy source is 1. The feed material itself has Fe which can be utilized by the Fe oxidizing microorganisms for bio-oxidation 2. The external Fe added to the medium might be an additional amount and not be used completely by the microorganism 3. Fe precipitation and jarosite formation is a common factor which influences the bioleaching yield 4. The extra Fe in bioleaching medium tends to enhance the possibility of jarosite formation which consequently increases the process cost due to the input of additional energy source, post residue treatment as well as the negative impact on Cu bioleaching efficiency. Most of the research suggests that addition of Fe might not be required during bioleaching of PCBs as the Fe which dissolves into the bioleaching medium from PCB can be utilized by the microorganisms. (Bryan et al., 2015; Wang et al., 2018).
The research on bioleaching of PCBs are on laboratory scale due few limitations of its largescale operations such as toxicity, time requirement as well as the cost. The present paper focuses on the current needs for the commercialization of the bioleaching system by comparing the bioleaching yield of Cu from waste MPPCBs in Fe free and supplemented growth medium for Fe oxidizing microorganisms. The paper also studied the bioleaching efficiency of only S oxidizing microorganisms. The bioleaching experiments were compared in respect of acid consumption, Cu recovery and time required for complete bio-oxidation of Cu.
Material and Methods
Mobile phone printed circuit boards (MPPCBs)
Printed circuit boards were separated by manual sorting of the waste mobile phones collected from the mobile phone repair shops in Alwar, Rajasthan, India. The crushing of MPPCBs was carried out at the Mineral Processing department of CSIR-Institute of Minerals and Materials Technology, Bhubaneswar, India. The PCBs were ground to a particle size below 250 µm in an impact crusher followed by sieving. The elemental composition of this grounded material (feed).
Table 1: Metal content (%) in mobile phone printed circuit boards (MPPCBs).
| MPPCB
Metal content (%) |
Cu | Si | Ca | Br | Al | Fe | Sn | Ni | P | Zn | S | Ti | Pb |
| 26.3 | 21.7 | 14.7 | 9.6 | 6.1 | 5.3 | 3.1 | 1.1 | 1.2 | 1.1 | 0.8 | 0.8 | 0.7 | |
| Ag | Mg | Sb | Cr | Ta | Sr | Cl | Nd | Mn | K | Au | Ga | Co Nb | |
| 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 0.01 |
was analyzed by X-Ray Fluorescence analyzer (Bruker)(Table-1).
The XRF analysis revealed that Cu was 26.3% of the total metal constituents in the feed material. The recovery of Cu was then focused along with Ni (1.08%) and Zn (1.08%). The X-ray diffraction (XRD) and Scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS) (Nova Nano FE-SEM 450 (FEI) analysis was done to determine the mineralogical phases and elemental weight percentage (%) of the feed material (Figure 1 and 2).
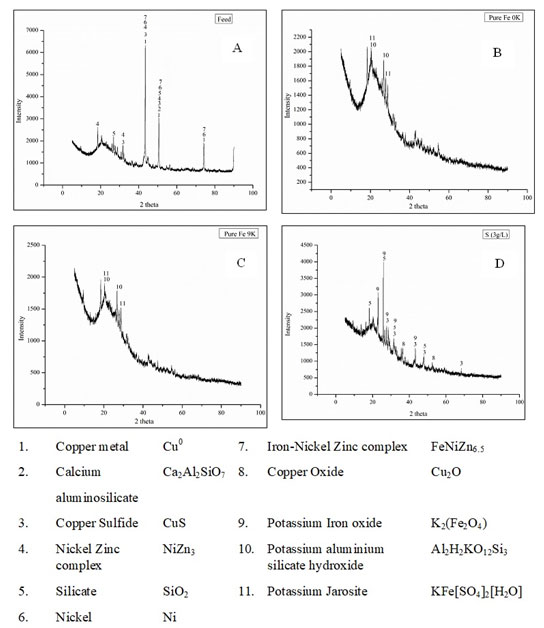 |
Table 1a |
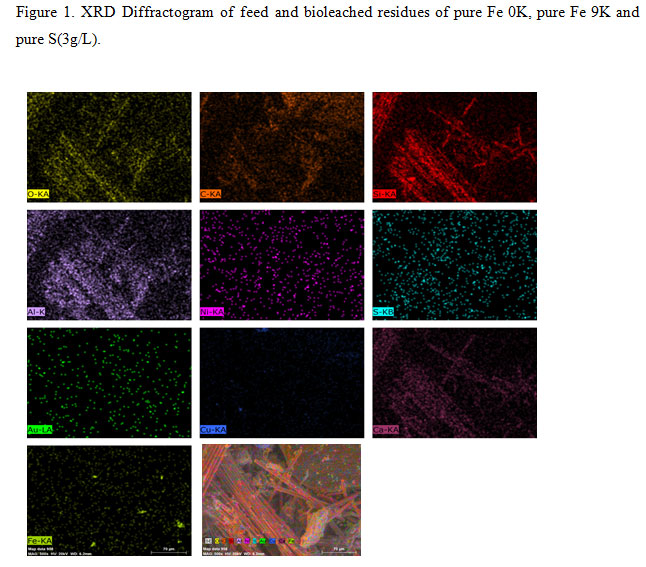 |
Figure 1: XRD Diffractogram of feed and bioleached residues of pure Fe 0K, pure Fe 9K and pure S(3g/L). |
 |
Figure 2: SEM-EDS mapping profiles (point scan) and EDS micrograph of feed. |
The small dotted arrangement patterns The same pattern of arrangement found in the Sulphur (S), Copper (Cu) and Iron (Fe) indicates the presence of the copper mineral complex with iron and sulfur, which was also observed in the mineralogy obtained from the XRD studies (Figure 1and 2)
Bioleaching experiments
The bioleaching of crushed MPPCBs was done in three sets of experiments. Among three two bioleaching experiments namely pure Fe 0K and pure Fe 9K were done with pure culture of Leptospirillum dominated Fe oxidizing bacteria in bioleaching medium without Fe supplement (0g/L, Fe) and with Fe supplement (9g/L, Fe) respectively. Another bioleaching experiment Pure S (3g/L) was done with At. thiooxidans dominated pure S oxidizing bacterial culture supplemented with 3g/L of elemental sulfur (S0).The inoculums used for bioleaching experiments were the activated microbial cultures grown in their respective mediums. The Fe oxidizing microbial culture was grown in a 0K medium [(NH4)2SO4, 3.0 g/L; KCl, 0.1 g/L; K2HPO4, 0.5 g/L; MgSO4·7H2O, 0.5 g/L; Ca(NO3)2·4H2O, 0.01 g/L] (Silverman and Lundgren, 1959), supplemented with 9g/L of ferrous (Fe2+) in the form of ferrous sulfate (FeSO4.7H2O) as an energy source. The pH was controlled at 1.5 and the temperature at 30°C. On the other hand, At. thiooxidans was grown in a X-Umea basal salt medium (Na2SO4.10H2O, 3.2g/L; (NH4)2SO4, 3.0 g/L; KCl, 0.1 g/L; K2HPO4, 0.5 g/L; MgSO4·7H2O, 0.5 g/L; Ca(NO3)2·4H2O, 0.01 g/L) supplemented with 3g/L of elemental sulfur (S0) as the energy source. The growth of S oxidizing microorganisms was started at 3 pH and 30°C. The growth of Fe oxidizing microorganisms was analyzed by the regular measurement of pH, redox potential, ferrous (Fe2+) and total iron. The maximum value of oxidation-reduction potential (ORP) and zero residual Fe2+ ion was indicated activated Fe oxidizers. The increasing sulfate ion (SO42-) concentration and decreasing trend in pH values were marked in the activated S oxidizing microbial culture.
The bioleaching experiments were done with a working volume of 1L (v/v), 90% (v/v) of growth medium and 10% (v/v) of the inoculums. The pH of the bioleaching medium was maintained at pH 1.5 by addition of 5M H2SO4 for the activity of Fe oxidizing microorganisms. The bioleaching medium composition was the same as the growth medium for pure S oxidizing microorganisms. No pH adjustments were made during the bioleaching of S-oxidizing microorganisms. Temperature for both the bioleaching experiments was maintained at 35ºC. 50g/L of the PCB powder was added in the bioleaching medium (growth medium + activated culture) in the starting of all the three experiments. The daily addition of H2O compensated the evaporation loss of water (H2O). The changes in pH during bioleaching experiments were regularly monitored by a pH meter (Eutech). The changes in Fe2+ concentration and ferrous/ferric (Fe2+/Fe3+) couple during bioleaching with pure Fe oxidizing microorganisms was monitored by a titrimetric method using cerium sulfate with 1,10-Phenanthroline as an indicator and an ORP meter having platinum electrode with Ag, AgCl reference electrode. The concentration of SO42- was analyzed by using a turbidimetric method by visible spectrophotometric method (420 nm) described in the American Public Health Association, 1975 (APHA) (Kolmert, Wikström and Hallberg, 2000).
The metal ion concentration in all the three experiments was determined by atomic absorption spectrophotometer (AAS) (Thermo Scientific iCE 3000 SERIES). A bright field microscope did the planktonic viable microbial cell count at 100X magnification on a Neubauer hemocytometer. After constant values of metal ion concentration and other parameters, the bioleaching experiments were harvested by solid-liquid separation. Bioleached residue was washed thoroughly using 1.5 pH water and air dried. The mineralogical analysis of both feed and the bioleached residue was determined by “PANanalytical Powder XRD.” The samples for XRD were pulverized first to ensure the homogeneity then the diffraction patterns were measured at angles between 10º to 90º, the step size was 0.02 angle/sec. The crystalline phases were identified by using the joint committee for powder diffraction standards (JCPDS) file.
Results and Discussion
The pH of without Fe supplement (0K) bioleaching medium was high during the initial three days of the experiment due to the alkaline nature of the PCBs and bio-oxidation of Fe2+ into Fe3+(Figure 3).
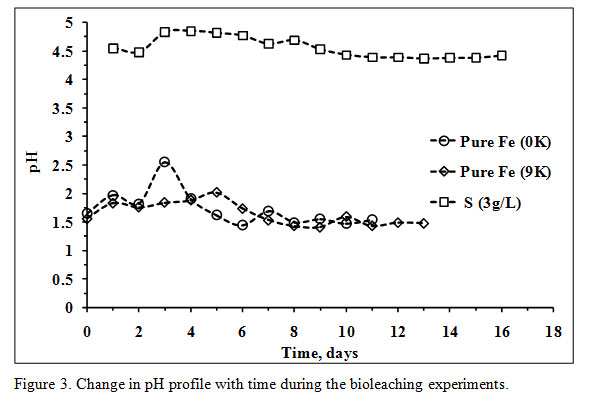 |
Figure 3: Change in pH profile with time during the bioleaching experiments. |
After that, no significant increase in pH was observed. The highest concentration of Fe3+ was marked on the 4th day of the experiment with a notable rise in ORP values after that the ORP was in an increasing trend along with microbial cell count. The bioleaching experiment in 0K medium was initiated with a total iron concentration of 0.92g/L and reached 3.51g/L on the 3rd day (Figure 4) which indicates complete dissolution of Fe presented in the feed (2.66g/L).
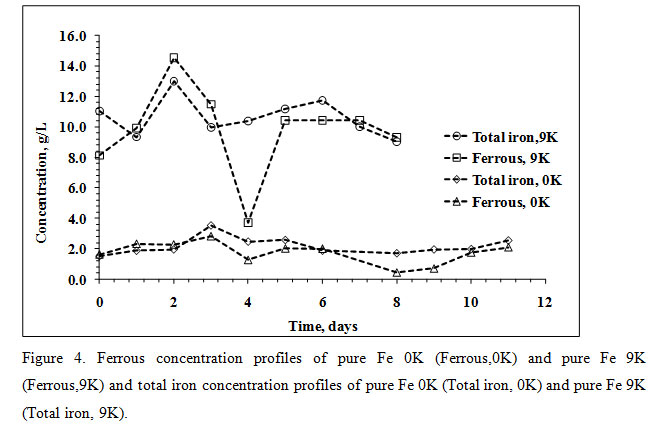 |
Figure 4: Ferrous concentration profiles of pure Fe 0K (Ferrous,0K) and pure Fe 9K (Ferrous,9K) and total iron concentration profiles of pure Fe 0K (Total iron, 0K) and pure Fe 9K (Total iron, 9K). |
The decreasing trend in total Fe concentration with a notable low pH value after the 3rd day of the experiment was due to formation jarosite. The presence of potassium jarosite peaks (characteristic in a Fe oxidizing microbial bioleaching system) in XRD data of bioleached residue confirms jarosite formation (Figure 1B). The significant increase in pH values in Fe supplemented medium (9K) was observed from the 4th day with increasing values of ORP and microbial cell count. The acid was mainly added during initial days to maintain a pH value of 1.5 which is accounted for the growth of Leptospirillum ferriphilum and highest Cu recovery ( Wang et al., 2018).
The pH values in both the experiments were low during the last days, and no more acid was added. The ORP values in both the systems were unstable after addition of PCBs in the bioleaching medium; hence, hard to evaluate. The stable ORP values from the 1st day of the experiments were low and started increasing from 4th and 5th day respectively. At the end of the operations, the ORP value of 0K bioleaching medium was 406mV while, 438mV in the 9K bioleaching medium (Figure 5).
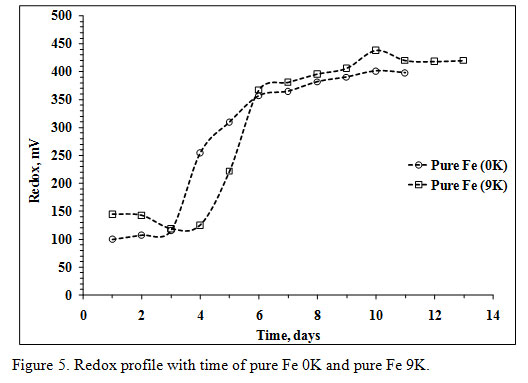 |
Figure 5: Redox profile with time of pure Fe 0K and pure Fe 9K. |
As soon as the PCB powder was added in bioleaching medium the initial microbial cell count of the 0K and 9K bioleaching medium was decreased from a value of 2.80E+08 to 7.20E+06 and 4.80E+06 respectively (Figure 6).
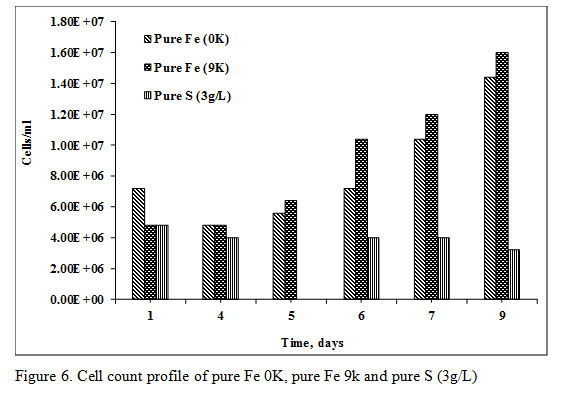 |
Figure 6: Cell count profile of pure Fe 0K, pure Fe 9k and pure S (3g/L) |
The microbial cell count was recovered after the 5th day in the amount of 1.60E+07 cells/mL of bioleaching medium. The total amount of acid consumption was 559.05 kg/ton and 579.65kg/ton in 9K and 0K bioleaching medium respectively. Complete recovery of Cu (100% recovery) took place in 8 days and six days of Pure Fe 0K medium and pure Fe 9K medium respectively. The recovery of Zn and Ni in pure Fe 0K and 9K systems was 75.57%, 98.14%, 75.12%, and 22.10% respectively (Figure 7).
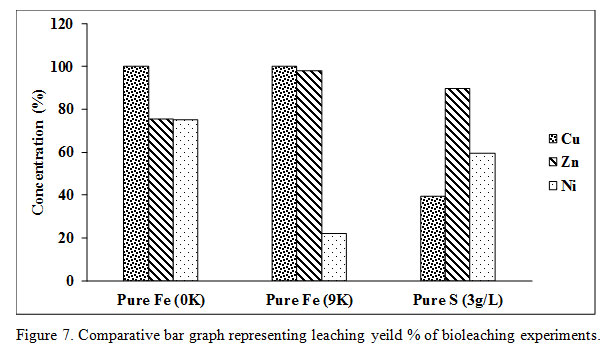 |
Figure 7: Comparative bar graph representing leaching yeild % of bioleaching experiments. |
The Cu dissolution rate was 0.128g/L/h in pure Fe 9K system while it was 0.075g/L/h in pure Fe 0K system (Figure 8).
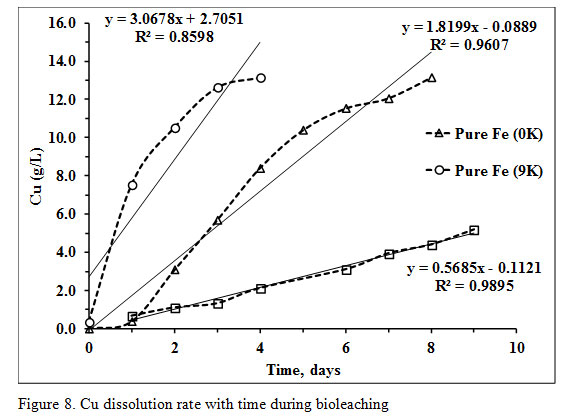 |
Figure 8: Cu dissolution rate with time during bioleaching |
The 50g/L PCB was added in X-Umea salt medium at pH 1 and initial sulfate (SO42-) concentration of 6.14g/L (inoculum+ media). The initial cell count of the bioleaching media was decreased from 6.64E+08 to 4.80E+06 as soon as PCB was added in the medium (Figure 6). The microbial cell count, pH as well as the sulfate concentration was not recovered with time. The acid was also added in initial few days to maintain the pH at three optimized for the growth of S oxidizing microorganisms. At pH 3, Fe does not remain in the soluble form which ensures only biogenic proton leaching of PCB. The SO42- contributed from 18.19M of H2SO4 with a purity of 96% and a density of 1.84g/mL was 6.93g/L. The total sulfate detected at the end of the experiment was 11.20g/L which was lesser than the total added SO42- concentration. The Cu recovery in pure S oxidizing system was only 39.41% which quite lower in comparison to the other two experiments with a slower dissolution rate of 0.023g/L/h. Recovery of the other two metals viz Ni and Zn was 59.38% and 89.68% respectively (Figure 7 and 8).
The low pH and redox values of 9K bioleaching medium during initial days indicate that as soon as PCB was added in the bioleaching medium the Fe3+ ion attacked on it and converted into Fe2+. The total Fe profile of 9K bioleaching medium shows that high Fe3+ ion concertation on the 0th hour was immediately reduced into Fe2+. The Fe2+ to Fe3+ bio-oxidation was then marked with an increasing ORP after a microbial growth lag phase of 4 days. The Fe2+ to Fe3+ bio-oxidation thus started in later days of the experiment. The results are consistent with a previous study stated that the Fe2+ to Fe3+ bio-oxidation is the rate-limiting step during bioleaching (Wu et al., 2018). The significant portion of the acid was added during the initial four days of the bioleaching without Fe supplements while in 9K medium bioleaching the acid addition was done during the experiment. This acid was required for the bio-oxidation of Fe2+ into Fe3+ which was high in the 9K medium. The more acid consumption by Fe oxidizing microorganisms in 9K medium shows that addition of Fe puts an extra burden on microbes as the Cu recovery in both the bioleaching mediums was similar with less consumption of acid in 0K (Table 2).
Table 2: Summary of bioleaching experiments.
| Pure Fe 0K | Pure Fe 9K | Pure S (3g/L) | |
| Feed weight, g | 50 | 50 | 50 |
| Bioleach residue, g | 35.44 | 35.06 | 48.9 |
| Acid Consumption, kg Conc. H2SO4/ton PCBs | 579.65 | 559.05 | – |
A study on PCBs found that 0.25g/L of Fe2SO4 is sufficient to mobilize Cu from 50g/L of PCBs in a multistage system, (S. Wang et al., 2018). The Fe2+ to Fe3+ bio-oxidation and jarosite formation are the two main reasons behind low pH values during the last days in 0K medium which can be confirmed by an increasing trend of redox potential, viable cell count, decrease in Fe2+ ion concentration. Hence, the Fe content in PCBs itself was sufficient to be utilized by the microorganisms for bioleaching.
The changes in pH, SO42- as well as the cell count profile of bioleaching in the presence of pure S oxidizing bacteria, was not significant. The bioleaching experiment was initiated with an SO42- concentration of 6.14g/L. The increased SO42- in the bioleaching system was contributed by the acid addition. The acid production by the S oxidizing microorganisms was insignificant. According to the previous study, the S oxidizers are more sensitive for the higher metal concentration as well as the toxicity related to PCBs (Wang et al., 2009). A previous study on bioleaching of PCBs also concluded the Cu recovery in presence to At. thiooxidans was less in comparison to the abiotic chemical leaching (Hong and Valix, 2014). The results were persistent with our study also where Ni and Zn dissolution were high in comparison to Cu. The XRD data of pure S oxidizers bioleached residue reveals the presence of CuS and Cu2O which according to a previous study tends to precipitate Cu, forms a passivation layer and results in an incomplete Cu dissolution (Hong and Valix, 2014). The faster Cu recovery rate in 9K medium shows that the presence of Fe3+ during first few hours helped in Cu dissolution. However, the bio-oxidation of Fe2+ into Fe3+ took a time of three days, and an increased amount of Fe3+ was again noted on the 4th day of the experiment. The metal dissolution in pure S oxidizers was due to proton leaching by addition of H2SO4 and not by the biogenic acid.
Conclusion
The present study was done to check the feasibility of MPPCBs bioleaching by using pure Fe oxidizing microorganisms in with and without Fe supplemented medium and by pure S oxidizing microorganisms. The Cu recovery was 100% in both the experiments with a faster dissolution rate of Cu in the 9K medium. The Cu dissolution rate was 0.1278g/L/h and 0.075g/L/h in pure Fe 9K and 0K medium whereas 0.023g/L/h in S oxidizers. The complete recovery of Cu took six days and 8days in pure Fe 9K and 0K medium respectively. The acid consumption in 0K medium was less in comparisons to 9K medium. The recovery of Ni and Zn was also satisfactory in bioleaching pure Fe 0K medium. The bioleaching with pure Fe oxidizers in 0K medium was found to be more feasible in terms of metal recovery, acid consumption, leaching time and total cost of the process. The pure S oxidizing microorganisms supplemented with 3g/L of S0 were found inefficient for the bioleaching of 50g/L of MPPCBs.
Acknowledgments
The authors want to acknowledge DST SERB for providing funds for the research. The authors also wish to thank DST INSPIRE for offering fellowship to Ph.D. The authors want to pay their gratitude to the Central University of Rajasthan for providing infrastructure facility. Last but not the least the author thanks to IMMT, CSIR, Bhubaneswar India and Material research center (MRC), MNIT, Jaipur for availing the instrumentation facilities.
References
Arinanda, M., van Haute, Q., Lambert, F. and Gaydardzhiev, S. (2019) Effects of operational parameters on the bio-assisted leaching of metals from pyrolized printed circuit boards, Minerals Engineering. Elsevier, 134, pp. 16–22.
Arshadi, M. and Mousavi, S. M. (2014) Simultaneous recovery of Ni and Cu from computer-printed circuit boards using bioleaching: Statistical evaluation and optimization, Bioresource technology. Elsevier, 174, pp. 233–242.
Bryan, C. G., Watkin, E. L., McCredden, T. J., Wong, Z. R., Harrison, S. T. L. and Kaksonen, A. H. (2015) The use of pyrite as a source of lixiviant in the bioleaching of electronic waste’, Hydrometallurgy. Elsevier, 152, pp. 33–43.
Cui, J. and Zhang, L. (2008) Metallurgical recovery of metals from electronic waste: a review’, Journal of hazardous materials. Elsevier, 158(2–3), pp. 228–256.
Erüst, C., Akcil, A., Gahan, C. S., Tuncuk, A. and Deveci, H. (2013) Biohydrometallurgy of secondary metal resources: a potential alternative approach for metal recovery’, Journal of Chemical Technology & Biotechnology. Wiley Online Library, 88(12), pp. 2115–2132.
Hong, Y. and Valix, M. (2014) ‘Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria’, Journal of Cleaner Production. doi: 10.1016/j.jclepro.2013.08.043.
Johnson, D. B. (2014) Biomining—biotechnologies for extracting and recovering metals from ores and waste materials, Current opinion in biotechnology. Elsevier, 30, pp. 24–31.
Kolmert, Å., Wikström, P. and Hallberg, K. B. (2000) A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures, Journal of microbiological methods. Elsevier, 41(3), pp. 179–184.
Mishra, D. and Rhee, Y.-H. (2010) Current research trends of microbiological leaching for metal recovery from industrial wastes Curr Res Technol Educ Topics Appl Microbiol Microb Biotechnol, 2, pp. 1289–1292.
Mostafavi, M., Mirazimi, S. M. J., Rashchi, F., Faraji, F. and Mostoufi, N. (2018) ‘Bioleaching and Kinetic Investigation of WPCBs by ferrooxidans, A. thiooxidans and their Mixtures’, Journal of Chemical and Petroleum Engineering. University of Tehran, 52(1), pp. 81–91.
Quatrini, R. and Johnson, D. B. (2019) Acidithiobacillus ferrooxidans Trends in microbiology. Elsevier, 27(3), pp. 282–283.
Rawlings, D. E. (2005) Characteristics and adaptability of iron-and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates, Microbial cell factories. BioMed Central, 4(1), p. 13.
Shah, M. B., Tipre, D. R. and Dave, S. R. (2014) Chemical and biological processes for multi-metal extraction from waste printed circuit boards of computers and mobile phones, Waste Management & Research. Sage Publications Sage UK: London, England, 32(11), pp. 1134–1141.
Silva, R. A., Park, J., Lee, E., Park, J., Choi, S. Q. and Kim, H. (2015) Influence of bacterial adhesion on copper extraction from printed circuit boards, Separation and Purification Technology. Elsevier, 143, pp. 169–176.
Silverman, M. P. and Lundgren, D. G. (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields, Journal of bacteriology. American Society for Microbiology (ASM), 77(5), p. 642.
Wang, J., Bai, J., Xu, J. and Liang, B. (2009) Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture, Journal of Hazardous Materials. Elsevier, 172(2–3), pp. 1100–1105.
Wang, L., Li, Q., Li, Y., Sun, X., Li, J., Shen, J., Han, W. and Wang, L. (2018) A novel approach for recovery of metals from waste printed circuit boards and simultaneous removal of iron from steel pickling waste liquor by two-step hydrometallurgical method’, Waste Management. Elsevier, 71, pp. 411–419.
Wang, S., Chen, L., Zhou, X., Yan, W., Ding, R., Chen, B., Wang, C.-T. and Zhao, F. (2018) Enhanced bioleaching efficiency of copper from printed circuit boards without iron loss’, Hydrometallurgy. Elsevier, 180, pp. 65–71.
Wang, Y., Li, K., Chen, X. and Zhou, H. (2018) Responses of microbial community to pH stress in bioleaching of low grade copper sulfide, Bioresource Technology. Elsevier, 249, pp. 146–153. doi: 10.1016/J.BIORTECH.2017.10.016.
Wu, W., Liu, X., Zhang, X., Zhu, M. and Tan, W. (2018) Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron–sulfur-oxidizing bacteria, Bioresources and Bioprocessing. Springer Open, 5(1), p. 10.
Xiang, Y., Wu, P., Zhu, N., Zhang, T., Liu, W., Wu, J. and Li, P. (2010) Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage’, Journal of hazardous materials. Elsevier, 184(1–3), pp. 812–818.
Yang, Y., Chen, S., Li, S., Chen, M., Chen, H. and Liu, B. (2014) Bioleaching waste printed circuit boards by Acidithiobacillus ferrooxidans and its kinetics aspect, Journal of biotechnology. Elsevier, 173, pp. 24–30.


