1Asst. Prof of Human Anatomy, Department of Anatomy. Ibn Sina National College for Medical Studies, Jeddah. Saudi Arabia.
2Associate Professor and Dean of the College, Ibn Sina National College for Medical Studies, Jeddah. Saudi Arabia.
3Professor of Surgery. Department of Surgery. Ibn Sina National College for Medical Studies, Jeddah. Saudi Arabia.
4*General Science Department. Physics Division, Ibn Sina National College for Medical Studies, P.O. Box No. 31906, Jeddah 21418, Saudi Arabia.
Corresponding author email: dr.syedismailahmad@gmail.com
Article Publishing History
Received: 15/02/2020
Accepted After Revision: 28/03/2020
As the revolution in technologies and industries continues in the modern world, there is a diverse process of evolution in electromagnetic field (EMF) induced by appliances that include laptops, mobiles, cellular base station, etc. The electromagnetic field has more negative effects on the living things, but can also be used in regenerating the nerve. Radiation is reported to influence isolated nerve preparations, the central nervous system, chemistry and histology of the brain, and the blood-brain barrier. Peripheral nerve injury occurs due to nerve crushing and are the most common lesions within the nerve injury. The low frequency non-ionizing EMFs vibrates are able to modify the tissues structures of the nerves due to their thermal effects. The effects of pulsating EMFs on nerves has been a subject of research in humans and animal by studying their behavior and nerve electrical properties. This review gives a brief introduction to types of EMFs and addressess the biologic consequences of electromagnetic field on the nervous system with special focusing on the peripheral nerves. In this review recovery characteristics of soft electromagnetism currents in nerve injury and regeneration of nerve, the therapeutic process associated with it has been discussed.
Electromagnetic field; Biotic effect; Nervous system; Peripheral nerve; Nerve regeneration
Dafalla S. E, Kashgari R. H, Suliman S. I, Ahmad S. I. Effect of Non-Ionizing Electromagnetic Field on Peripheral Nerve and its Functional Disorders – A Review. Biosc.Biotech.Res.Comm. 2020;13(1).
Dafalla S. E, Kashgari R. H, Suliman S. I, Ahmad S. I. Effect of Non-Ionizing Electromagnetic Field on Peripheral Nerve and its Functional Disorders – A Review. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/39nzYim
Copyright © Dafalla et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
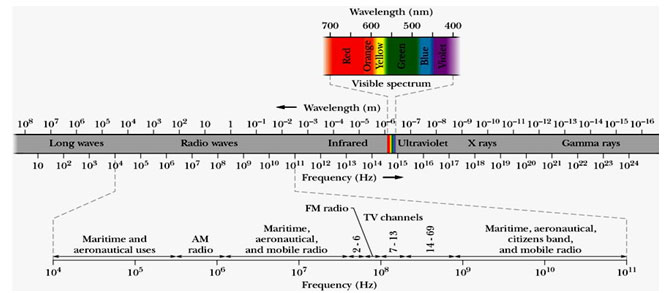
Electromagnetic field (EMF) consists of oscillating electric and magnetic field perpendicular to each other and perpendicular to direction of propagation. The energy of the electromagnetic waves are quantized by quanta called Photons. The electromagnetic spectrum consist of various regions frequency of photons ranging from 101 Hz to 1024 Hz. The region of frequency 4×1014 to 7.5×1014 Hz is called visible regions and others are invisible, Fig1. Visible and invisible EMF exist everywhere in our surroundings in the environment due to manmade and natural sources. High frequency EMF consist of photons of high energy, they ionize the materials that they pass through. Low frequency EMF are non-ionizing emanates from many man-made electrical and industrial devices. Furthermore, based on frequencies these EMF are classified as extremely low-frequency electromagnetic fields (ELEMF) frequency ranging from 1 to 300 Hz, intermediate frequency EMF from 300 Hz to 100 kHz, commonly called as Low-frequency EMF. Further the high frequency but non-ionizing EMF can be classified as Radio frequency EMF frequency ranging 100 kHz- 300 MHz, Micro waves 300 MHz -30 GHz, millimeter waves 30 GHZ -300 GHz, and Terahertz waves frequency ranging from 300 GHz to 10 or 30 THz. EM radiations from different regions of EM spectrum leaves different effect of absorbing materials. When atoms or molecules absorb the electromagnetic energy from terahertz regions, then they are transferred to higher energy levels. The electrons are promoted to higher orbital by visible or ultra violet radiations, vibrations are excited by infrared radiation and rotations are excited by microwaves. The atomic absorption spectroscopy measures the concentration of an element in a sample; whereas atomic emission spectroscopy measures the concentration of elements in samples (Ahmad, 2010; Ahmad 2014, Ju 2015; Terzi 2016, Ross 2019, Lin 2020).
General public is mainly exposed to the low frequency EMFs in microwave regions from 300 MHz-3 GHz due to indoor electrified appliances particularly microwave oven, TVs, smart phones, computers, science games, induction cooking heats vessels, and fluorescent tubes, etc. The magnetizing vibrates releases frequency from the mentioned instruments ranges from Low to very high frequency domains. Electromagnetic vibrates is an extra area of energy created by electrical devices. Several research groups are giving attention to the possible effects of the EMFs with biological relationships, particularly in the light of recent study proposing that EMFs may contribute to degenerative nerve illness. Several searches have shown the EMF influences on human health but still there is no clear cut evidence to relationship effects caused by EMF exposure. EMFs have improved in human daily life and this are useful even in medical field for diagnostic and treatment purposed. The harmful effects of EMF exposure are most commonly skin problems including ruddiness, tickling and burning feel as well as nervous breakdown indications in a mode of fatigue, concentricity lose, whirling sensation, and motion troubles. Large number of experiments done on the EMF impacts on nerve system and associated sensory apparatus. It is reported that EMF can lead to chemical, morphological, and electrical changes in the nerve system (WHO, 2002; Terzi, 2016; Peter Lyttkens 2018 Lin 2020).
Figure 1: Electromagnetic spectrum; (Ahmad 2010)
The biotic effects of contact with Low frequency EMF (LEMF) results from such devices rely on the distance of the servant from them. Prolong exposure of LEMF by electronic gadgets such as micro wave devices and outdoor sources power bases, high-voltage overhead and underground power lines used for the electricity traffic cause Electro-hypersensitivity (EHS) called microwave syndrome. Some of the symptoms of EHS are headache, fatigue, insomnia, tinnitus, photophobia, loss of memory, sense of cognitive dysfunction, irritability, pain at different sites and sometimes cardiovascular abnormalities (Liakouris, 1998; Khurana, 2010; WHO, 2013; Jhonson, 2015; Carpenter, 2015;.Dekun Gao et al 2018).
Biological Effects of Low Frequency EMF
When an Low frequency electromagnetic radiation is applied to metal it will be reflected, where as it get attenuated and fall off exponentially in biological subjects. The effects of these electromagnetic forces on the biological systems have been primarily associated to thermal and non-thermal attributes. An EMF in microwave region on biological subject can considered as imposing time-varying forces on charged ions, molecules, regions of bonds between molecule having dipoles and monopoles in tissue by virtue of its oscillating electric field.
Thermal Effects: All electromagnetic forces possibly transformed into thermal performance during interactions with materials; thus, all electromagnetic force has thermal effects on living systems. The frequency of electromagnetic pulsing have thermal consequences as it affects the permeation into biological systems. At first, it has been thought that low frequency EMFs did not have sufficient power to make significant heating, and could not lead to any potential alterations in the biological complexes. Recently many studies have shown that electromagnetic vibrates are able to modify living tissues structures due to its thermal effects (Binhi, 2002; Gajšek, 2016).
An endothelium of a large blood vessel or tissue boundary of a mucous membrane of the stomach has an electrically polarized cell membranes with a regular coulomb structure, the orientation of the membrane dipoles are completely randomly oriented in the absence of EMF. But when an EMF is applied coherent microwave beam producing a standing E-field wave, this radiation wave pattern can diffract stirring charged particles into highly organized patterns. When charges are rotated a torque will acts on them in phase with the EMF, the work done is converted in thermal energy (Nairz, 2001; Williams, 2016; Ahmad, 2018).
Non-Thermal Effects: Non-thermal effects are not caused by temperature changes but by some other changes in the tissues as the magnetizing force is absorbed into the body. The leading mechanisms of the interaction between electromagnets and biological complexes at non-thermal levels are still unclear (Binhi, 2002; Gajšek, 2016). Many studies have reported that both thermal and non-thermal effects of EMF can affect nervous system and peripheral nerves.
Figure 2: Neuron Histology – Degeneration and Regeneration of nerve (Grinsell, 2014)
EMF and Nervous System
The Peripheral nerve in human body has been proved to be an ultimate electromagnetic system. It works by releasing different chemicals and producing heat that creates electromagnetic fields in the body. Different exposure conditions can been used to study the effect of EMF on biological and immune system. Radiation that allowed to expose on the nerve includes continuous-wave (CW) or pulsed-wave RF radiation of different frequencies and with power densities having different specific absorption rates (SARs) in biological material. Researchers use continuous and/or intermittent pulse exposures, some groups argue that the intermittent exposures are more efficient in causing biological effects, (Rannug, 1994) while, some other suggest peaked EMF exposures. The comparison of homogeneous and gradient static EMF exposures indicates that the gradient EMF is more biologically effective (Hirose, 2003).
Some studies suggest that EMF and Radio Frequency radiation will have no direct effect on DNA, some conditions exert a biological effect reminiscent of heat shock and/or stress. This effect is weak but much dependent on the state of cell homeostasis prior to radiation exposure. Studies suggest that the effects of EMF exposures are mild in comparison to other ionizing radiation, heat shock, nutrient deprivation, etc. and unpredictable. The effects of electromagnetic pulsing on nerves have been a subject for research since alterations in animal behavior and nerve electrical properties were first reported in the end of the nineteenth century. Radiation is reported to influence isolated nerve preparations, the central nervous system, chemistry and histology of the brain, and the blood-brain barrier (National Research Council (US), 1993).
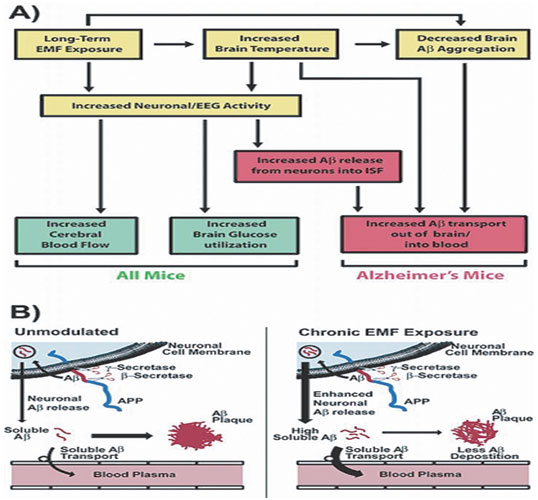
Figure 3: Mechanism of action for the intellectual effect of long exposure period to EMF in standard and AD Tg mice
(Arendash 2012)
The transmission of signals in biological systems occurs through complex electrochemical events. The biological effects of EMF is more pronounced in pathway of the plasma membrane, through which the sinusoidal EMF signal induces a voltage change. The signal exerts forces on free ions which are present on either sides of the plasma membrane leading it to travel across the cell surface through trans membrane proteins. These free ions often generate an intracellular vibration which is responsible for the influx of extracellular Ca2+ ions and the binding affinity of calmodulin (CaM), which is the basic pathway to the secondary messengers, cAMP and cGMP, that have been found to influence inflammatory pain (Christina, 2016). The nature of the electrical communication in humans and animals suggests a potential interactive target for influences from external EMFs. Some research groups described the thermal nature of MW energy absorption, some other groups associate non-thermal or specific MW effects at the molecular and cellular level. The description of the effect of MW energy of 3 to 30 GHz in the centimeter range on the conditional response activity of animals was experimentally studied by Gordon et al. (Gordon, 1955; Martin 2016).
In subsequent years, the study of non-thermal effects of MWs gradually taken the main role in electrophysiological studies by many researchers. It is reported that single and repeated exposures of MW of power density 50 to 150 W/m2 to rats, led to weakening the excitation process and decreased the functional mobility of cells in the cerebral cortex of rats. Edematous changes due to aqueous accumulations were found throughout the cortex. The greatest number of altered cells was noted with repeated exposures at 150 W/m2 (Yakovleva, 1968; Novitskiy, 1971). Studies have made it is clear that the amount of energy absorbed due to exposure of MW depends on factors such as frequency and wavelength, body shape and size, and orientation in the fields. On the whole, the evidence for differential nervous system or behavioral responses to continuous or pulsed-wave MWs is fairly weak (Frey, 1975; Durney, 1986; Gandhi, 1990; Rocha 2015).
Furthermore, some research groups studied the comparison of pulsed and CW EMF in MW region and can be found that exposure of MWs can cause conditioned reflex activity and functional changes in the activity of CNS which is reversible. In a study by some other group, proliferative reaction of glial cells shows that, even at high peak powers and wideband exposures of MWs, no evidence was observed of differences between CW and PW (Sherry, 1995).
EMF and Peripheral Nerves
Biological stimulation of nerve by electromagnetic fields can greatly modify the functions of nervous tissue. It accelerate the regenerative capacity of the tissue. The Peripheral nervous scheme consist of sensory neurons and motor, the neurons are composed of cell bodies in the spinal cord and axons. The axons are the sensory argons, grouped together in a set of spatially sensory bundles called fascicles. A groups of fascicles are enclosed within a peripheral nerve encircling a connective tissue layer called epineurium. The internal epineurium separates fascicles, while external epineurium surrounds all the fascicles in the nerve. The epineurium is sutured in nerve repair and nerve grafting and contributes about half of the total cross sectional area of a peripheral nerve. Exterior of this layer consists the mesoneurium, providing the blood supply to the nerve. A fine but fragile network of capillaries exists at the endoneurial level which can be easily disrupted due to tension or trauma at the nerve repair (Sunderland, 1948; Flores, 2000; Siemionow, 2009;).
The Pulsed electromagnetic field (PEMF) is widely used as a non-invasive procedure and efficacious treatment for resuscitation of peripheral nerve, it has been proved to be promoting extension of neurites in vitro. Thus it can be taken into account as a novel pretreatment modality for crush injury cases. Numerous studies have been carried out by various research groups on the electromagnetic effects on the peripheral nerve mechanism and functionality. This literature review is a brief outlines within the context of certain published writings of correlated studies.
A study done by Nari Seo and et al. to assess the effects of the Pulsed EMF on mesenchymal stem cells (MSC) multiplication and on nerve resuscitation. Result of pre-therapy with Pulsed EMF showed an increase in cell multiplication process along with increasing in Glial fibrillary acidic protein expression. Additionally, it was found to promote the release of growth factors like NGE and BDNF were observed. In addition to this Histological investigation showed an increase in total of axon number and density, suggesting an axonal regeneration (Seo, 2018). Boise et-al applied pulse EMF stimulation on human bone marrow MSC cultured on a substrate of nano-structured TiO2 to study the effect of surface nano-topography with exposure of low-frequency Pulse EMF on cells differentiation, with a special focus on behavior of Ca2+ related cell metabolism. It is reported that the osteogenic differentiation of hBM-MSCs occurs in complex manner, it is not the simple sum of each isolated effect. Surface nanostructure, OM treatment and PEMF stimulation have been confirmed to alter cellular calcium homoeostasis, the overall effect of an integrated treatment is strongly non-summative (Aubin, 2001; Bloise, 2018; Unal, 2018, Ross 2019).
A second study by Jensen and et al applied Bipolar electromagnetic pulsing stimulation applied to the brain (T-PEMF) therapy to improve function of fine motor skills of Parkinson’s disease patient, reduced muscle rigidity, lower muscle spasms and tingle, and less tiredness through the time of T-PEMF therapy. They reported improvement in fine motor skills functioning, and acknowledged T-PEMF therapeutic as a potential long-term therapy (Jensen, 2018). The study of pulse EMF on microcirculation and angiogenesis by Pan Y et-al using a model of acute hind limb ischemia in diabetic rats showed that the pulse EMF has increased the acute hind limb ischemia-related perfusion and angiogenesis, which is associated with up-regulating FGF-2 expression and activating the ERK1/2 pathway in diabetic rats. It concluded the pulse EMF might be important for the treatment of diabetic patients with ischemic injury (Pan, 2013; Ross 2019).
Wei-Hong Hei., et al. (Hei, 2016) conducted a study on immortalized schwann cells derived from rat to assess the effects of exposure frequency of pulse EMF on neuro regeneration. The study concluded that the irradiation of 50 Hz EMF in pulse mode for 1 hour led to enhancement of peripheral nerve resuscitation. This enhancement has attributed to Schwann cell multiplication along with increase of expression rate in S100 gene in neurotrophic factors level (Hei, 2016). Studies demonstrated that the exposure of pulse EMF has increased iSCs mRNA expression of S100 and brain-derived neurotrophic factor (BDNF). Further studies showed that pulse EMF exposure improved BDNF expression in vivo both in dorsal root gangion (DRG) and nerve segment. EMF increased in vitro and in vivo angiogenesis via endothelial release of FGF-2. It is reported that the Pulsed EMF enhances BDNF expression through L-type voltage-gated Ca2+ channel-and Erk-dependent signal pathways in rats dorsal root ganglion neurons (Kim, 2014; Tepper, 2004; Li, 2014).
Furthermore investigation carried out by Kolosova to evaluate the recovery characteristics of soft electromagnetism currents in nerve injury by using the popular model rat sciatic nerve. These study have shown that electromagnetism currents had an impetus effects on recovery performance of operated rats (Kolosova, 1996; Lin, 2020). In contrast, Michael Kelleher and his group carried a study on adult sheep to illustrate the impacts of introducing stable magnetized domain on sensory nerves medicament after nerve injury to these nerves and repair. The study concluded introduction to fixed magnetized domains make no improvement move of peripheral nerves (Kelleher, (2006; Grinsell 2014). Behavioral studies on EMFs reported no significant effects on cochlear and brainstem auditory operation, these study revealed cross result on involuntary and elicit brain electrical action. The conflicting conclusion could be false positive for different evaluations and thus appropriate study design and data analysis considering various comparisons and effect size are needed to minimize controversy in this important area of research (Kwon, 2011; Joan 2019).
A research conducted to determine the thermal marks concerning electromagnetic radiation emitted from mobiles of the seventh Cranial Nerve (CN VII) and surrounding soft-tissue. The study concluded that The electromagnetic radiation arising out of mobiles could be a matter for unstable disorders of Cranial Nerve VII through heat erratic increasing on the framing soft-tissue of the CN VII (Acar,2009). Results of recent studies have been achieved to identify the preliminary effects of the Electromagnetic Fields on Peripheral Nerve and its functions, previous studies have yielded inconsistent findings. Most of the previous research have shown that EM pulsing has the potential to be recommended as an appropriate and effectual therapeutic for peripheral nerve cure in clinical applications. Some studies did not show any recovery pattern for electromagnetic pulsing in peripheral nerve injure. Furthermore, it is essential rating lowest frequencies of magnetized fields proved to be efficient for injured nerve recovery. Moreover studies should consist of morphological and Ultra structural properties by the molecular techniques to determine the exact effects (Lei, 2013; Terzi, 2016).
Regeneration of nerve: Non-ionizing EMF are being used for regeneration of nerves. Some studies have used the crushed sciatic nerve in rat are used as model to study the functional, biochemical and morphological properties. An improvement in the regeneration was observed after exposure of LFMF sinusoidal waves of magnetic flux densities of about 0.5 mT (Rusovan,1991; Rusovan, 1992; Bervar, 2005). A rotating magnetic field using Helm-holtz coils has been used to delivered variable magnetic flux densities on the animals, depending on the position of exposure coils. Various flux densities, all at frequency 40 Hz, have been used in the study, and the highest interval (150–300 µT) showed the largest improvement to regeneration compared to control condition. An Improved regeneration of the muscle and nerve in mice due to crush injury of the upper part of thigh (Pulse EMF about 50 Hz) has been reported. A positive effects on hemi-sectioned spinal cord in rats due to sinusoidal 50 Hz wave of magnetic flux 17.96 µT have also been observed (Suszyński, 2014; Das, 2012; Stölting, 2016). Survey of literature reveals studies considering the effect of Pulse EMF on spinal cord regeneration was covering both in vivo as well as in vitro studies. It observed that the regenerative effect depends on the signal that have used initially reduce inflammation, then regeneration proceed. These positive effects are reported with Pulse EMF at frequencies under 100 Hz and flux densities below 5 mT (Ross, 2017).
Literature reveals the Pulse EMF promote peripheral nerve regeneration to an degree comparable to that with hormones, conditioning lesions, and growth factors. Exposure of pulsed EMF prior to treatment on crush injury has resulted in acceleration of axonal regrowth, and was in consistent with a spur of regenerative neurite outgrowth increased outcomes like walking behavior, promotes neutrite growth invitro (Greenebaum, 2007; Baptisa, 2008; Walker 1994). But in some studies it has been observed that prolonged Pulse EMF treatment resulted to delayed histological peripheral nerve regeneration and increased oxidative stress but no loss of function recovery (Baptisa, 2009; Zang, 2019). These contradictory results could be due to procedural differences. A study by Minoo Shadel et-al reveals the exposure of pulse EMF on the whole body of wistar rats could speed up functional recovery after nerve allografting in sciatic nerve (De Lahunta, 2009; Minoo, 2017; Alvites, 2018).
This may have clinical implications for the surgical management of patients after nerve transection. In this study rats were divided in to normal, allograft, and PEMF treated group. In the allograft group the left sciatic nerve was exposed through a gluteal muscle, while for PEMF group the whole body was exposed to pulse EMF of 0.3 mT and frequency of 2Hz for 4h/day within 1-5 days (Khan, 2014; Faroni, 2015; Lin, 2020).
CONCLUSIONS & RECOMMENDATIONS
EM waves exists anywhere in the environment around us. Due to the use of household appliances and outdoor electromagnet paths and towers that induce non-ionizing pulsating electromagnetic field has increased markedly all over the world. The electromagnetic field has more negative effects on the living things, but can also be used in regenerating the nerve. Peripheral nerve injury occurs due to nerve crushing and are the most common lesions within the nerve injury. It could represent the limit between the lesions inclined to spontaneous regeneration and those that require essential surgical intervention for regeneration to occur. Peripheral nerve crush lesions generally occur linked with compressive forces and fractures and could affect the neighboring tissues, leading to a difficult conditions. The important factor for nerve regeneration in is the concern of exuberant inflammatory reactions, the adhesions of the nerve with surrounding tissues, occurrences of axonal misdirecting and failures in demyelization. Pulsed EMF and used in regeneration of nerve. There are some positive sides of these pulses. Studies shows that the pulsed EMF have successfully used in authentic clinical scopes to improve the regeneration performances and to supply sustainable health conditions of peripheral nerves. Many of the preceding research have shown that EM pulsing has the potential to be recommended as an appropriate and effectual therapeutic for peripheral nerve cure in clinical applications.
On the contrary, some studies did not display any recovery pattern for electromagnetic pulsing in peripheral nerve injure. Thus, a proper investigation design and appropriate data interpretation are Key factors that determine the exact effects of these therapy approach. Furthermore, it is essential rating lowest frequencies of magnetized fields efficient for injured nerve recovery. Moreover studies should consist of morphological and Ultra structural properties by the molecular techniques to determine the exact effects. All previous data suggest further research are highly required to judge the role and function of pulse and continuous EMF in therapeutics in clinical scopes. Clinical application of Low frequency Electromagnetic fields are emerging multidisciplinary field. Tissue engineering is the process of developing methods that associated with nerve regeneration by applications of EMFs. The peripheral nerve regeneration involves basic Science research inorder to address the issues such as nerve growth factor of selected nerve, release kinetics related to regeneration etc.
REFERENCES
Acar, G.O; Yener, H.M; Savarun,F.K (2009). Thermal effects of mobile phones on facial nerves and surrounding soft tissue. The Laryngoscope. Vol. 119. No.3. Pages. 559-562.
Ahmad, S.I. (2018). Fundamentals of Applied Engineering Physics Applied Engineering Physics for Scientist and Engineers. Isbn: ISBN13: 978-613-9-93127-9. Publisher: LAP Lambert Academic Publisher.
Ahmad, S.I; Syed I A, Ravi P. P. Ahmad A.(2014). Quantitation of urea in urine by Fourier transforms infrared spectroscopy. Der Pharma Chem., Vol 6 (1). Pages 90-96.
Ahmad, S.I. Studies on some biophysical aspects of human renal excretory fluid, Ph.D. dissertation, Department of Physics, Jawaharlal Nehru Technological University, Hyderabad, India, 2010.
Alvites, R; Caseiro, A. R; Pedrosa, S.S; Branquinho, M.V; Ronchi, G; Geuna, S; Varejão, A.S.P; and Maurício, A.C. (2018), Peripheral nerve injury and axonotmesis: State of the art and recent advances. Alvites et al., Cogent Medicine. Vol.5: 1466404 https://doi.org/10.1080/2331205X.2018.1466404.
Arendash, G. W; Mori,T; Dorsey,M; et-al. Electromagnetic Treatment to Old Alzheimer’s Mice Reverses β-Amyloid Deposition, Modifies Cerebral Blood Flow, and Provides Selected Cognitive Benefit. (2012). PLoS One. 7(4): e35751. doi: 10.1371/journal.pone.0035751
Aubin, J. E. (2001). Regulation of Osteoblast Formation and Function. Rev Endocr Metab Disord. Kluwer Academic Publishers. Vol. 2. Pages. 81–94. https://doi.org/10.1023/A:1010011209064
Baptista, A.F; Gomes, J.R; Oliveira, J.T; Santos, S.M; Vannier Santos, M.A. et al. (2008) High- and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J Peripher Nerv Syst. Vol. 13. No.1. pages. 71-80.
Baptista, A.F; Goes, B.T; Menezes, D; Gomes, F.C.A; Zugaib, J; Stipursky, J; Gomes, J.R.S; Oleveria, J.T; Vannier-Santos, M.A and Martinez, A.M.B. (2009). PEMF fails to enhance nerve regeneration after sciatic nerve crush lesion. J. Peripheral Nerv. System. Vol. 14. No 4. Pages 285-293. https://doi.org/10.1111/j.1529-8027.2009.00240.x
Binhi, V.N., Repiev, A and Edelev, M. (2002). Magnetobiology: Underlying Physical Problems.San Diego: Academic Press. Pages. 1-16.
Bervar M. (2005). Effect of weak, interrupted sinusoidal low frequency magnetic field on neural regeneration in rats : functional evaluation. Bioelectromagnetics. Vol.26. Pages. 351–356. doi:10.1002/bem.20108
Bloise, N; Petecchia, L; Ceccarelli, G; Fassina, L; Usai, C; Bertoglio, F. et al. (2018) The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces. PLoS ONE. Vol. 13. No.6: e0199046. https://doi.org/10.1371/journal.pone.0199046
Carpenter, D.O. (2015). The microwave syndrome or electro-hypersensitivity: historical background. Rev. Environmental Health, 30(4), Pages 217–222. DOI: https://doi.org/10.1515/reveh-2015-0016.
Christina L. R; Teli, T; Harrison, B.S. (2016). Electromagnetic Field Devices and Their Effects on Nociception and Peripheral Inflammatory Pain Mechanisms. Alternative Therapies. Vol. 22. N0. 3: Pages 34-47.
Das, S; Kumar, S; Jain, S; Avelev, V.D and Mathur, R. (2012). Exposure to ELFmagnetic field promotes restoration of sensori-motor functions in adult rats with hemisection of thoracic spinal cord. Electromagn Biol Med. Vol.31. No.3. Pages180–194. doi:10.3109/15368378.2012.695706
De Lahunta, A; Glass, E; and Kent, M. (2009). Lower motor neuron: Spinal nerve, general somatic efferent system. Veterinary
Dekun Gao , Hui Sun , Jin Zhu, Yinda Tang, Shiting Li, (2018) CXCL12 induces migration of Schwann cells via p38 MAPK and autocrine of CXCL12 by the CXCR4 receptor. Int. J. Clin. Exp. Pathol;11(6):3119-3125
Durney C.H; Massoudi H and Iskander M.F. (1986). Radiofrequency Radiation Dosimetry Handbook, 4th ed., Report USAFSAM-TR-85-73, USAF School of Aerospace Medicine, Brooks AFB, TX.
Faroni, A; Mobasseri, S. A; Kingham, P. J and Reid, A. J. (2015). Peripheral nerve regeneration: Experimental strategies and future perspectives. Advanced Drug Delivery Reviews, 82–83, 160–167. doi:10.1016/j.addr.2014.11.010
Flores,A.J; Lavernia, C.J and and Owens, P.W. (2000). Anatomy and physiology of peripheral nerve injury and repair. The American Journal of Orthopedics Vol. 29, No. 3. Pages 167–173.
Frey, A.H and Feld, S.R. (1975). Avoidance by rats of illumination with low-power nonionizing electromagnetic energy, J. Comp. Physio. Psych. Vol.89. Pages.183–188.
Gajšek, P; Ravazzani, P; Grellier, J; Samaras, T; Bakos, J and Thuróczy, G. (2016) Review of Studies Concerning Electromagnetic Field (EMF) Exposure Assessment in Europe: Low Frequency Fields (50 Hz–100 kHz), Int. J. Environ. Res. Public Health Vol.13. Pages 875-887.
Gandhi O.P. (1990). Electromagnetic energy absorption in humans and animals, in: Biological Effects and Medical Applications of Electromagnetic Energy, Gandhi O.P., Ed., Prentice Hall, Englewood Cliffs, NJ. Pages. 174–195.
Gordon, Z.V; Lobanova, Y.A and Tolgskaya, M.S. (1955). Some data on the effect of centimeter waves (experimental studies), Gig. Sanit. (USSR) 12:16.
Greenebaum, B and Sisken, B.F. (2007) Does direction of induced electric field or current provides a test of mechanism involved in nerve regeneration? Bioelectromagnetics. Vol 28. No 6. Pages 488-492.
Grinsell, D; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. (2014). Biomedical Res. 698256. doi: 10.1155/2014/698256
Hirose, H., Nakahara, T., Zhang, Q.M., Yonei, S., and Miyakoshi, J. (2003). Static magnetic field with a strong magnetic field gradient (41.7 T/m) induces c-Jun expression in HL-60 cells, In Vitro Cell Dev. Biol. Anim. Vol. 39 (8–9). Pages. 348–352.
Hei; Wei-Hong; Soo-Hwan Byun; Jong-Sik Kim; Soochan Kim; Young-Kwon Seo; Joo-Cheol Park; Soung-Min Kim; Jahng, J,W. and Lee, J.H. (2016). Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: in vitro and in vivo study. International Journal of Neuroscience. Vol. 126. No. 8. Pages 739-748.
Johansson, O. (2015) Electro hypersensitivity: a functional impairment due to an inaccessible environment. Rev Environ Health. Vol.30. No 4. Pages 311-321. doi:10.1515/reveh-2015-0018.
Joan M. S; Oury, M; Farnaz, A; Chantel T. D. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. (2019). Headache. doi: 10.1111/head.13479
Jensen, B.R; Malling, A.S.B; Morberg, B.M; Gredal O; Beach, P and Wermuth L. (2018). Effects of Long-Term Treatment with T-PEMF on Forearm Muscle Activation and Motor Function in Parkinson’s Disease. Case Rep. Neurol. Vol. 10. Pages 242–251.
Ju, D.T; Liao, H.E; Shibu MA, Ho T.J; Padma, VV, et al. (2015) Nerve Regeneration Potential of Protocatechuic Acid in RSC96 Schwann Cells by Induction of Cellular Proliferation and Migration through IGF-IRPI3K-Akt Signaling. Chin J Physiol 58(6): 412-419.
Khan, A. A; Faruqi, N. A and & Ansari, M. S. (2014). Effects of hydrocortisone on the sciatic nerve crush injury in adult rat-a light microscopic study. Current Neurobiology, Vol.5. No.1.
Khurana, V.G; Hardell, L, Everaert, J; Bortkiewicz, A; Carlberg, M and Ahonen, M. (2010). Epidemiological evidence for a health risk from mobile phone base stations. Int. J. Occup. Environ Health. Vol. 16. Pages 263-267.
Kim Y.T; Hei W.H; Kim, S. et al. (2014). Co-treatment Effect of Pulsed Electromagnetic Field (PEMF) with Human Dental Pulp Stromal Cells and FK506 on the Regeneration of Crush Injured Rat Sciatic Nerve. International Journal of Neuroscience. Vol. 125. No. 10. Pages. 1-27.
Kolosova, L. I., et al. (1996). Effect of low‐intensity millimeter wave electromagnetic radiation on regeneration of the sciatic nerve in rats. Bioelectromagnetics: Journal of the Bioelectromagnetics Society, The Society for Physical Regulation in Biology and Medicine, The European Bioelectromagnetics Association. Vol. 17. No.1. Pages. 44-47.
Kelleher, M. O; Al-Abri, R. K; Lenihan, D. V and Glasby, M. A. (2006). Use of a static magnetic field to promote recovery after peripheral nerve injury. Journal of neurosurgery. Vol.105. No.4. Pages 610-615.
Kwon; Myoung Soo and Heikki Hämäläinen. (2011). Effects of mobile phone electromagnetic fields: critical evaluation of behavioral an neurophysiological studies. Bioelectromagnetics. Vol. 32. No.4. Pages253-272.
Liakouris, J.A.G. (1998) Radiofrequency (RF) sickness in the Lilienfeld study: an effect of modulated microwaves? Arch Environ Health 53:226-228.
Lin QM, Tang XH, Lin SR, Chen BD, Chen F. Bone marrow-derived mesenchymal stem cell transplantation attenuates overexpression of inflammatory mediators in rat brain after cardiopulmonary resuscitation. (2020). Neural Regen Res;15(3) Pages: 24-31. DOI: 10.4103/1673-5374.265563
Li, Y; Yan, X; Liu, J. et al. (2014). Pulsed electromagnetic field enhances brain-derived neurotrophic factor expression through L-type voltage-gated calcium channel-and Erk-dependent signaling pathways in neonatal rat dorsal root ganglion neurons. Neurochemistry international. Vol. 75. Pages. 96-104.
Lei, T; Jing, D; Xie, K; Jiang, M; Li, F; Cai, J and Guo, W. (2013). Therapeutic effects of 15 Hz pulsed electromagnetic field on diabetic peripheral neuropathy in streptozotocin-treated rats. PLoS One, 8(4), e61414.
Martin, L.P. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. (2016) J. Chem. Neuroanatomy,B.7. Pages 43-51. https://doi.org/10.1016/j.jchemneu.2015.08.001
Minoo, S; Payman, T and Rahim, M. (2017). Effects of Pulsed Electromagnetic Fields on Peripheral Nerve Regeneration Using Allografts in Sciatic Nerve: An Animal Model Study. Biomed J Sci & Tech Res. Vol. 1. No.6. MS.ID.000509. DOI : 10.26717/BJSTR.2017.01.000509.
Nairz, O., Brezger, B., Arndt, M. and Zeilinger, A. (2001). Diffraction of Complex Molecules by Structures Made of Light. Physical Review Letters. Vol. 87. No.16. 401.1 – 401.4 (online article #160401).
National Research Council (US) Committee on Assessment of the Possible Health Effects of Ground Wave Emergency Network (GWEN). (1993). Assessment of the Possible Health Effects of Ground Wave Emergency Network. Washington (DC): National Academies Press (US); 1993. 6, Effects of Electromagnetic Fields on Organs and Tissues. Available from: https://www.ncbi.nlm.nih.gov/books/NBK208983/
Novitskiy, Y.I; Gordon Z.V; Presman A.S and Kholodov, Y.A. (1971). Radio Frequencies and Microwaves, Magnetic and Electrical Fields, National Aeronautics and Space Administration (NASA TT F-14.021), Washington, D.C.
Pan, Y; Dong, Y; Hou, W; Ji, Z; Zhi, K; Yin, Z. et al. (2013). Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics. Vol.34. No.3. Pages. 180–188.
Peter Lyttkens. Electromagnetic field and neurological disorders Alzheimer´s disease, why the problem is difficult and how to solve it. (2018). Upasala University, PhD Thesis. Brain – Functions, Diseases and Mysteries I, 7.5 ECTS credits.
Rannug, A., Holmberg, B., Ekstrom, T., Mild, K.H., Gimenez-Conti, I., and Slaga, T.J. (1994). Intermittent 50 Hz magnetic field and skin tumor promotion in SENCAR mice, Carcinogenesis. Vol.15. No.2. Pages 153–157.
Rocha, S; Melo, L; Boudoux, C; Foerster, Á;Araújo, D; Monte-Silva K. (2015). Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: A pilot randomized controlled trial. J. Neurol Sci.349 (1-2):33-39
Ross, C.L; Syed, I; Smith, T.L and Harrison, B.S. (2017) The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med. Vol. 36. No. 1. Pages. 74–87. doi:10.3109/15368378.2016.1160408
Ross, C.L; Ang, D.C; Almedia-Porado, G. Targeting Mesenchymal Stromal Cells/Pericytes (MSCs) With Pulsed Electromagnetic Field (PEMF) Has the Potential to Treat Rheumatoid Arthritis.(2019). Front. Immunol. 10: 266. . doi: 10.3389/fimmu.2019.00266. eCollection 2019
Rusovan, A and Kanje M. (1991). Stimulation of regeneration of the rat sciatic nerve by 50 Hz sinusoidal magnetic fields. Exp Neurol. Vol.112, No.3. Pages. 312–316. doi:10.1016/0014-4886(91)90132-V
Rusovan, A; Kanje, M and Mild, K.H. (1992). The stimulatory effect of magnetic fields on regeneration of the rat sciatic nerve is frequency dependent. Exp Neurol. Vol.117. No.1. Pages.81–84. doi:10.1016/0014-4886(92)90113-5
Sherry, C.J; Blick, D.W; Walters, T.J; Brown, G.C and Murphy, M.R (1995). Lack of behavioral effects in non-human primates after exposure to ultra wide band electromagnetic radiation in the microwave frequency range, Radiat. Res. Vol.143. Pages. 93–97
Siemionow, M and Brzezicki,G (2009). Chapter 8- Current techniques and concepts in peripheral nerve repair. International Review of Neurobiology, Vol. 87, No. C, Pages. 141–172.
Sunderland, S and Ray, L.J. (1948). The intraneural topography of the sciatic nerve and its popliteal divisions in man. Brain. Vol. 71, part 3, Page. 242–273.
Seo, N.R; Lee, S.H; JU, K.W; Woo, J.M; Kim, B.J; Kim, S.M; Jahng, J.W and Lee, J.H. (2018). Low-frequency pulsed electromagnetic field pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve. Neural Regen. Res. Vol. 13.N0. 1. Page.145–153.
Suszyński, K; Marcol, W; Szajkowski, S. et al. (2014). Variable spatial magnetic field influences peripheral nerves regeneration in rats. Electromagn Biol Med. Vol. 33. No.3. Pages.198–205. doi:10.3109/ 15368378.2013.801351
Stölting, M.N.L; Arnold, A.S; Haralampieva, D; Handschin, C; Sulser, T and Eberli, D. (2016). Magnetic stimulation supports muscle and nerve regeneration after trauma in mice. Muscle Nerve. Vol.53. No.4. Pages 598– 607. doi:10.1002/mus.24780
Terzi,M; Ozberk,B; Deniz, O,G and Kaplan,S. (2016) The role of electromagnetic fields in neurological disorders. Journal of Chemical Neuroanatomy. Vol 75-B. Pages 77-87. http://dx.doi.org/10.1016/j.jchemneu.2016.04.003.
Tepper, O.M; Callaghan, M.J; Chang, E.I. et al. (2004). Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. The FASEB journal. Vol.18. No.11. Pages. 1231-3.
Terzi, Murat, et al. (2016). The role of electromagnetic fields in neurological disorders. Journal of chemical neuroanatomy. Vol. 75. Pages. 77-84.
Unal, M; Creecy, A and Nyman, J.S. (2018). The Role of Matrix Composition in the Mechanical Behavior of Bone. Curr Osteoporos Rep. https://doi.org/10.1007/s11914-018-0433-0 PMID: 29611037
Walker, J.L; Evans, J.M; Resig, P; Guarnieri, S; Meade, P, et al. (1994) Enhancement of functional recovery following a crush lesion to the rat sciatic nerve by exposure to pulsed electromagnetic fields. Exp Neurol.Vol.125. No.2. pages 302-305.
Williams, J.M. (2016). Biological Effects of Microwaves: Thermal and Nonthermal Mechanisms. Biological Thermal and Nonthermal Mechanisms. Vol. 4.7.
WHO (2002). Radiation and environmental health department of protection of the human environment. Establishing a dialogue on risks from electromagnetic fields. Geneva WHO. Electromagnetic Field and public Health.
WHO (2013). Electromagnetic fields and public health: Electromagnetic hypersensitivity. http://www.who.int/peh-emf/publications/facts/fs296/en/.
Yakovleva, M.I; Shlyafer I.P and Tsvetkova I.P. (1968). On the question of condition cardiac reflexes, the functional and morphological state of cortical neurons under the effect of superhigh-frequency electromagnetic fields, Zh. Vyssh. Nervn. Deyat. (USSR) 18:973.
Zhang, P.X; Han, N; Kou, Y.H; et-al. Tissue engineering for the repair of peripheral nerve injury. (2019). Neural Regen. Res;14:Pages 51-8. DOI: 10.4103/1673-5374.243701