Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
Corresponding author email: iullah@kau.edu.sa
Article Publishing History
Received: 01/10/2019
Accepted After Revision: 25/12/2019
Brassica napus is a heavy metal hyperaccumulator plant which can tolerate high levels of heavy metals as compared to other plants. In the present study, B. napus was exposed to 20 and 50 mg/kg of Zn and 0 mg/kg of Zn treated plants, which were used as controls. The results showed that high Zn concentration reduced plant shoots length, roots length, chlorophyll contents and biomass in a dose-dependent manner as compared to controls. However, inoculation of bacterial isolated, Serratia sp. IU01 significantly improved (p < 0.05) all plant growth attributes as compared to uninoculated plants. The estimation of Zn accumulation through inductively coupled plasma mass spectrometry (ICPMS) in shoot and roots showed that a higher concentration of Zn was accumulated in roots as compared to shoot and inoculation significantly improved (p < 0.05) the Zn accumulation in roots as compared to uninoculated plants.
Zinc toxicity, Heavy metal, Immobilization, Brassica napus, Growth Attributes
Ullah I. Accumulation of Cytotoxic Zinc and its Detoxification by Brassica napus, Mediated by Endophytic Bacteria. Biosc.Biotech.Res.Comm. 2019;12(4).
Ullah I. Accumulation of Cytotoxic Zinc and its Detoxification by Brassica napus, Mediated by Endophytic Bacteria. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/3538rRu
Copyright © Ullah This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Endophytes are plants symbiotic microbes (bacteria and fungi), provide physiological support to the plants. They help plants in development and growth promotion (Jeger and Spence, 2001). The main benefit of the endophytic bacteria is assumed to be the stress resistant and make the plants stress resistant as well, (Sturz et al., 2000). The heavy metal stressor are very common in present age (Jeger and Spence, 2001; Bharti et al., 2016). From soil to plant, transport of heavy metals depends on total amount of heavy metals, in soil and rate of transfer of heavy element from soil to plant roots (Buendía-González et al., 2010). Numerous studies have described that endophytic bacteria can apply for agricultural purposes to enhance the phytoremediation role of the heavy metal accumulator plants (Sturz et al., 2000). In addition endophytes perform nutrients mobilization such as nitrogen production and phosphorus solubilization (Sharma et al., 2013), providing plant hormone such as auxin and gibberellin (Hardoim et al., 2008) and protect the diseases caused by soil-borne pathogens and hence plants are enabled to tolerate that heavy metal toxicity and go for phytoremediation, (Bahadir et al., 2007; Ullah et al., 2019).
The heavy metal such as Zn has been a major threat to animals, plants, and human life due to their toxicity in living organisms. Contamination of soil with heavy metals enhances plant uptake leading to accumulation in various parts of the plants (Berg and Hallmann, 2006). Larger agricultural soil has been contaminated with heavy metals due to mining activities, industrial discharges and the application of agrochemicals and lime products (Bahadir et al., 2007). Severe heavy metal contamination in the soil may cause a variety of problems, including low crop yield and toxicity in plants, animals, and humans. A trace amount of few heavy metals such as Cu and Zn are essential for plant growth and natural development because they are used as co-factors for many enzymes (Ullah et al., 2015).
However, high concentrations of both essential and unnecessary heavy metals in the soil can lead to toxic symptoms and growth inhibition in most of the plants (Dahmani-Muller et al., 2000). At the cellular level, excessive amounts of toxic heavy metal ions stimulate many stress responses and damage various cell components such as cell membranes, proteins and nucleic acid (Fidalgo et al., 2011; Chauvin et al., 2017).
Bacterial mediated phytoremediation have been used to detoxify the heavy metal such as zinc (Zn) by immobilization and turn them harmless to the soil, water or air (Buendía-González et al., 2010). Contaminants such as minerals, pesticides, solvents, explosives, crude oil, and derivatives have been detoxified by bacterial mediated phytoremediation of plant such as Brassica napus (Fidalgo et al., 2011). Phytoremediation technique is a method of treatment that takes advantage of the ability of hyper-accumulator plants to accumulate heavy metals and toxic compounds from the environment and metabolize them in their tissues (Garbisu and Alkorta, 2001). B. napus has been used in studies of the effects of heavy metals such as cadmium hyper-accumulator (Ullah et al., 2013; Xia et al., 2016).
MATERIALS AND METHODS
Plant growth, Zn contamination and bacterial inoculation
Brassica napus plants were grown in pots using (Wei et al., 2009) commercial soil. The plants were put in a growth chamber at 25 ± 2 °C. Two different Zn concentrations: 20 mg/kg and 50 mg/kg of soil were used as treatments and 0 mg/kg was (without Zn) was used as control. The experiment lasted for 4 weeks. Three replications were used and ten plants per replication were used in the experiment. Previously isolated bacterial strain Serratia sp. IU01 (Ullah et al., 2019) was used in the present study as an inoculum to enhance plant phytoremediation capability. The IU01 was grown in LB broth of for 72 h at 37°C and inoculum was used to plants treated with different concentrations 0, 20 and 50 mg/kg of soil. Whereas, 0 mg/kg of Zn was used as control to compare the treated and untreated results.
Estimation of growth attributes of B. napus
The experiment was harvested after 50 days and plant growth attributes of Zn treated and control plant was determined. The total length of the plants (Zn treated and control) was measured as root length and shoot lengths. Fresh biomass plant after harvesting was measured and then plants were dried at 100°C for 10 min and dried biomass was measure. The chlorophyll contents of the treated and control plants were also determined using chlorophyll meter (SPAD- 502, Minolta, Japan).
Zinc accumulation in B. napus
The oven-dried plant samples were crushed into powder using mortar and pestle and the 100 mg of powder were subjected to acid digestion. The solution, HNO3-HClO4 was used to digest the sample powder. The concentration of Zn samples digested with acid was estimated through double plasma spectral analysis (ICP, PerkinElmer, USA).
Assessment of proline contents
Proline contents of the plants under Zn stress and control were estimated through spectrophotometer at OD 765 nm. The plant was crushed in 0.5 mL methanol and the extract was added with 10% Folin-Ciocalteu reagent (2.5 mL). The mixture was dissolved in a solution of 7.5% Na2CO3 in 2 mL water. The blank was made as same without plant sample. The samples were incubated at 45°C for 45 min and absorbance was measured at 765 nm.
Statistics
The data were analyzed obtained from three independent experiments and analysis using Duncan’s multiple range test.
RESULTS AND DISCUSSION
Estimation of growth attributes of B. napus
The plants grown under different Zn concentration i.e., 20 and 50 mg/kg and effects of Zn stress was determined on plant. The growth attributes such as chlorophyll contents, roots and shoot lengths, fresh biomass and dry biomass was determined as compared to 0 mg/kg of soil, used as control. The results revealed that Zn stress at 20 and 50 mg/kg significantly reduced (p < 0.05) the chlorophyll contents, roots length, and shoots length, dry biomass and fresh biomass as compared to control. However, inoculation of isolate IU01 improved the shoots length, roots length, chlorophyll contents, fresh and dry biomass as compared to plant exposed to different concentration of Zn as well as control (Table 1). The growth reduction compared to the control was evident at higher concentrations of Zn. The length of roots and shoots were significantly affected in a dose-dependent manner compared to the length of the control plants.
Heavy metal resistant bacteria, e.g., Morganella sp., Providencia spp., and Stenotrophomonas sp., were reported to have great bio-sorption and mobilization potential (Kartik et al., 2016). Previous studies have shown that bacterial endophytes were not only capable of detoxifying heavy metal toxicity but also promoting plant growth (Jabeen et al., 2009; Ullah et al., 2013; Fidalgo et al., 2011). It has also been reported a Zn tolerant bacterial isolate, from different plants promoted plant growth and Zn tolerance in Brassica plants (He et al., 2013). Heavy metal resistant and plant growth promoting bacteria: Azomonas sp. RJ4, Bacillus sp. RJ16, Xanthomonas sp. RJ3, Bacillus sp. RJ31 and Pseudomonas sp. RJ10 have been reported to have effective in plant growth promotion and biomass enhancement in B. napus and B. juncea (Mata et al., 2002; Sheng et al., 2008; Ma et al., 2013; Wan et al., 2012; Liu et al., 2009; Kurzbaum et al., 2014).
Table 1: Plant growth characteristics of Brassica napus under various concentrations of Zn
| Zn Treatment (mg/kg) | Shoot length (cm) | Root Length (cm) | Chlorophyll contents (SPAD) | Fresh biomass | Dry biomass | |
| 0 | Uninoculated
IU01 |
12.46 ± 1.22a
11.96 ± 1.52a |
12.33 ± 2.81a
13.35 ± 2.53a |
22.11 ± 2.23a
23.53 ± 3.13a |
60.54 ± 3.22a
62. 43 ± 2.46a |
21.62 ± 1.35a
23.41 ± 2.33a |
| 10 | Uninoculated
IU01 |
8.40 ± 1.38b
10.96 ± 1.52a |
08.00 ± 1.43b
11.00 ± 1.36a |
15.21 ± 1.67b
21.74 ± 2.62a |
40.33 ± 1.53b
57. 88 ± 2.76a |
12.29 ± 0.66b
20.56 ± 2.45a |
| 50 | Uninoculated
IU01 |
5.38 ± 0.57c
9.84 ± 1.71a |
6.25 ± 0.56c
10.71 ± 1.58a |
12.56 ± 2.67c
20.56 ± 2.72a |
15. 83 ± 1.45c
52. 37 ± 2.41d |
09.25 ± 0.56c
18.86 ± 2.75d |
Mean ± SD values are presented in each column different letters represent the significant difference (p < 0.05) as analyzed by Duncan’s multiple range test.
Assessment of Zn accumulation in shoots and roots of the B. napus
The level of Zn accumulations in shoots and roots of the plants grown in 20 and 50 mg/kg of Zn were evaluated. The results revealed that the B. napus plant accumulated significantly higher concentration in roots then shoot. In addition, the inoculation of IU01 markedly improved the Zn accumulation the roots of B. napus (Table 2). The accumulation of heavy metals in parts of plants i.e., shoot and roots has presumably attributed to the extensive root of the plants. Biomass increase through endophytic bacteria confined to the roots of the plants (Sheng et al., 2008). Same results of Zn accumulation were also reported by Wei et al. (2013), their report showed that an increase in Zn in the soil was associated with higher amounts of Zn accumulating in roots of the B. napus. The studies conducted by Malandrino et al., (2006), Wan et al., (2012) showed that Zn accumulation was higher in the roots of plant as compared to shoots. Similarly Wan et al. (2012) reported that the level Zn accumulated in the roots was many-fold higher as compared to the shoots of the B. napus.
Table 2: Fresh and dry biomass of B. napus after treatment with different concentrations of Zn (mg/kg of soil).
| Zn Treatment (mg/kg) |
Treatment |
Zn concentration mg/kg of Plant DW* | |
| Shoot Root | |||
| 0 | Uninoculated
IU01 |
ND*
ND |
ND
ND |
| 10 | Uninoculated
IU01 |
250.86 ± 5.95a
121.37 ± 3.54b |
453.54 ± 5.57a
216.28 ± 7.25b |
| 50 | Uninoculated
IU01 |
327.28 ± 6.53c
211.76 ± 5.54d |
752.34 ± 8.52c
527.23 ± 14.42d |
ND* = represents not detected and DW* = represents dry weight. The values are expressed as the mean ± SD and different letters represent significant differences (p < 0.05).
Effects Phenolic contents on plant under Zn stress
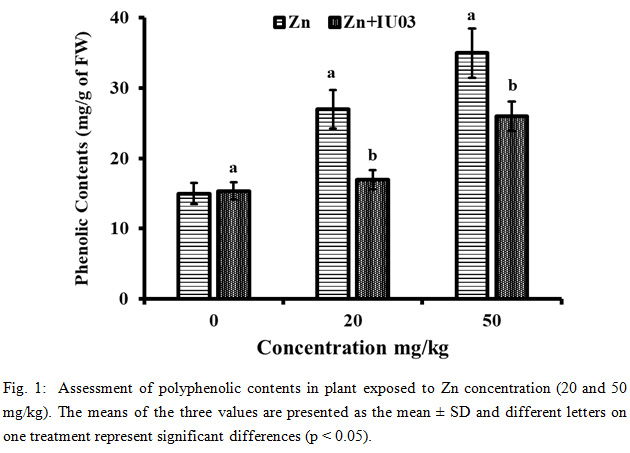
The results revealed that high concentration of Zn markedly increase the phenolic content of plants treated with 20 and 50 mg/Kg of Zn as compared to control. The IU01 inoculation significantly (p < 0.05) decrease the total phenolics in plants stressed with Zn various concentration such as 20 and 50 mg/kg as compared to uninoculated plant (Fig. 1). Zinc stress in plants was neutralized by phenolic contents produced by plants under the plant defense system (Ahmad et al., 2014; Hossain et al., 2012).
Application of strain IU01 on B. napus plant treated with different Zn concentration, mediated the antioxidant activities significantly (p < 0.05) as compared to uninoculated plants. Previously, it has been reported that phenolic contents in plants were increased when exposed to high level of heavy metal; however, application of IU01 significantly reduced the phenolic contents (Wang et al., 2008). Moreover, the reduction antioxidant activities was presumed to be because of increased biomass in bacterial inoculated plants (Ullah et al., 2019).
CONCLUSION
Zinc is a toxic heavy metal to plants and animals including human being even at low concentration. The Zn contamination has been a main problem in recent decades. Phytoremediation mediated ny bacteria is among the most important strategy through which the heavy metals are eliminate from the soil. In the present study, B. napus inoculated with IU01 was used in Zn contaminated soil. The IU01 was assessed to enhance the Zn accumulation predominantly in roots.
ACKNOWLEDGMENTS
The authors are extremely thankful all those who supported the study during experimentation and drafting.
REFERENCES
Bahadir, T., Bakan, G., Altas, L. ,Buyukgungor, H. (2007). The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme and Microbial Technology, 41: 98-102.
Berg, G. ,Hallmann, J., 2006. Control of plant pathogenic fungi with bacterial endophytes. Microbial root endophytes. Springer, pp. 53-69.
Bharti, N., Pandey, S.S., Barnawal, D., Patel, V.K. ,Kalra, A. (2016). Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Scientific Reports, 6: 34768.
Buendía-González, L., Orozco-Villafuerte, J., Cruz-Sosa, F., Barrera-Díaz, C.E. ,Vernon-Carter, E.J. (2010). Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresource Technology, 101: 5862-5867.
Chauvin, J., Judée, F., Yousfi, M., Vicendo, P. ,Merbahi, N. (2017). Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Scientific Reports, 7: 4562.
Dahmani-Muller, H., van Oort, F., Gelie, B. ,Balabane, M. (2000). Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ Pollut, 109: 231-238.
Fidalgo, F., Freitas, R., Ferreira, R., Pessoa, A.M. ,Teixeira, J. (2011). Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and post translationally in Cd-induced stress. Environmental and Experimental Botany, 72: 312-319.
Garbisu, C. ,Alkorta, I. (2001). Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol, 77: 229-236.
Hardoim, P.R., van Overbeek, L.S. ,van Elsas, J.D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends in microbiology, 16: 463-471.
He, H., Ye, Z., Yang, D., Yan, J., Xiao, L., Zhong, T., Yuan, M., Cai, X., Fang, Z. ,Jing, Y. (2013). Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere, 90: 1960-1965.
Jabeen, R., Ahmad, A. ,Iqbal, M. (2009). Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. The Botanical Review, 75: 339-364.
Jeger, M.J. ,Spence, N.J., 2001. Biotic interactions in plant-pathogen associations. CABI.
Kartik, V.P., Jinal, H.N. ,Amaresan, N. (2016). Characterization of cadmium-resistant bacteria for its potential in promoting plant growth and cadmium accumulation in Sesbania bispinosa root. Int J Phytoremediation, 18: 1061-1066.
Kurzbaum, E., Kirzhner, F. ,Armon, R. (2014). A hydroponic system for growing gnotobiotic vs. sterile plants to study phytoremediation processes. Int J Phytoremediation, 16: 267-274.
Liu, Y.W., Gao, J.L., Guan, J., Qian, Z.M., Feng, K. ,Li, S.P. (2009). Evaluation of antiproliferative activities and action mechanisms of extracts from two species of Ganoderma on tumor cell lines. J Agric Food Chem, 57: 3087-3093.
Ma, Y., Rajkumar, M., Luo, Y. ,Freitas, H. (2013). Phytoextraction of heavy metal polluted soils using Sedum plumbizincicola inoculated with metal mobilizing Phyllobacterium myrsinacearum RC6b. Chemosphere, 93: 1386-1392.
Malandrino, M., Abollino, O., Giacomino, A., Aceto, M. ,Mentasti, E. (2006). Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. J Colloid Interface Sci, 299: 537-546.
Mata, J.A., Martinez-Canovas, J., Quesada, E. ,Bejar, V. (2002). A detailed phenotypic characterisation of the type strains of Halomonas species. Syst Appl Microbiol, 25: 360-375.
Sharma, S.B., Sayyed, R.Z., Trivedi, M.H. ,Gobi, T.A. (2013). Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus, 2: 1.
Sheng, X.F., Xia, J.J., Jiang, C.Y., He, L.Y. ,Qian, M. (2008). Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut, 156: 1164-1170.
Sturz, A., Christie, B. ,Nowak, J. (2000). Bacterial endophytes: potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences, 19: 1-30.
Ullah, I., Al-Johny, B.O., Al-Ghamdi, K.M.S., Al-Zahrani, H.A.A., Anwar, Y., Firoz, A., Al-Kenani, N. ,Almatry, M.A.A. (2019). Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicol Environ Saf, 174: 197-207.
Ullah, I., Khan, A.L., Ali, L., Khan, A.R., Waqas, M., Hussain, J., Lee, I.J. ,Shin, J.H. (2015). Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J Microbiol, 53: 127-133.
Ullah, I., Khan, A.R., Park, G.-S., Lim, J.-H., Waqas, M., Lee, I.-J. ,Shin, J.-H. (2013). Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Science and Biotechnology, 22: 25-31.
Wan, Y., Luo, S., Chen, J., Xiao, X., Chen, L., Zeng, G., Liu, C. ,He, Y. (2012). Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere, 89: 743-750.
Wei, S., Hu, Y., Srivastava, M., Zhou, Q., Niu, R., Li, Y., Wu, Z. ,Sun, T. (2009). Seed germination of a newly discovered hyperaccumulator Solanum nigrum L. affected by illumination and seed-soaking reagent. J Hazard Mater, 170: 1256-1259.
Xia, Z.-K., Ma, Q.-H., Li, S.-Y., Zhang, D.-Q., Cong, L., Tian, Y.-L. ,Yang, R.-Y. (2016). The antifungal effect of silver nanoparticles on Trichosporon asahii. Journal of Microbiology, Immunology and Infection, 49: 182-188.