1Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Neurosciences Research Center (NSRC), Tabriz University of Medical Sciences, Tabriz, Iran
Corresponding author Email: rashtchizadeh@rocketmail.com
Article Publishing History
Received: 17/12/2017
Accepted After Revision: 19/03/2017
Dyslipidemia,oxidative stress,inflammation and apoptosis are common features in atherosclerosis disease leading to stroke. However there are numerous diagnostic tools indicating atherosclerotic lesions status such as imaging tools and evaluation of circulating indexes such as atherogenic index of plasma (AIP). But these markers don’t definitely reflect vessel inflammation and apoptosis signaling pathways in early stages. We therefore considered which circulating risk factors have stronger association with apoptosis related proteins in the carotid tissues. Hence the associations between the expression of inflammation or apoptosis related proteins content and antioxidant enzymes activity, serum levels of oxidized low density lipoproteins (OxLDLs) or AIP were assessed and compared. Twenty male wistar rats aged 8 weeks were randomly divided into two groups (n=10) and fed the following diets for 8 weeks: normal diet, (ND); a high-cholesterol diet (HD) 2%.Immunoblotting technique was applied to assay of expression of B-cell lymphoma 2 (Bcl2) and cleaved caspase 3(c-caspase 3) proteins as well as phosphorylation of p38 mitogen activated protein kinase (MAPK) in carotid artery homogenate. Plasmatic lipid profile consist of triglyceride (TG), total cholesterol (TC), HDL-C and LDL-C were measured using colorimetric technique in end point manner. The serum levels of Ox-LDL were measured by ELISA.Log (TG/HDL-C) as aatherogenic index of plasma (AIP) was calculated. Correlations were assessed by use of the nonparametric Spearman correlation coefficient. After 8 weeks feeding with high cholesterol diet, the mean of lipidic profile including TC,LDL-C, TG, OxLDL and AIP, MDA were higher in HD vs. ND group (P<0.05 in all) as well as the immunoreactivity of p- p38 and c-caspase 3 were elevated in HD vs. ND(P<0.05 in both). Antagonistically, the expression of bcl2, the activity of SOD and GPx as well as the capacity of total antioxidants were reduced in HD vs. ND(P<0.05 in all). The association (with the expression of p- p38 or c-caspase 3 )were significant also positive for variables of OxLDL and MDA (strongest; r= 0.874) but negative for SOD, GPx and TAC(P<0.05 in all). The association (with the expression of bcl2 )were significant also negativefor variables of OxLDL and MDA but positive for SOD, GPx and TAC(P<0.05 in all). There is no significant association between AIPand the expression of c-caspase 3, p- p38 or bcl2 (r=0.45; p=0.08, r=0.44; p=0.08, r=-0.38; p=0.14 respectively). Findings suggest that MDA,anti oxidant enzymes activity and ox LDL are powerful predictor and monitoring tools for carotid tissue inflammation and apoptosis , two common feature for atherosclerotic lesions.
Atherosclerosis, Bcl2 Protein, Cholesterol, Caspase 3,Oxidized Low Density Lipoprotein, P38 Mitogen Activated Protein Kinase
Bayatmakoo R, Rashtchizadeh N, Yaghmaei P, Farhoudi M, Karimi P. Which one More Reflects Atherosclerotic Lesion Status in Rat Carotid? Oxidized Low Density Lipoprotein, Activity of Plasmatic Antioxidant Enzymes or Atherogenic Index of Plasma: A Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1).
Bayatmakoo R, Rashtchizadeh N, Yaghmaei P, Farhoudi M, Karimi P. Which one More Reflects Atherosclerotic Lesion Status in Rat Carotid? Oxidized Low Density Lipoprotein, Activity of Plasmatic Antioxidant Enzymes or Atherogenic Index of Plasma: A Comparative Study. Biosc.Biotech.Res.Comm. 2017;10(1). Available from: https://bit.ly/2JHltMh
Introduction
Atherosclerosis as a chronic inflammatory underling disease (Yang et al., 2012) is accompanied by some changes in plasmatic lipid profile and redox status (Tie et al., 2014). Generally, the hyperlipidemias are of interest to the physician in the context of risk factors for cardiovascular diseases. Among lipid profile, The strong predicting value for the ratio of triglyceride (TG) to high density lipoprotein (HDL-C) has been shown in (Watt
et al., 2016). The log of (TG/HDL-C) is commonly called as atherogenic index of plasma (AIP) (Nwagha et al., 2010, Klafke et al., 2015).
High density lipoprotein cholesterol (HDL-c) is the most important particle among the five major lipoprotein particles involved in esterification and reverse transporting of cholesterol from the peripheral tissues to the liver (Nwagha et al., 2010). HDL-c particles possess multiple anti-atherogenic activities such as anti-inflammatory, anti-oxidant, anti-thrombotic, anti-apoptotic and vasodilator effects (Vavrova et al., 2015 and Klafke et al., 2015). Despite numerous reports on the relationship between HDL-c concentration and inflammatory and oxidative stress biomarkers, there is controversial data in some population (Vavrova et al., 2015).
In addition, a large body of studies investigated the associations between HDL-c concentration and status of inflammation, endothelial activation and oxidative stress biomarkers (Silva et al., 2011). Recently, Klafke et al study has showed that TG concentrations can reflect the enhanced advanced oxidation protein products, proinflammatory markers such as high-sensitivity C-reactive protein, endothelial dysfunction indicator like nitric oxide and ischemia-modified albumin (Klafke et al., 2015). Oxidised low-density lipoprotein (OxLDL) as an cholesterol induced oxidative stress injures the vascular endothelium, a key step in the pathogenesis ofatherosclerosis (Watt et al., 2016).
Circulating levels of OxLDL in blood are increased generally following dislipidemia and particularly hypercholesterolemia (Levitan et al., 2010). There are conflicting data on whether The levels of circulating OxLDL correspond to the severity of vascular damage. However accumulative evidence have shown the atherogenic effect of OxLDL, there are too many controversies in use of plasma levels of OxLDL as a atherogenic index. Tertov et al. study in 1998, showed that atherogenicity of the levels of plasmatic LDL does not depend on the degree of lipid peroxidation in LDL particles (Tertov et al., 1997).Marchesi et al. study indicated that, the use of biological markers of in vivo LDL oxidation (antioxidatively modified LDL autoantibody titers) could be used to evaluate the clinical setting of high-risk carotid atherosclerosis both in screening and in follow-up studies (Chiesa et al., 1998). Another study has been showed that enhanced LDL oxidation correlates to the intima media thickness (IMT) in carotid arteries of hypertensive patients.3 The relationship between circulating Ox-LDL levels and foam cell formation has been shown (Liu et al., 1996).
Another study has been indicated that enhanced LDL oxidation correlates to the intima media thickness (IMT) in carotid arteries of hypertensive patients (Marchesi et al., 1996). in other hand the results of studies suggest that OxLDL initiates and accelerates the development of atherosclerosis by endothelial injury through change the expression of antioxidant enzymes such as extracellular – superoxidedismutase (EC-SOD) (Makino et al., 2016), inducible nitric oxide synthase (iNOS) (Luoma et al., 1998), glutathione peroxidase (GPx) (Ma et al., 2015) and inducing the oxidative stress markers such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) (Zhang et al., 2016).
Cao et al. have showed negative partial correlation between total anti oxidant status and arterial stiffness in elderly hypertensive patients that suggests the decline in antioxidant capacity may be responsible for vascular damage and arterial elasticity decrease in elderly essential hypertension patients (Cao et al., 2013). Antioxidant enzymes activity associate with inflammatory index. As shown in a Vasamsettiet al recent study a intracellular glutathione GSH contents as an antioxidant marker regulates monocyte-to-macrophage differentiation and inflammation (Vasamsetti et al., 2016).
Nevertheless, there is few study investigating the correlation between antioxidant enzyme activity and biomarkers of vessel inflammation. p38 mitogen activated protein kinase (MAPK) is a well known stress-induced protein kinases in general and subsequent of modified LDLin (Chapple et al., 2013) cells in particular. Studies of advanced atherosclerotic lesions revealed a strong correlation between incidence of vessels inflammation and programmed cell death (Brown and Jessup., 1999). Several plasmatic inflammatory biomarkers have been already candidate to reflect the status of atherosclerotic injury (Karakurt et al., 2013). Heretofore, High sensitivity C-reactive protein(hsCRP) (Gupta et al., 2013), TNFá and Interleukin-6 (IL6) have represented the inflammation in general but any of them could not present the real feature of vessels atherosclerotic lesions (Silva et al., 2011 and Gupta et al., 2013). Recently, evidence showed that estimation of activity of related kinases are more specific and reliable indicator of tissue inflammation and apoptosis as two common features of atherosclerotic lesions (Chapple et al., 2013 and Zhang et al., 2013). The phosphorylation of p38 MAPK and expression of apoptosis related proteins can exactly reflect the degree of involvement of vessels but based on location and unavailable nature of these proteins, they have not been used as diagnostic markers yet. So any correlation between an available plasmatic marker such as AIP, circulating OxLDL or antioxidant enzymes activities and expression of these proteins in vessels tissue can be helpful in monitoring of real status of atherosclerotic lesions.
Therefore, the aim of this study was to investigate the relationship between plasma OxLDL,AIPor antioxidant enzymes activity with expression of inflammatory and apoptosis related proteins in carotid tissue. To our knowledge, this is the first study to consider these associations and our results provide data that enable physicians to really evaluate atherosclerotic lesion status by using circulating indexes.
Reagents
All compounds were of the purest quality available andwere purchased from Sigma Chemical (St. Louis, MO,USA) or Merck (Darmstadt, Germany).All required antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Animals
Twenty wistar male rats, obtained from our local breeding colony (neurosciences research center laboratories, tabriz university of medical sciences, tabriz, Iran), underwent controlled light (12 h light/dark), humidity (45–65%) and temperature (21–23°c) conditions with free access to standard (chow diet) or to high-cholesterol diet which is composed of 2% cholesterol plus 0.5% cholic acid and tape water .
Rats were divided into two experimental groups (n=10 per group): one normal diet group (ND) and another high-cholesterol dietgroup received chow diet or high-cholesterol diet until 8 weeks respectively. Agreement of experimental protocolwith the ethics of Guidelines of National Institute of Health for the Care and Use of Laboratory Animals (NIH Publications No.80-23) was confirmed by the local institutional animal care anduse committee (Approval Number:A125345).
Sample Collection And Storage
At the end of the 8 weeks treatmentby high cholesterol diet, rats were anesthetized by Xylazine (Parke-Davis, Ann Arbor, MI, USA) and ketamine hydrochloride (Parke-Davis, Ann Arbor, MI, USA).Following vascular access and isolation of the common carotid artery (CCA),Blood samples were collected positively from heart of rats and poured in anticoagulant free tube then left to clot formation in 2 hours at room temperature. centrifugation (Beckman model L centrifuge) 3000 × g for 20 min was performed to serum separation. sera immediately subjected to biochemical analyses. The CCAs were stored in -80◦C deep freezer for immunoblotting analysis.
Biochemical Measurments
The photometric assay (VITROS 5600 Autoanalyser; (Ortho-Clinical Diagnostics Inc. USA). were used to determinestandard lipid panel including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) andtriglyceride (TG) by using pars azmoon kits (Tehran, Iran).The levels of Low-density lipoprotein cholesterol (LDL-C) were calculated by usingFriedewald’s formula (Abo El-Khair et al., 2014) asdescribed: LDL-C= TC– (TG/5) –HDL-C .Atherogenic index of plasma (AIP)); log (TG/HDL–C) was calculated as a significant predictor ofatherosclerosis (Nwagha et al., 2014). Plasma levels of Ox-LDL were detected by a competitive enzyme-linked immunosorbent assay (ELISA) using a commercial specific ELISA kit (MBS729489, My Bio Source. Ltd, USA).GPxactivity was measured in hemolysate usingRansel kit (randoxLaboratories Ltd. Admore, Northern Ireland, UK) in which GSH-Px degraded H2O2 in the presence of GSH, decreasing the GSH. SOD activity was also determined in hemolysate using Ransod kit (randox Laboratories Ltd. Admore, Northern Ireland, UK) in which SOD inhibited the generation of nitrite from oxidation of hydroxylamine by superoxide anion (O2-) produced by the xanthine/xanthine oxidase system.
The units of measurement for both of them were expressed as U/g Hb. Plasma Total Antioxidant Status (TAS) of serumwas determined by using randox kit (randoxLaboratories Ltd.Admore, Northern Ireland, UK). Serum levels of MDA was assayed with the thiobarbituric acid (TBA) method in which the reaction of MDA with thiobarbituric acid toproducethiobarbituric acid-reactive substances (TBARS), and the resultant data was expressed as nanomolesper milliliter of serum.
Immunoblotting Analysis
Western blot analysis was used to determine of the content of phosphoP38MAPK, P38 MAPK,Bcl2 and cleaved caspase3in carotid tissue. Santa Cruz online protocol was applied as previous study (Faramoushi et al., 2016) all over experiment.Briefly, A 10% w/v carotid tissue homogenate was prepared in ice-cold lysing buffer (50 mMTris–HCl, pH 7.4, NP-40 1%, Triton X-100 1%, 50 mMNaCl, sodium deoxycholate 1%, 0.5 mMEDTA) containing protease inhibitor cocktail (Sigma Chemical Co. MO, USA). The protein concentration was measured by Bradford assay using commercial available kit(Sigma Chemical Co. MO, USA). Denaturing SDS/polyacrylamidegel10% were used to separate proteins.Protein bands were transferred to Hybond ECL nitrocellulose membrane (Sigma Chemical Co. MO, USA). After blocking of membranewith 3% nonfat milk (sigma) in Tris-buffered saline (TBS) 1x-Tween 20 0.05%, the membrane was blotted overnight at4 °C with the rabbit polyclonal primary antibodies(1:500; Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) againstBcl-2 (N-19) (sc-492) Cleaved caspase-3 p11 (h176)-R (sc-22171-R), antiP-p38 Antibody (Tyr 182) (sc-101759) and anti p38 antibody (sc-535) and B-actin (sc-47778) then probed by HRP-conjugated anti-rabbit secondary antibodies (1:5000;Santa Cruz Biotechnology Inc.) for one hour at 4 °C. The membranes werestripped (Restore Western Blot Stripping buffer, Pierce Biotechnology, Rockford, IL, USA). The bandswere detectedusingECL kit (GE Healthcare Europe) following the manufacturer’sinstruction. B-actin was used as loading control.
Statistical Analysis
Data are expressed as means±standard error of the mean (SEM). Kolmogorov smirnov test was used to determine distribution of variables. Differences between groups were evaluated with the Mann-Whitney U test for variables. Spearman correlation coefficient was applied to correlation analysis. P value less than 0.05was considered as statistically significant. The data were analyzed using SPSS statistical package, ver 26 (SPSS).
Results and Discussion
The baseline parameters of the study rats are shown inTable 1.The levels of serum total cholesterol in HD group were approximately three-fold higher than ND group where as the levels of serum LDL-C in HD group were more than ten-fold higher than ND group
(table 1). Based on resultant data, a significant increase in triglyceride levels were observed in the HD group, when compared to ND group (p < 0.05).However a slight and non significant decrease observed in HDL-cholesterol (P = 0 .36), the ratios of HDL to LDL were significantly decreased (approximately four times).As shown in table 1 serum OxLDL levels was three times higher in HD compared to ND.An antagonized effect of cholesterol was observed on redox system of rats. The antioxidant capacity including SOD, GPx and TAC were significantly decreased in HD vs.ND but a two fold increase wasseen in MDA levels as a lipid peroxidation marker (table 1).The expression of inflammation related protein, p-p38 MAPK, and proapoptotic protein,c-caspase 3, were diminished in HD vs. ND but The expression of anti apoptotic protein,Bcl2, was raised in HD in compare to ND (table 1).Moreover,a 150% increase also were observed in AIP index in HD vs. ND group (table 1).
| Table 1: Baseline parameters of the twenty rats studied. Data are expressed as mean and SD or percentages. ND: normal diet rats; HD: hypercholesterol diet rats. Data were analyzed statistically using non parametric two- independent -sample test. Categorical data were summarized as percentages.*P < 0.05when compared to ND group. **P < 0.01when compared to ND group. | |||
| ND | HD | P value | |
| Total serum cholesterol (mg/dl) | 67.89±5.14 | 229.35±13.26** | <0.001 |
| LDL-cholesterol (mg/dl) | 15.20±2.34 | 177.39±10.38** | <0.001 |
| HDL-cholesterol (mg/dl) | 33.66±2.90 | 35.27±4.69 | NS |
| HDL/LDL | 1.96±.14 | .55±.12** | <0.001 |
| Triglyceride (mg/dl) | 50.12±7.16 | 65.41±10.66** | <0.001 |
| Serum-oxLDL (ng/dl) | 69.13±9.92 | 214.42±17.46** | <0.001 |
| OxLDL to LDL ratio (ug/mg) | 4.48±.84 | 1.20±.05** | <0.001 |
| total antioxidant (mmoL/L) | 1.74±.53 | 1.17±.24** | <0.001 |
| Hemolysate superoxide dismutase (U/gHb) | 829.14±65.90 | 681.68±73.65 | <0.001 |
| SERUM MDA (umol/l) | 2.95±.68 | 6.37± .857 | <0.001 |
| Hemolysate Glutathione peroxidase (U/gHb) | 85.29±2.62 | 71.08±9.32** | <0.001 |
| c-caspase 3/Bactin(% of control) | 100 | 147.63 ± 6.89** | <.001 |
| p-p38/total p38(% of control) | 100 | 235.56± 6.95** | <.001 |
| Bcl-2/B-ACTIN(% of control) | 100 | 6.95 ± 5.02* | <0.05 |
| AIP | .17±.06 | .26±.09* | <0.05 |
CORRELATION BETWEEN CIRCULATINGOx-LDLANDc-caspase 3,pP38MAPKANDBcl2.
According to resultant datathere were significant, positive,and strong correlation between serum levels of Ox-LDL and expression both of c-caspase 3 (r=0.768, p<0.001) and pP38MAPK (r = 0.760, p<0.001) (Fig.1A,B).Correlation between serum levels of Ox-LDL and expression ofBcl2 was reverse and significant (r=-0.82, p<0.001) (Fig.1C).
 |
Figure 1a |
 |
Figure 1b |
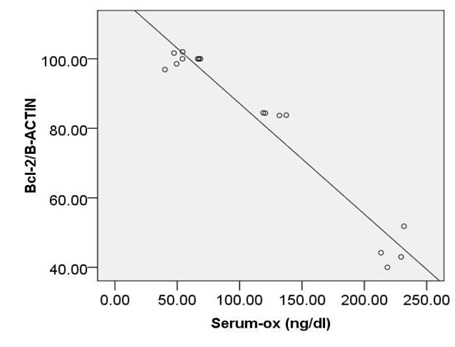 |
Figure 1c |
CORRELATION BETWEEN CIRCULATINGSODANDc-caspase 3, pP38MAPK AND Bcl2.
As shown in (Fig. 2A,B, C) significant, moderate and reverse correlation were found between SOD activity and expression of c-caspase 3 ( r=-0.62, p<0.01) and pP38MAPK (r=-0.593, p <0.016),where as positive correlation was observed between SOD activity and expression of Bcl2(r= 0.593, p<0.016).
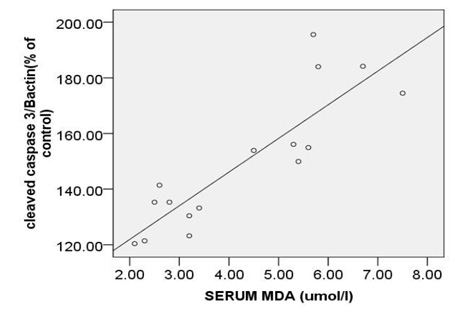 |
Figure 2a |
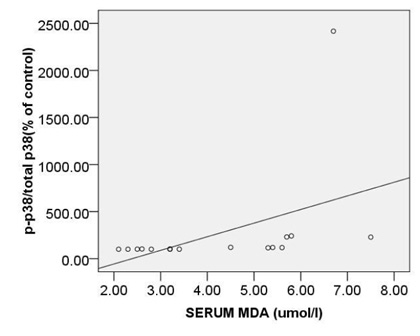 |
Figure 2b |
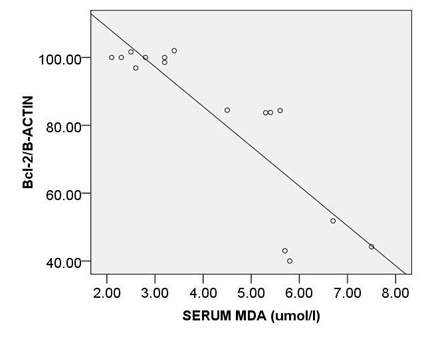 |
Figure 2c |
CORRELATION BETWEEN CIRCULATINGGPxANDc-caspase 3, pP38MAPK AND Bcl2.
Considration of the relationship between GPx activity and c-caspase 3 or pP38MAPK revealed reverse correlation (r= -0.59, p < 0.01 and r=-0.51, p < 0.04 respectively) (Fig. 3A,B). No considrable correlation was observed between GPx and expression of bcl2 (r= 0.41, p = 0.11) (Fig. 3C).
 |
Figure 3a |
 |
Figure 3b |
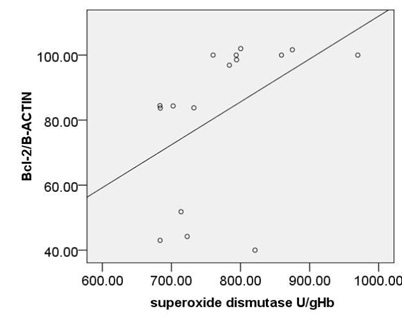 |
Figure 3c |
CORRELATION BETWEEN CIRCULATINGTACANDc-caspase 3, pP38MAPK AND Bcl2.
When the association of TAC with c-caspase 3, pP38MAPK were considered, it was noted that the correlation between them is reverse and significant (r= -0.77, p < 0.00 and r=-0.77, p < 0.00) (Fig. 4A,B). moreover, we observed significant, also strong, correlations between TAC and bcl2 (r= 0.78, p<0.00) (Fig. 4C).
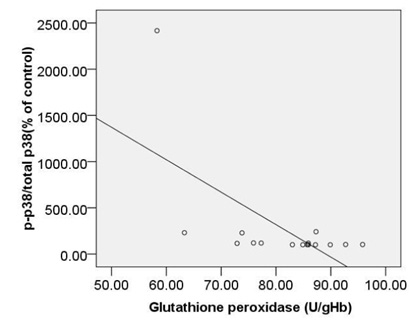 |
Figure 4a |
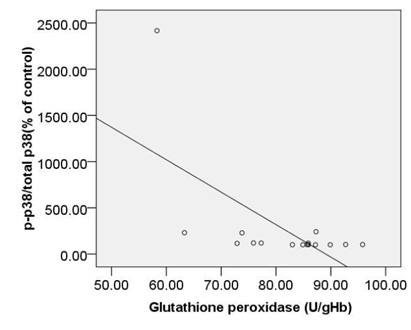 |
Figure 4b |
CORRELATION BETWEEN CIRCULATINGMDAANDc-caspase 3, pP38MAPK AND Bcl2.
The strongest also positive correlation was observed between the levels of circulating MDA and expression ofc-caspase 3 (r= 0.873, p < 0.000) (Fig. 5A) and between the levels of circulating MDA and expression ofpP38MAPK (r= 0.874, p < 0.000) (Fig. 5B). Morovercorrelation between circulating MDA and expression of Bcl2 was strong but reverse (r=-0.832, p < 0.000) (Fig. 5C).
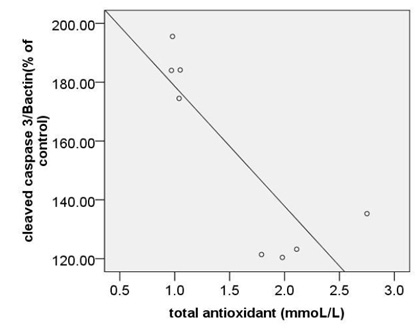 |
Figure 5a |
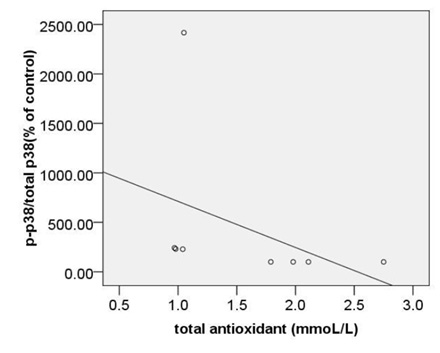 |
Figure 5b |
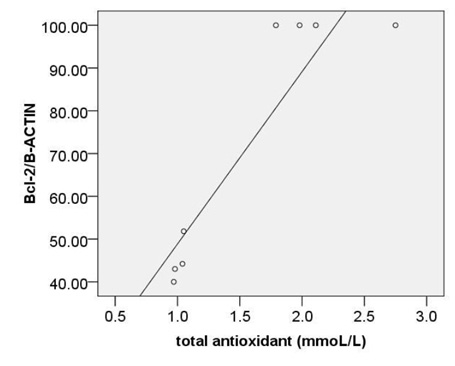 |
Figure 5c |
CORRELATION BETWEEN AIP ANDc-caspase 3, pP38MAPK ANDBcl2.
Based on our finding no significant correlation were observed between calculated AIP and expression of c-caspase 3 (r=0.45, p=0.08), p -p38MAPK (r=0.44, p=0.08) or bcl2 (r=-0.38, p=0.14) (Fig. 6A, B, C).
Atherosclerosis is a major cause of stroke in developing countries (Georgiadi et al., 2013). Historically, Atherosclerosis was believed as a simple accumulation of lipids in sub intima and was not thought to be an inflammatory disease but there is growing evidence supporting the fact that inflammation are essential contributing factors in the development of atherosclerotic lesions (Niemann-Jonsson et al., 2000). This fact that Risk factors for atherosclerosis, such as oxidative stress (Watt et al., 2016), inflammation (Bretscher et al., 2015), hypercholesterolemia (Niemann-Jonsson et al., 2000) and central obesity (Verreth et al., 2004) commonly co-exist suggest that we can use some circulating indexes to monitoring of arterial inflammation or apoptosis regarding to association of between them.
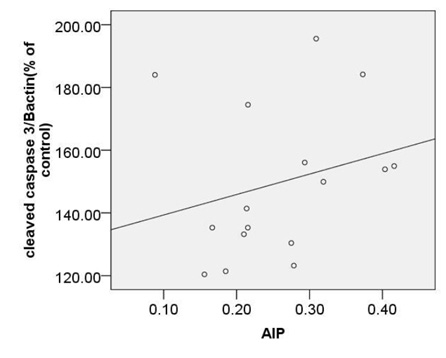 |
Figure 6a |
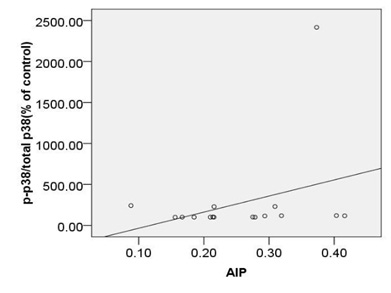 |
Figure 6b |
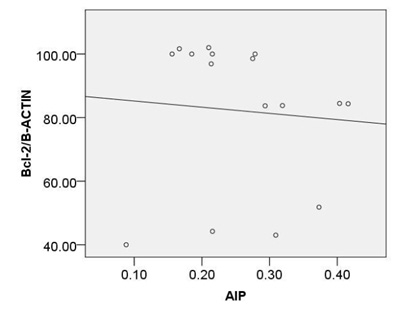 |
Figure 6c |
The current study is the first in vitro work which highlights the association between various markers of arterial inflammation or apoptosis with circulating oxidative stress or atherogenic indexes and compare considerable associations to know which one is more strong and reliable to monitoring of carotid tissue inflammation and apoptosis status.
The present study indicates that the markers of circulating antioxidant status and lipoprotein oxidation including TAC, SOD, GPx, MDA, OxLDL respectively reflect arterial inflammation and apoptosis status accessed by the expression of pP38MAPK, c-caspase 3 and bcl2 in carotid arteries of hyper cholestrolemic model rats. In this experiment, we made a moderate atherosclerotic rat model by administration of 2% cholesterol (TC = 229.35±13.26 mg/dl) We used this model to investigate the expression of inflammation and apoptosis related proteins in carotid of hypercholestrolemic rats. As shown in Ntchapda et al study, extensive atherosclerotic plaques were created even by administration of 1% cholesterol, which was not the case with of the normocholesterolemic rats (NC) (Ntchapda et al., 2015).We found significantly higher level of OxLDL, MDA in serum of hypercholestrolemic rats in comparison to normal control group. Further the study shows the decrease in the serum levels of TAC, AIP and activity of antioxidant enzymes containing SOD and GPx which is also confirmed by (Lluis et al., 2013).
The results indicated the strongest relationship between the serum levels of MDA and the expression of pP38MAPK, c-caspase 3or bcl2, suggesting a role of this measurement in monitoring of arterial inflammation and apoptosis status. MDA is an important end product of lipid peroxidation and a widely-used indicator of reactive oxygen species (ROS) production playing a critical role in the pathogenesis of both the micro and macrovascular complications (Karakurt et al., 2013). In other hand, The increase of MDA activity is an indirect proof that atherogenic conditions could elevate the oxidative stress (Levitan et al., 2010). Since we did not find any study investigating the correlation between MDA and the expression of pP38MAPK we have to compare previous study in correlation of MDA and circulating inflammatory cytokines.
In line of our study, Gupta et al showed a significant, positive, also strong correlation between MDA concentration and tumor necrosis factor á (TNFá) as an inflammatory index in diabetic chronic kidney disease (Gupta et al., 2013). Also, previous researchers found the association of genotype of IL-1á as another inflammatory factor with MDA and showed that there was significant difference in MDA level in nephropathy with diabetes and nephropathy without diabetes group vis-a-vis control (Dabhi et al., 2015). Moreover, the relevance of MDA epitopes in human pathologies by inflammatory processes in atherosclerosis was reported in Papac et al.’s study (Papac-Milicevic et al., 2016). In contrary of our result, Karakurt Arıtürk in a study on atherosclerosis in familial Mediterranean fever (FMF) showed that Serum MDA levels were the same between the FMF and healthy control group( Karakurt Ariturk et al., 2013). Based on resultant data, there was also significant decrease in GPx and SOD activity and serum levels of TAC in HD group vis-à-vis control group. Mentioned that low activity of antioxidant enzymes may increase the susceptibility of arteries to oxidative injury.
In agreement with this study the concentrations of SOD were significantly low in high cholesterol diet fed group as compared to the control group (Balkan et al., 2002). The results also indicated powerful association between serum TAC content, activity of SOD with the expression of pP38MAPK, c-caspase 3or bcl2. Moreover a moderate association was observed between GPx activity and the expression of pP38MAPK, c-caspase 3 or bcl2.Similar to our study, Baez-Duarte et als study indicated that SOD activity is associated with metabolic syndrome in Mexican subjects (Odds ratio: 167.1; P < 0.01) (Baez-Duarteet al., 2016).
Furthermore in a recent study, the extracellular superoxide dismutase methylation frequency of case group was reported lower than the control group. That suggest methylation status is associated with the size of cerebral infarction, degree of cerebral arteriosclerosis and severity of neurological impairment (Zhou et al., 2016). Also, Pearson’s correlation analysis showed that SOD and total antioxidant status were negatively related to AP-1 as a responding to a variety of inflammatory cytokines in elderly patients with mild-to-moderate essential hypertension (Liu et al., 2016). In present study serum levels of OxLDL were higher in cholesterol rich diet group in compare to normal diet fed group that has been frequently confirmed by previous studies (Chatauret et al., 2014 and Canas et al., 2015 ).
Moreover, a strong correlation was observed between OxLDL concentration and expression of pP38MAPK, c-caspase 3 or bcl2.Oxidative modifications in low-density lipoprotein are associated with intima media thickness of carotid artery in athletes (Fonseca et al., 2016).Despite of significant increase in AIP index in HD group, no considerable association was found between calculated AIP and mentioned inflammatory and apoptotic markers. As afore mentioned, the point of current study is a concomitantly comparison of spearman correlation coefficient of several atherosclerosis risk factor including OxLDL, TAC, SOD, GPx, MDA or AIP with expression of pP38MAPK, c-caspase 3 or bcl2.The experiment showed that the strongest association is belonged to MDA ( Fig. 5). The correlation coefficient of SOD (Fig. 2) and TAC (Fig. 4), OxLDL (Fig.1) also was strong and near to MDA.A moderate correlation coefficient was calculated for GPx (Fig. 3) and finally no significant correlation was observed for AIP
(Fig. 6).
Conclusion
Findings suggest that MDA and anti oxidant enzymes activity and ox LDL are powerful predictor and monitoring tools for carotid tissue inflammation and apoptosis , two common feature for atherosclerotic lesion.
Acknowledgments
We thank the Dr. farhoudi as head of Neurosciences Research center (Tabriz university of medical sciences, Tabriz, Iran) for providing the facilities for experiments.
Conflict of Interest
None to declare.
References
- Abo El-Khair DM, El-Safti Fel N, Nooh HZ, El-Mehi AE.(2014) A comparative study on the effect of high cholesterol diet on the hippocampal CA1 area of adult and aged rats. Anatomy & cell biology. ;47(2):117-26.
- Baez-Duarte BG, Zamora-Ginez I, De Jesus KL, Torres-Rasgado E, Gonzalez-Mejia ME, Porchia L, (2016) Association of the Metabolic Syndrome with Antioxidant Defense and Outstanding Superoxide Dismutase Activity in Mexican Subjects. Metabolic syndrome and related disorders. 14(3):154-60.
- Balkan J, Kanbagli O, Hatipoglu A, Kucuk M, Cevikbas U, Aykac-Toker G (2002). Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Bioscience, biotechnology, and biochemistry. 66(8):1755-8.
- Bretscher P, Egger J, Shamshiev A, Trotzmuller M, Kofeler H, Carreira EM (2015) Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO molecular medicine.
- Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. (1999) 142(1):1-28.
- Canas JA, Ross JL, Taboada MV, Sikes KM, Damaso LC, Hossain J. (2015) A randomized, double blind, placebo-controlled pilot trial of the safety and efficacy of atorvastatin in children with elevated low-density lipoprotein cholesterol (LDL-C) and type 1 diabetes. Pediatric diabetes. 16(2):79-89.
- Cao J, Wang HY. ( 2013)Association between total antioxidant status and atherosclerosis in elderly patients with essential hypertension. Zhonghua xin xue guan bing za zhi.;41(10):857-61.
- Chapple SJ, Cheng X, Mann GE.(2013) Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox biology. 1:319-31.
- Chatauret N, Favreau F, Giraud S, Thierry A, Rossard L, Le Pape S (2014) Diet-induced increase in plasma oxidized LDL promotes early fibrosis in a renal porcine auto-transplantation model. Journal of translational medicine. 12:76.
- Chiesa R, Melissano G, Castellano R, Astore D, Marone EM, Grossi A (1998) In search of biological markers of high-risk carotid artery atherosclerotic plaque: enhanced LDL oxidation. Annals of vascular surgery. 12(1):1-9.
- Dabhi B, Mistry KN. (2015) Oxidative stress and its association with TNF-alpha-308 G/C and IL-1alpha-889 C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene. 562(2):197-202.
- Faramoushi M, Amir Sasan R, Sari Sarraf V, Karimi P.(2016) Cardiac fibrosis and down regulation of GLUT4 in experimental diabetic cardiomyopathy are ameliorated by chronic exposures to intermittent altitude. Journal of cardiovascular and thoracic research.;8(1):26-33.
- Fonseca HA, Bittencourt CR, Fonseca FA, Monteiro AM, Santos PR, Camargo L (2016)Non-linear Optical Responses of Low-Density Lipoprotein are Associated with Intima-Media Thickness of Carotid Artery in Athletes. Cell biochemistry and biophysics. 74(2):253-62.
- Georgiadi A, Wang Y, Stienstra R, Tjeerdema N, Janssen A, Stalenhoef A (2013) Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arteriosclerosis, thrombosis, and vascular biology. 33(7):1529-37.
- Gupta S, Gambhir JK, Kalra O, Gautam A, Shukla K, Mehndiratta M. (2013) Association of biomarkers of inflammation and oxidative stress with the risk of chronic kidney disease in Type 2 diabetes mellitus in North Indian population. Journal of diabetes and its complications. 27(6):548-52.
- Karakurt Ariturk O, Ureten K, Sari M, Yazihan N, Ermis E, Erguder I. (2013)Relationship of paraoxonase-1, malondialdehyde and mean platelet volume with markers of atherosclerosis in familial Mediterranean fever: an observational study. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 13(4):357-62.
- Klafke JZ, Porto FG, Batista R, Bochi GV, Moresco RN, da Luz PL(2015) Association between hypertriglyceridemia and protein oxidation and proinflammatory markers in normocholesterolemic and hypercholesterolemic individuals. Clinica chimica acta; international journal of clinical chemistry. 448:50-7.
- Levitan I, Volkov S, Subbaiah PV. (2010) Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxidants & redox signaling. 13(1):39-75.
- Lluis L, Taltavull N, Munoz-Cortes M, Sanchez-Martos V, Romeu M, Giralt M (2013) Protective effect of the omega-3 polyunsaturated fatty acids: Eicosapentaenoic acid/Docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids in health and disease. 12:140.
- Liu SX, Zhou M, Chen Y, Wen WY, Sun MJ. (1996)Lipoperoxidative injury to macrophages by oxidatively modified low density lipoprotein may play an important role in foam cell formation. Atherosclerosis. 121(1):55-61.
- Liu Q, Han L, Du Q, Zhang M, Zhou S, Shen X. (1993)The association between oxidative stress, activator protein-1, inflammatory, total antioxidant status and artery stiffness and the efficacy of olmesartan in elderly patients with mild-to-moderate essential hypertension. Clinical and experimental hypertension (New York, NY : 1993). 38(4):365-9.
- Luoma JS, Stralin P, Marklund SL, Hiltunen TP, Sarkioja T, Yla-Herttuala S. (1998) Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arteriosclerosis, thrombosis, and vascular biology. 18(2):157-67.
- Ma L, Zhu XF, Wu YY, Chen KJ, Shi DZ, Yin HJ. (2015) Protective effect of propyl gallate against oxidized low-density lipoprotein-induced injury of endothelial cells. Chinese journal of integrative medicine. 21(4):299-306.
- Makino J, Asai R, Hashimoto M, Kamiya T, Hara H, Ninomiya M (2016) Suppression of EC-SOD by oxLDL During Vascular Smooth Muscle Cell Proliferation. Journal of cellular biochemistry. 16 21
- Marchesi E, Martignoni A, Salvini M, Catalano O, Maggi E, Negro C (1996) Carotid intima-media thickening and in vivo LDL oxidation in patients with essential hypertension. Journal of human hypertension. 10(9):577-82.
- Niemann-Jonsson A, Dimayuga P, Jovinge S, Calara F, Ares MP, Fredrikson GN (2000) Accumulation of LDL in rat arteries is associated with activation of tumor necrosis factor-alpha expression. Arteriosclerosis, thrombosis, and vascular biology. 20(10):2205-11.
- Ntchapda F, Djedouboum A, Talla E, Sokeng Dongmo S, Nana P, Adjia H (2015) Hypolipidemic and anti-atherogenic effect of aqueous extract leaves of Ficus glumosa (Moraceae) in rats. Experimental gerontology. 62:53-62.
- Papac-Milicevic N, Busch CJ, Binder CJ.(2016) Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis. Advances in immunology.131:1-59.
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK (2004) Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 110(20):3259-69.
- Zhou X, Xu Y, Xie Z, Xu S, Bi J.(2016) Association of extracellular superoxide dismutase gene methylation with cerebral infarction Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chinese Journal of Medical Genetics. 33(3):378-82.


