Federal State Budgetary Educational Institution of Higher Education, N.P. Ogarev
Mordovia State University, Bolshevistskaya Str. 68, Saransk 430005, Russia
Article Publishing History
Received: 15/10/2020
Accepted After Revision: 08/12/2020
Background & objectives: Endogenous intoxication and, as a consequence, the development of multi-organ failure are extreme forms of the pathological process. The aim of the study is to look into the DNA modifications during endotoxic “pollution” of the body and to understand the mechanisms of development of dysregulation processes mediated by structural changes in DNA. Methods: The rate of endogenous intoxication was assessed by determining the generally accepted laboratory-clinical and biochemical parameters. The mononuclear cell fraction was collected by gradient centrifugation on Ficoll-Paque™ (ρ=1,077). The isolation of DNA from blood mononuclear cells was performed by using Laura-Lee Boodram. UV-spectroscopic assays of DNA solutions were carried out using a spectrophotometer UV-3600 Shimadzu (Japan). Interpretation & conclusions. The paper is concerned with the study of changes in biochemical composition and structure of genomic DNA of mononuclear cells of venous blood in patients with endogenous intoxication. The FT-IR spectra of genomic DNA of patients’ venous blood mononuclear cells reveal that the band at a frequency of 1337 cm-1, shifts to a higher frequency. The absorption band at 1491 cm-1, becomes wider and has a more pronounced character. The absorption intensity increases at a frequency of 863 cm-1, characteristic of the type of twisting in N-type sugars. The intensity of the band 1086 cm-1, decreases. The corresponding alterations in the spectrum point to changes in the mutual orientation of DNA phosphate groups, resulting in changes in DNA spatial structure, and the increased proportion of DNA in the A-form. UV spectra of the DNA of the experimental samples showed a slight shift of the maximum and a small hyperchromic effect.
Endogenous Intoxication, Dna Configuration, Dna Conformation, Fourier Infrared Spectroscopy, Uv Spectroscopy, Reactive Oxygen Intermediate, Lipid Peroxidation.
Trofimov A.V, Trofimov V.A, Sidorov D.I, Kadimaliev D.A, Vlasov A.P. The Study of Modifications of Genomic DNA in Endotoxemia. Biosc.Biotech.Res.Comm. 2020;13(4).
Trofimov A.V, Trofimov V.A, Sidorov D.I, Kadimaliev D.A, Vlasov A.P. The Study of Modifications of Genomic DNA in Endotoxemia. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/3mLFwKM”>https://bit.ly/3mLFwKM</a>
Copyright © Trofimov et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Endogenous intoxication and, consequently, the development of multi-organ failure are extreme forms of the pathological process and require immediate and radical repairing, therefore, the study of the molecular mechanisms of the pathogenesis of endogenous intoxication syndrome is a topical issue (Savelyev et al., 2008; Sánchez-Tapia et al., 2020; Vlasov et al., 2009).Endogenous intoxication syndrome is characterized by accumulation of toxic endotoxins in tissues and biological fluids, organs damage, disruption of the functional systems of the body due to ischemia, necrotic cell death, release of microorganisms, their metabolic products and decay from the focus of invasive infection into the body, impaired excretion of endotoxins from the body and secondary toxic aggression (Koos et al., 2020).
The most important triggers for the development of the pathological process leading to the development of endogenous intoxication are free radicals, which are formed in excess in the body during various pathological processes induced by inflammation and ischemia. The involvement of new biomolecules, including proteins and lipids in the free radical process coupled with poor antioxidant activity in the body leads to deep destructive changes in the cells and tissues of the body, the development of dysregulation processes, which ultimately aggravates the pathological process in the body and leads to the development of multi-organ failure (Guevara‐Cruz et al., 2019; Halliwell and Chirico, 1993; Valko et al., 2007). Endotoxins are the metabolites accumulating in large quantities in the tissues of the body, modified cytotoxic biomolecules, free radicals, including reactive oxygen and nitrogen intermediates having a high reaction activity (Baeuerle, 2020).
DNA, like other important structural and functional biomolecules of the body – proteins and lipids, is also exposed to various oxidative modifications, but changes in the DNA covalent structure are offset by enzymes of the body’s repair system (Van Houten et al., 2018; Shimizu, 2014). However, endogenous intoxication nourishes all necessary conditions for the “long-term” presence of structural changes in the genomic DNA that can affect the DNA functional activity and the implementation of genetic processes, primarily gene transcription (Sánchez-Tapia et al., 2020).
This paper presents the results of an experimental study of modifications that occur in genomic DNA of mononuclear cells in venous blood of patients with endogenous intoxication syndrome. The aim of the study is to investigate the DNA modifications during endotoxic “pollution” of the body and to understand the mechanisms of development of dysregulation processes mediated by structural changes in DNA.
MATERIAL AND METHODS
In laboratory studies were used samples of venous blood of 15 patients with endotoxicosis syndrome against the background of acute pancreatitis, after giving patient informed consent. The examined group: age – 48,3 ± 2,1 years, men – 7 (46,7%), women – 8 (53,3%). Control blood 10 samples were obtained from 15 to 60 years. Studies conducted in the period: January – May 2019.The rate of endogenous intoxication was assessed by determining the generally accepted laboratory-clinical and biochemical parameters (Karpishchenko, 2014).
To determine nuclease activity isolated genomic DNA was used as the substrate of the reaction. Hydrolysis was carried out in the presence of MgCl2, incubated at 37 ° C during 2 hours.The mononuclear cell fraction was collected by gradient centrifugation on Ficoll-Paque™ (ρ=1,077). The received ring of mononuclear cells was taken to clean Eppendorf-type test tubes and cleansed from Ficoll impurities by phosphate-saline buffer (PBS) and concentrated in Hanks’ solution. Cell viability was determined by the amount of penetration of stain into the cells (1% of trypan blue or 5% eosin solution).
The isolation of DNA from blood mononuclear cells was performed by using Laura-Lee Boodram technique (Boodram, 1999-2006). The cells were lysed with 10% of sodium dodecyl sulfate, then dispensed with 20% of proteinase K and incubated at 55ºC during 2 hours. Proteins were salted out with 5,3 M NaCl solution. To deposit DNA in the supernatant cold isopropanol was used. The DNA precipitate was washed with 70% ethanol and resuspended in 0,1 M Tris-HCl, pH 8.5.
UV-spectroscopic assays of DNA solutions (maximum absorption at λ260), quantitative determination of medium-mass molecules (λ280 and λ254 nm), diene conjugates in the extracted heptane-isopropanol mixture (1:1, by volume) of lipid fractions (λ232 and λ220 nm) were carried out using a spectrophotometer UV-3600 Shimadzu (Japan). The DNA precipitate was lyophilized by freeze-drying FreeZone Plus. The DNA preparation was mixed with KBr to form pills. The IR Fourier spectra of DNA preparations were recorded on IRPrestige-21 SHIMADZU spectrometer (Japan) in the range of 400 cm-1 – 4000 cm-1. The obtained findings were statistically processed by the method of variation statistics using the Student t-test.
RESULTS AND DISCUSSION
The experiments utilized patients’ blood with acute endotoxicosis, which is a complication of patients with acute pancreatitis, accompanied by significant changes in the biochemical and blood cell composition (Fig.1).
The content of the average mass molecules – oligopeptides with molecular masses in the range between 500 – 5000 Da (MFM: λ280 and λ254) in patients’ blood exceeded the normal values (249,2±19,08 and 296,4±22,17 relative units x 10-3, respectively) on average by 240% and 233% (p<0.05), which is a characteristic sign of the development of endogenous intoxication. In the blood of endogenously intoxicated patients, the content of lactic and pyruvic acids was increased by 252% and 169% compared to the control samples (0,25±0,022 and 0,035±0,003 mmol/g protein, respectively) (p<0,05). As it was shown above in the blood of patients with endogenous intoxication, there is a more significant increase in the content of lactic acid in comparison with increased concentration of pyruvic acid, which points to the development of toxic enzymopathy. Also, blood plasma of patients with endogenous intoxication, has a high activity of DNAse equal to 240,2±2,36 activity units/l.
One of the indicators of molecular instability in the body is a high level of lipid peroxidation. In patients with endogenous intoxication syndrome, the content of the primary products of lipid peroxidation – diene conjugates (DC) on average exceeded the normal values (0,45±0,01 rel.units/mg. of lipids) by 3,3 times, and TBA-reactive substances, which include malon dialdehyde, exceeded the control parameters (4,5±0,5 nmol/ml) by an average of 220% (p<0,05). To study the properties of genomic DNA, we utilized the blood of endogenously intoxicated patients whose core biochemical parameters (markers) were higher or lower than the corresponding normal values. By using spectral methods of analysis in IR and UV regions of the spectrum we studied structural modifications of genomic DNA (changes in chemical structure, conformations, packing density) in endogenous intoxication
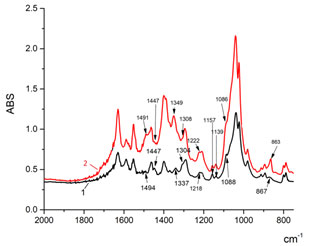
Analysis of absorption spectra of control and experimental samples in the IR region (Fig. 1) showed the presence of DNA-specific bands in the frequency range 2960 – 2850 cm-1(A), 1500 – 1250 cm-1 (B), and 1150 – 750 cm-1 (C), which are usually attributed to the bands caused by vibrations in the bonds of CH2-groups, bonds between the bases and sugars (the fluctuation region of the groups in the sugar-phosphate backbone of DNA) and symmetrical vibrations in the bonds of phosphate groups -PO2, respectively (Stuart 2004; Zhizhina and Oleynik, 1972). At the same time, the IR spectra of the genomic DNA in the control and experimental samples differed by the intensity of absorption of characteristic bands and the shift of absorption bands.
Figure 1: IR-spectra changes in DNA of donors’ blood (1) as opposed to patients with endogenous intoxication syndrome (2)
Of particularly note are changes in the IR-spectrum of the DNA blood of patients with the syndrome of endogenous intoxication in the areas 1500-1250 cm-1 and 1150-750 cm-1, as they may indicate rearrangements in the sugar phosphate backbone of the DNA, associated in particular with changes in efficiency of the ionic interactions between the functional groups of separate molecular fragments, which maintain a specific DNA conformation (Spirin, 1958; Taillander et al., 1985).
As it can be seen from Fig. 1, experimental samples, as opposed to control samples, in the range between 1500-1250 cm-1, have a decreased absorption intensity in the region 1447 cm-1 and 1308 cm-1, an increased intensity and shifting of the band at a frequency of 1337 cm-1, characterizing the vibrations of -CH2 group, the vibrations of deoxyribose-nitrogen base bonds, glycoside dihedral angles into the region of higher frequencies – 1349 cm-1. The absorption band of genomic DNA of patients with endogenous intoxication 1491 cm-1, reflecting the movements of purine atoms and the corresponding C=N guanine bond (Polyanichko et al., 2004; Eichhorn and Shin, 1968), is getting wider and has a more obvious character. In the region 1150-800 cm–1, where we have vibrations of the sugar-phosphate backbone, noticeable shifts of the absorption bands are not observed. At the same time, the absorption intensity caused by symmetric vibrations of the bonds in the group O=P=O at frequencies 1086 cm-1, 1157 cm-1 and 1222 cm-1 decreases, and increases at a frequency of 1139 cm-1 and the absorption intensity at a frequency of 863 cm-1, characteristic of n-type sugars increases significantly.
It is known that the spatial structure of DNA is polymorphic, that is, it can take different conformations, including three main types of structures – A-, B- and Z- forms. For each of these forms in the IR spectra there are so-called marker absorption bands, which can tell us about the geometry of the DNA macromolecule, the possible transitions between conformations, the stability of the structure (Zhizhina and Oleynik, 1972; Stuart, 2004; Taillandier and Liquier, 1992). Normally, the DNA isolated from the control samples is mainly represented by the B-form: absorption bands are observed at frequencies of 970, 896 and 837 cm−1, characteristic of DNA in the B-form (Spirin, 1958) and partially in the A-form – absorption band at a frequency of 863 cm-1 (Taillandier and Liquier, 1992; Tsuboi, 1969).
As it was shown above, in DNA, isolated from samples, the band shifts at a frequency of 1337 cm-1 (vibrations of methylene radicals) to the region of higher frequencies – 1349 cm-1, as well as there is a noticeable increase in the absorption intensity at a frequency of 863 cm-1, (absorption band regular of the type of twisting in n-type sugars -C3′-endo/anti, 870-865 cm-1, marker of A-form) (Stuart, 2004). In addition, the intensity of the 1086 cm-1 band related to the symmetric vibrations of the bonds of O=P=O backbone decreases (Spirin, 1958; Taillander et al., 1985). Such changes in the spectrum point to changes in the mutual orientation of the DNA phosphate groups, which is a consequence of the local unwinding of the double helix, the redistribution of hydrogen bonds between the nitrogen bases, the appearance of bends in it, resulting cumulatively in a change in the spatial structure of DNA. In genomic DNA, isolated from the blood of patients with endogenous intoxication syndrome, most likely, there is a relative increase in the proportion of DNA in the A-form. According to the literature, under physiological conditions (low salt concentration, high degree of hydration), the dominant structural type of DNA is a B-form. An A-form is formed under conditions of lesser hydration and a higher ion content. Apparently, during evident endotoxicosis, a complex of conditions is formed under which DNA modification occurs, consisting in structural changes and conformational rearrangements.
One of the methods of detecting changes in the structure of DNA molecules is the record of absorption spectra in the UV region. Firstly, by absorption ratio at wavelengths of 260 nm and 280 nm (λ260/λ280) one can estimate the purity of the DNA preparation. The preparation is considered pure if the ratio of 260 nm/280 nm is approximately 1,8 for DNA. Secondly, by changing the nature of absorption at a wavelength of 260 nm, one can judge the stability of the bonds between complementary pairs, ensuring the maintenance of the necessary DNA conformation (Doshi et al., 2009). Intensive absorption of nucleic acids in the UV region of the spectrum is caused by purine and pyrimidine bases. Absorption spectra of select bases merge into one wide band and give a characteristic absorption of DNA with a maximum absorption at λ= 260 nm.
Our studies have shown that the UV spectra of genomic DNA isolated from donors’ blood and patients with endogenous intoxication syndrome are different (Fig 2).
Figure 2: UV spectra of DNA from donors’ blood (1) against patients with the syndrome of endogenous intoxication (2)
Figure 2 shows that in the UV absorption spectra of DNA, the absorption ratio at wavelengths of 260 nm and 280 nm of control and experimental samples lies within 1,8, which indicates a sufficiently high purity of the isolated macromolecules. At the same time, in the DNA spectra of the experimental samples there is a relative displacement of the maximum and a slight hyperchromic effect.
Quantum-mechanical calculations show that the intense absorption of light by purine and pyrimidine bases at 260 nm is linked with p-p and to a certain extent with n-p transitions. The optical properties of chromophore groups depend on the conformation of DNA. The phenomenon associated with an increased optical density is called the hyperchromic effect, and vice versa, the reduced absorption of native DNA preparations – hypochromic effect. Such changes in the spectra may also be attributed to changes in the DNA structure of blood cells in patients with endogenous intoxication syndrome. We would like to emphasize that DNA spatial structure disorders can be the result of accumulation (penetration) of products, including those of toxic ones, in the nucleic acid microenvironment, formed because of impaired balance of biochemical reactions during intoxication. In its turn, changes in the DNA conformation can be the cause of disruption of genetic processes, including gene expression and, consequently, lead to the development of dysregulation processes in the body of patients.
During various inflammatory conditions, one of the undesirable stages of the disease is the development of endogenous intoxication syndrome and, consequently, the development of multi-organ failure.During endogenous intoxication, toxic endotoxins accumulate in the body, causing aggravation of the patient’s condition and the formation of this condition, that requires timely diagnosis and immediate repair. According to the obtained findings during the development of endogenous intoxication syndrome in patients’ blood cells there occur changes in the structure of genomic DNA. The changes in the genomic DNA spatial structure in the syndrome of endogenous intoxication can also be revealed through changes in the UV spectra of DNA samples, observed as a slight shift of the maximum and a small hyperchromic effect. The observed changes in the UV spectrum of the DNA medicine isolated from the blood of patients with endogenous intoxication syndrome are usually attributed to the binding of metal ions to the DNA molecule at N7 guanine position (Kasyanenko et al., 1989; Koos et al., 2020). Such binding leads to destabilization of hydrogen bonds between complementary pairs, and with a significant number of such binding sites, there is a disruption of base-stacking, accompanied by a hyperchromic effect in the DNA absorption spectrum (Kasyanenko et al., 2014; Sánchez-Tapia et al., 2020).
Several papers have shown (Doshi et al., 2009; Khan Asia et al., 2006; Doshi et al., 2010) that the saturation of DNA solutions with oxygen leads to an increased absorption with a maximum at λ260. Such hyperchromism is attributed to one-electron oxidation of DNA by singlet oxygen (Baeuerle, 2020; Kanvah et al., 2009) and the formation of its complex with superoxide anion, DNA+•O2–, by a product of singlet oxygen reduction.It is known that singlet molecular oxygen initiates some biological processes that lead to oxidative stress, accompanied by hyperproduction of reactive oxygen species, which can damage nucleic acid molecules. DNA is one of the main targets for reactive oxygen species, which have genotoxicity and lead to mutations. Modification of DNA effectuated by singlet oxygen is expressed almost exclusively as oxidation of purines. Whereas OH-radical, effectively interacting with deoxyribose, purine and pyrimidine bases leads to more serious changes in the DNA structure. Oxidized purines and pyrimidines are products of the induced damages that are very common in DNA under oxidative stress (Kanvah et al., 2009; Kim et al., 2020).
It was noted above that during endogenous intoxication syndrome, the concentration of lipid peroxidation products (LPP) increases in patients’ blood, and accumulation of acids creates prerequisites for reducing the pH values. The initiators of LPO are reactive oxygen intermediates. In particular, singlet oxygen can be formed following the interaction of hydroxyl radicals with superoxide anion radicals, which in turn can be formed as a response to high concentrations of ions, for instance K+, and contribute to the development of a vicious circle of free-radical modification of biomolecules, cells and tissues damage.During lipid peroxidation, a variety of cytotoxic products accumulates, including aldehydes. In particular, malondialdehyde (MDA) is formed as a result of peroxidation of fatty acids containing three or more double bonds (linolenic and arachidonic acids, respectively). The reaction of MDA with primary amines leads to the formation of Schiff bases. MDA can bind to the DNA nitrogenous bases, leading to a cross-linking of DNA chains, therefore MDA is assigned to the role of a mutagenic, genotoxic, and carcinogenic compound (Freeman and Crapo, 1984Uzbekov, 2014; Guevara‐Cruz et al. 2019 ).
Earlier we have shown that under conditions of increased activity of lipid peroxidation, cells respond by modification of chromatin lipids, coupled with changes in the amount of separate lipids and degree of their oxidation (Trofimov et al., 2013 Sánchez-Tapia et al., 2020). Therefore, on the basis of research findings and data of scientific literature it can be assumed that in severe forms of inflammatory diseases accompanied by endogenous intoxication, hyperproduction of reactive oxygen species, intensification of free radical processes, including lipid peroxidation, impairs the barrier and regulatory properties of the nucleus membranes, resulting in penetration of excess ions and other strange to karyoplasm substances penetrate karyoplasm, local changes in pH of the medium and in degree of hydration (Kim et al., 2020).
The interaction of reactive oxygen species with DNA, most likely and primarily with the nitrogen guanine bases, leads to modification of the macromolecule structure and conformational rearrangements: as evidenced by the change in the vibrations of methylene groups, vibrations in the sugar-base bond groups, changes in glycosidic dihedral angles (band shift at a frequency of 1337 cm-1 into the region of higher frequencies – 1349 cm-1), changes in vibrations of the C=N guanine bond (shift and increase in the absorption band at 1491 cm-1, reflecting the movements of purine atoms) (Polyanichko et al., 2004; Eichhorn and Shin, 1968). These changes to a certain degree are provoked by interaction of lipid peroxidation products with nitrogen bases (Baeuerle, 2020).
CONCLUSION
The paper discusses the analysis of improvements in the biochemical composition and structure of the genomic DNA of venous blood mononuclear cells in patients with endogenous poisoning. The FT-IR genomic DNA spectra of the venous blood mononuclear cells of patients show that the band shifts to a higher frequency at a frequency of 1337 cm-1, characterizing the fluctuations of the CH2 group and sugar-base bonds. The absorption band at 1491 cm-1, reflecting the movement of purine atoms and corresponding to –C=N guanine bond, becomes wider and has a more pronounced character. The absorption intensity increases at a frequency of 863 cm-1, characteristic of the type of twisting in N-type sugars. The intensity of the band 1086 cm-1, capturing the symmetric vibrations of the O=P=O stroma bonds decreases.
The related spectrum changes reflect changes in the reciprocal orientation of the phosphate groups of DNAs arising from the topical untwisting of the double helix, the redistribution of the hydrogen bonds, the presence of bends on the helix resulting in changes in the spatial structure of DNA, and the increased proportion of DNA in the A-form. The DNA UV spectra of the experimental samples demonstrated a slight maximum shift and a minor hyperchromic effect. The observed changes in the spatial structure of DNA may be due to the accumulation (penetration) of microenvironmental DNA materials, including toxic ones, resulting from impaired biochemical reaction equilibrium during intoxication. In turn, variations in DNA conformation may be the cause of genetic process disorders, including gene expression disorders, leading to the creation of dysregulation processes in the bodies of patients.
REFERENCES
Baeuerle EC (2020). Evaluation of Microbiome-Targeting Interventions on Metabolic Endotoxemia and Insulin Sensitivity in Obese Humans (Doctoral dissertation, The University of Texas Health Science Center at San Antonio).
Boodram LL (2006). Extraction of genomic DNA from whole blood. Your Lab’s Reference Book – online database of research protocols in a variety of life science fields [Electronic resource]. URL: http://www.protocol-online.org/prot/Protocols/Extraction-of-genomic-DNA-from-whole-blood-3171.html.
Doshi R, Day PJR, Carampin P, Blanch E, Statford IJ, Tirelli N. (2010). Spectrophotometric analysis of nucleic acids: oxygenation-dependant hyperchromism of DNA. Anal Bioanal Chem 396: 2331-9.
Doshi R, Day PJR, Tirelli N (2009). Dissolved oxygen alteration of the spectrophotometric analysis and quantication of nucleic acid solutions. Biochemical Society Transactions; 37: 466-70.
Eichhorn GL, Shin YA (1968). Interaction of metal ions with polynucleotides and related compounds. XII. The relative effect of various metal ions on DNA helicity. J Am Chem Soc; 90: 7323–8.
Freeman BA, Crapo JD. (1984). Biology of disease: Free radicals and tissue injury. Adv. Biol. Disease. 1: 26–40.
Guevara‐Cruz M, Flores‐López AG, Aguilar‐López, M, Sánchez‐Tapia M, Medina‐Vera I, Díaz D, & Torres N (2019). Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. Journal of the American Heart Association, 8(17), e012401.
Halliwell B, Chirico S (1993). Lipid peroxidation: its mechanism, measurement, and significance. Amer J Clin Nutr; 57: 715-25.
Kanvah S, Joseph J, Schuster GB, Barnett RN, Cleveland CL, Landman U. (2009). Oxidation of DNA: damage to nucleobases. Accounts of Chemical Research; 43: 280-7.
Karpishchenko AI (2014). Medical laboratory diagnostics: softwares and algorithms: guidelines for doctors. 3rd edition. Moscow: GEOTAR-Media Publ;.
Kasyanenko NA, Diakonova NE, Frisman EV (1989). Study of the DNA molecule conformation in its interaction with divalent ions in solution. Molecular biology; 23: 835–41.
Kasyanenko NA, Varshavskyi MS, Zhang Zusi, Alekseev GV, Bakulev VM (2014). Revisiting metallization of DNA in solution. Bulletin of Saint Petersburg State University; 1(59): 498-507.
Khan A, Khan F, Siddiqu AA, Ali R (2006). Increasing the immunogenicity of plasmid DNA under the action of singlet oxygen. Biochemistry; 71: 1074-82.
Kim BS, Tilstam, PV, Arnke K, Leng L, Ruhl T, Piecychna M, & Lindenblatt N (2020). Differential regulation of macrophage activation by the MIF cytokine superfamily members MIF and MIF‐2 in adipose tissue during endotoxemia. The FASEB Journal, 34(3), 4219-4233.
Koos B, Moderegger EL, Rump K, Nowak H, Willemsen K, Holtkamp C, & Rahmel T (2020). LPS-Induced Endotoxemia Evokes Epigenetic Alterations in Mitochondrial DNA That Impacts Inflammatory Response. Cells, 9(10), 2282.
Polyanichko AM, Andrushchenko VV, Chikhirzhina EV, Vorob’ev VI, Wieser H (2004). The effect of manganese (II) on DANN structure: electronic and vibrational circular dichroism studies. Nuc Ac Res; 32: 989–96.
Savelyev VS, Filimonov MI, Burnevich SZ (2008). Pancreatonecrosis. Medical inform agency Publ; 258.
Shimizu I, Yoshida Y, Suda M, Minamino T (2014). DNA Damage Response and Metabolic Disease. Cell Metabolism; 20: 967-77.
Spirin AS (1958). Spectrophotometric determination of total nucleic acids. Biochemistry; 23: 657– 62.
Stuart BH. Infrared Spectroscopy: Fundamentals and Applications. Wiley 2004; 242.
Sánchez-Tapia M, Miller AW, Granados-Portillo O, Tovar AR, & Torres N (2020). The development of metabolic endotoxemia is dependent on the type of sweetener and the presence of saturated fat in the diet. Gut microbes, 12(1), 1801301.
Taillander E, Liquier J, Taboury JA (1985). Infrared spectral studies of DNA conformations. Advances in Infrared and Raman Spectroscopy; 12: 65–114.
Taillandier E, Liquier J (1992). IR spectroscopy of DNA. Method in Enzymology; 11: 307-35.
Trofimov VA, Aksenova ON, Dudko AA, Lopukhova EN, Trofimov AV (2013). Free radical lipid peroxidation in the nuclei of mouse liver cells under normal conditions and after partial hepatectomy. Bulletin of Experimental Biology and Medicine; 155: 436-8.
Tsuboi M (1969). Application of infrared spectroscopy to structural studies of nucleic acids. Appl Spectrosc; 3: 45–90.
Uzbekov MG. Lipid peroxidation and antioxidant systems in mental diseases. Social and clinical psychiatry 2014; 24: 97–103.
Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology; 39: 44–84.
Van Houten B, Santa-Gonzalez GA, Camargo M. (2018). This review comes from a themed issue on Oxidative Toxicology: Role of ROS. Current Opinion in Toxicology; 7: 9–16.
Vlasov AP, Trofimov VA, Krylov VG (2009). Systemic lipid distress syndrome in surgery. Nauka Publ; 224.
Zhizhina GP, Oleynik EF (1972). IR spectroscopy of nucleic acids. Chemistry advances – Uspekhi khimii; 41: 475-511.