Department of Molecular and Cellular Engineering, Jacob Institute of Biotechnology and Bioengineering, Sam Higginbottom University of Agriculture, Technology & Sciences, (SHUATS), Allahabad India
Corresponding author Email: btandon7@gmail.com
Article Publishing History
Received: 18/03/2018
Accepted After Revision: 25/04/2018
Biofuels are important source of renewable chemical energy, more precisely those that are available in liquid form. Algal biofuel offers the advantage of being a low-emission fuel. Algal biofuel offers a clean source of renewable energy. It burns without smoke and can be easily cultured in areas which are unsuitable for agriculture due to low production and installation costs. It releases only carbon dioxide and water on burning and carbon dioxide is one that is fixed from atmosphere during photosynthesis. In the present work, five algae samples were collected from different sites. According to the maximum growth of algae in BG11 media, it was selected for mass culture of potentially growing samples. They were kept for two weeks and optimized through various confirmatory tests. The flame test and transesterification test showed positive results for all five samples. These samples were mass cultured in pond and oil was extracted using Soxhlet extractor with ethanol as solvent. The oil was recovered and transesterification was performed to convert it to biodiesel.
Biodiesel, Biofuel, Algae, Culture, Bg11, Bolds Basal, Bristol, Soxhlet
Soni R, Henry S, Tandon B. The Potential of Certain Algal Species as Source of Biodiesel. Biosc.Biotech.Res.Comm. 2018;11(2).
Soni R, Henry S, Tandon B. The Potential of Certain Algal Species as Source of Biodiesel. Biosc.Biotech.Res.Comm. 2018;11(2). Available from: https://bit.ly/2TK9ukD
Introduction
Algae are a diverse group of eukaryotic organisms that belong to the phylum Protista. These organisms use light energy to convert CO2 and H2O into carbohydrates and other cellular products. During this process, oxygen released. Algae are found anywhere there is water, fresh water, salt water, and in the soil (Brown, 1969). Macro-algae are defined as the multicellular plants also known as seaweeds found in salt or fresh water (Kharkwal, 2012) whereas one of the miniscule plants known as Micro-algae contributes in the production of around 60 percent of the earth’s oxygen. Such organisms comprise of twenty five to thirty thousand species representing a range of forms and sizes that can exist from unicellular microscopic organism (microalgae) to multicellular large size (macroalgae) (Ugoala, 2012). Due to the fact that the oceans cover 70% of the earth’s surface, aquatic algae are major producer of oxygen and important users of carbon dioxide. All algae are primarily made up of proteins, carbohydrates, fats and nucleic acids in varying proportions. While the percentage can vary with the type of algae, some types of algae are made up of up to 40% fatty acids based on their overall mass. It is this fatty acid that can be extracted and converted into biofuel. Due to the high lipid content, algal strains are of great interest in the search for sustainable sources for the production of biodiesel. Reports proclaim that Macro-algae is composed of more than 2400 organic and chemical free products with a great commercial value in industries such as pharmaceutical, biomedical, neutraceutical, etc. (Saranya, 2013).
Fuel derived from plants, animals or algal resources can not only bring an initiative to preserve a healthful global environment but also shall prove to be a substitute in order to reduce our dependency on fossil fuels. High lipid content, ease of cultivation, cost effective and rapid growth rate is the factors that make microalgae to become a desired candidate for biofuel production (Alam, 2015). Over the past decade there have been plenty of advancements in algal technology for biofuel production. Algae is considered as a traditional food or feed and can be cultured in huge open ponds or closed photo bioreactors placed on non-arable land. Certain algal species have a high potential as the oil-producer as compared to oil crops. It can be isolated from various carbon (CO2) sources and further processed in a wide spectrum of products like biodiesel, gasoline replacements, green diesel, methane, bioethanol, heat, high protein animal feed, bio-oil, etc. (Yang, 2016).
 |
Figure 1: Different sites from where algal samples were collected |
 |
Figure 2: Steps involved in biodiesel production from algal biomass |
In the future of transport sector various biofuels, including bio-ethanol, -methanol, -diesel- and –hydrogen appear to be appealing options. Therefore, it is essential to look for novel feedstock sources which ar suitable for biofuel production and does not withdraw the supply of edible feedstock. Algae can be an alternative to the conventional crop. Algae or cyanobacteria based third generation technology contains an elevated oil mass fraction grown in ponds (Suganya, 2016). It is a favourable source of third generation renewable fuels. However, the procedure of biofuel production from the growth of microalgae till the last step is still not contemplated economically viable (Laamanen, 2016). Microalgae have various autotrophic capabilities which add an element of flexibility as compared to conventional fuels. They are also supported by fundamental nutrients which capture solar energy to fix the amount of carbon dioxide and split water (Hallenbeck, 2016). The production of biodiesel using algae demands the conversion of algal biomass through transesterfication (Kandiyoti, 2017). It is a process in which lipids are simultaneously separated and trans esterified from microalgae cell walls and trans esterification is generally used for heterotrophic microalgae (Veillette, 2017). Further research is needed to discover the most energy efficient, cost effective and high yield extraction process to amplify the economic viability of global algae biofuels sector. This will address all the crucial technical trials which affect the production processes involved. Hence, these aforementioned research when incorporated with government emission policies will fast-track the date when algal biofuel production can be a commercial enterprise. In the field of science and research to succeed fossil fuels, algae feedstock has appeared as a suitable candidate not only for its renewable and sustainable characteristics but also for its economic reliability based on the prospective to match up with the global demand for transportation fuels (Adeniyi, 2018).
In the present study, fresh water algae were collected from 5 below mentioned sites and denoted as R1, R2, R3, R4 and R5. (Orchid, SHIATS R1, Agriculture Department, SHIATS: R2, Forestry Department, SHIATS:R3, Arail Ghat, Allahabad:R4 and Saraswati Ghat, Allahabad: R5).
Algae culture was carried out in tissue culture bottles with 3 different growth media and one was in distilled water as control. 2 gm of algae was inoculated in each media and further in open land area in polybags, these media were following-Bolds Basal Medium (BBM) (Nichols and Bold, 1965), Bristol Medium, (Bold, 1949) and BG11 medium (Stanier, 1971). Oil extraction from algae using Soxhlet apparatus was performed as per the method of Franz von Soxhlet, (1879). The algae was dried by exposure to hot air oven at 60 0C for 1 hour. After complete drying, the algae were blended to get powder. A 25 gm sample of the dried algae was placed in the thimble, which was loaded in the main chamber of Soxhlet extractor. The Soxhlet extractor was placed onto a flask containing extraction solvent i.e. 95% ethanol. The solvent was heated to reflux, so it forms vapors, which travels up a distillation arm, and floods into the chamber housing the thimble of algae powder. Some of the desired compound will then dissolve in the warm ethanol. When the Soxhlet chamber was almost full, the chamber was automatically emptied by the siphon side arm, with ethanol running back to the distillation flask. This cycle was repeated for three to four times. During each cycle, a portion of the oil is dissolved in ethanol.
Confirmatory Tests for Biodiesel:-Transesterification is the most commonly used method for Biodiesel oil production from algae. Harvested biomass from these algal species was dried, ground and oil was extracted by Soxhlet extractor using ethanol as a solvent. The extracted oil was trans esterified to biodiesel using sodium methoxide as a catalyst.1ml of extracted algal oil was taken with 3ml of methanol and 0.075 g of NaOH and it was shaken vigorously and then kept in a shaker for overnight. Two layers were observed the next day in which the lower layer was glycerol and the upper layer was biodiesel. The upper layer i.e. biodiesel was extracted for further experiments.
Ideally, there were two distinct layers: an amber (ranging from very light to very dark depending on the oil used) biodiesel layer on top and a darker glycerol layer on the bottom (usually contaminated with catalyst, alcohol, or dust particulates). Sometimes, there will be a third or fourth layer between the glycerol and the biodiesel. These layers are soap from too much catalyst or water and often appear milky or yellowish. The property of a biodiesel is to be highly flammable when in contact of fire. So for the flame test of biodiesel we lit the matchstick in front of the upper layer of solution which was taken out by the process of transesterification.
Growth rate measurement: The tissue culture bottles were kept in open area where proper sun-light and aeration was available for the suitable growth of algal sample as shown in figure 3 and 4.
 |
Figure 3: Algal growth of R3 |
 |
Figure 4: Growth of algal sample in first week of inoculation |
 |
Figure 5 |
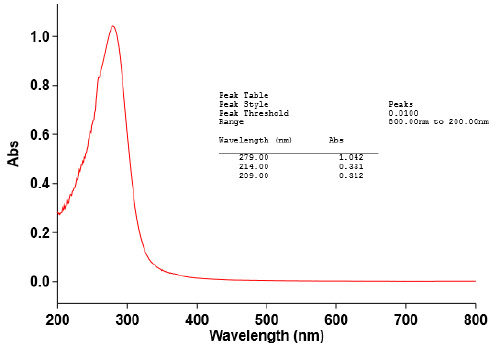 |
Figure 5a |
 |
Figure 6: Flame Test |
In all these above 4 growth medium BBM, Bristol, BG11 including one control (distilled water) BG11 media showed maximum algal growth, so it was taken for the mass culture of algal sample in open ground.
Identification: This process was carried out at department of biological sciences, SHIATS, Allahabad, using a compound microscope for analysis of temporary slides of Algae samples collected from different sites. Different Algae species were identified by observing their unique reproductive structures. The table 2 shows the results of identification of algae samples showing the type of species which constitute every sample.
| Table 2: List of the species present in the algal samples | |
| Samples | Present species |
| R-1 | Microcystis |
| R-2 | Vaucheria
Microcystis |
| R-3 | Microcystis |
| R-4 | Vaucheria
Microcystis Nostoc |
| R-5 | Microcystis |
After the transesterification process, a separate layer of biodiesel was obtained from the extracted algal oil. The upper layer contained biodiesel which was carefully extracted with pipette in a separate flask, and the lower layer was glycerol so it was discarded.
As Biodiesel has the highly inflammable property, it caught fire rapidly when a matchstick was passed over it.In the current study, 5 algae samples were used to extract oil and it was converted to biodiesel. The study revealed that algae are fast growing and effective organism for biodiesel production as these can be grown in normal water as well as in artificial media. Oil extracted from harvested biomass of these algae was trans esterified to biodiesel using sodium methoxide as a catalyst. It was found that properties of biodiesel so it can be blended with fossil fuels or can be used individually as an alternative of fossil fuels.
References
Adeniyi, Oladapo, Azimov, Ulugbek and Burluka, Alexey (2018) Algae biofuel: Current status and future applications. Renewable and Sustainable Energy Reviews, 90. pp. 316-335. ISSN 1364-0321
Alam F, Mobin S, Chowdhury H. (2015) Third generation biofuel from algae. Procedia Eng 2015; 105:763–8
Becker, E.W. (1994) Microalgae: biotechnology and microbiology. New York: Cambridge University Press; 181-182. Biotechnology: 235-240.
Brown, A., Knights, B. and Conway, E. (1969) Hydrocarbon content and its relationship to physiological state in the green algae Botryococcus braunii. Phytochem 8:543-547.
Chisty, Y. (2007) Biodiesel from microalgae. Biotechnology Advance 25:294-306.
Chisty, Y.(2007) Chlorella Isolated from Soil in the Algerian Sahara, International Journal Biotechnology Hydrogen Energy 4941-4946.
Douche, J, Syraka, F. and Livansky, (2005) Utilization of fuel gas for cultivation of micro algae in an outdoor open thin-layer photobio reactor. J. Appl Phycol 17:403.
Douskova, I., Kastanek, F., Maleterova, Y., Kastanek, P., Doucha, J., and Zachleder, V. (2010) utilization of distillery stillage for energy generation and concurrent production of valuable microalgal biomass in the sequence: biogas cogeneration-microalgae-products. Energy convers Manage 51:606-611.
Dukes, J.S. (2003) Burning buried sunshine: human consumption of ancient solar energy. Clim Change 61:31-41.
Fargione, J., Hill, J., Tilman, D., Polasky, S. and Hawthrone, P. (2008) Land clearing and the biofuel carbon debt. Science 319:1235-1238.
Folch, J., Lees,M., Stanley, G.H. (1957) A simple method for the isolation and purification of total lipids from animals tissues, Journal of Biological Chemistry:497-509.
Grima, M.E, Belarbi, E., Fernandez, F.A., Medina, A.R., and Chisti, Y. (2003) Recovery of algal biomass and metabolites: process options and economics. Biotechnol Adv 20:491-515.
Hallenbeck PC, Grogger M, Mraz M, Veverka D. (2016) Solar biofuels production with microalgae. Appl Energy 2016; 179:136–45 Vol-5 ISSN – 0974-2441
Harsh Kharkwal, DD Joshi, Preeti Panthari, Manish Kant Pant, Amit C Kharkwal (2012) Algae as future drugs. Academic Sciences Asian journal of pharmaceutical and clinical research
Johnson, M.B., and Wen, Z. (2009) Production of biodiesel fuel from microalgae Schichytrium limacinum by direct transesterification of algal biomass. Energy fuels 23:5179-5183.
Kim, S., and Dale, B., (2005) Global potential bioethanol production from wasted crops and crop residues. Biomass bioenergy 26:361-375.
Liu, J., Huang, J., Sun, Z., Zhong, Y., Jiang, Y., and Chen F. (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 120:106-110.
Laamanen CA, Ross GM, Scott JA. (2016) Flotation harvesting of microalgae. Renew Sustain Energy Rev 2016; 58:75–86
Muffler,K.and Ulber,R. (2005) Downstream processing in marine biotechnology.Adv biochem Eng biotechnology.
Olaizola, M. (2003) Phototrophic: efficient alternatives to land based crops for biofuels. Current opinion in eng: 459-466.
Pinto, M. M., Raposo M.F., Bowen,J., Young,A.J., and Min orais, R. (2001) Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvial: effect on astaxanth recovery and implication for bio-availability. J Appl Phycol 13:18-24
Rebolloso, F., Navaro, P., Garcia, C., Ramos, M. and Guil, G. (2001) Optimisation of Biodiesel Production by Sunflower oil transesterification’. J.Agric. Food. Chem: 2966-2972.
Sheehan, J., Cambreco, Duffield, J., Graboski, M., and Shapouri, H. (1998). An overview of biodiesel and petroleum diesel life cycles. US Department of agriculture and Energy Report; 1-35.
Stephenson, A. L., Kazamia, E Dennis, J. S. Hawe, C. J. Scott, S. A. and smith (2010) Lifecycle assessment potential algal biodiesel production in the United Kingdom: a comparison of raceways and airlift tubular bioreactor. Energy flues 24:4062-4077.
Saranya C, Girija K, (2013) Estimation of major pigment content in seaweeds collected from Pondicherry coast, The Experiment, 9(1), 2013, 522-525.
Suganya T, Varman M, Masjuki HH, Renganathan S. (2016) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sustain Energy Rev 2016; 55:909–41.
Ugoala, Emeka, Ndukwe G I, Mustapha K B and Ayo R I (2012) Constraints to large scale algae biomass production and utilization. J. Algal Biomass Utln. 2012, 3 (2): 14- 32
Veillette M, Giroir-Fendler A, Faucheux N, Heitz M. (2018) Biodiesel from microalgae lipids: from inorganic carbon to energy production. Biofuels 2018; 9:175–202
Wijffels, R.H. (2008) Advances in biochemical engineering/biotechnology. Trend. Biotechnol: 26-30.
Williams, P. R. D., Inman, D., Aden, A. Anheath, G. A. (2009) Environmental and sustainability factor associated with next generation befoul in U.S. Environment Science Technol.43:4763-4775.
Yang C. Hua, Q. and Shimizu, K. (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87-102.
Yuanjun Li (2011). Inexpensive culturing of freshwater algae in a simulated warm environment using chicken manure medium. Chalmers University of Technology (Master’s thesis).
Yang C, Li R, Cui C, Liu S, Qiu Q, Ding Y, et al. (2016) Catalytic hydroprocessing of microalgae-derived biofuels: a review. Green Chem 2016; 18:3684–99.


