Department of Biological Science, Faculty of Science, King Abdulaziz University, P, O, Box 80203, Jeddah 21589, Kingdom of Saudi Arabia
Corresponding author email: boaljohny@kau.edu.sa
Article Publishing History
Received: 17/10/2019
Accepted After Revision: 27/11/2019
Endophytic bacteria are plant associated bacteria living inside the plant tissues symbiotically with damaging the plant tissues. The endophytic bacteria play very important role in plant growth and development by adding variety of metabolites such as phytohormones, antimicrobial compound and other nutrients. In the present study, endophytic bacterium was isolated from Calotropis plant and was identified as Bacillus sp. IU103 through 16-sDNA sequencing. The IU103 was analyzed for indole-3-acetic acid through spectrophotometer. Application of IU103 showed that inoculation significantly improved plant growth attributes e.g., root and shoot length, biomass and chlorophyll contents. In addition, IU103 showed antibacterial and antifungal activities. All these characteristics revealed that IU103 has shown some plant growth promoting characteristic and can be used as plant growth promoting bacteria in agriculture.
Endophytic bacteria, Indole-3-acetic acid, antibacterial activity, antifungal activity
Al-Johny B. O. Synergetic Role of Endophytic Bacteria in Promoting Plant Growth and Exhibiting Antimicrobial Activities. Biosc.Biotech.Res.Comm. 2019;12(4).
Al-Johny B. O. Synergetic Role of Endophytic Bacteria in Promoting Plant Growth and Exhibiting Antimicrobial Activities. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/3392uAU
Copyright © Al-Johny This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Bacteria associated with plants are called endophytes and they are very helpful to the plants in the term of plant growth promotion and survival under stress to the host plants. Bacterial plant endophytes are existing in a broad species and various of plant organs (Jeger and Spence, 2001; Bharti et al., 2016), without any phenotype changes of plant or decrease of crop yield (Sturz et al., 2000). Numerous studies have described that endophytic bacteria can apply for agricultural purposes with following activities; plant growth promoting (Saharan, 2011), nutrients mobilizing such as nitrogen and phosphorus (Sharma et al., 2013), providing plant hormone such as auxin and gibberellin (Hardoim et al., 2008) and protect the diseases caused by soil-borne pathogens (Berg and Hallmann, 2006). In addition, endophytes produce numerous distinctive and useful secondary metabolites.Phytohormones are plant growth regulators that conduct signals to control cell division and therefore contribute growth promotion and development of plant (El-Tarabily and Sivasithamparam, 2006). Moreover, they also modulate plant responses to environmental changes, allowing the plant to tolerate environmental stresses (Gouda et al., 2018). Present research have established that certain endophytic microbes, including bacteria, enhance growth of the plants by pouring phytohormones e.g., cytokinins, indole-3-acetic acid (IAA) and gibberellins (Gouda et al., 2018). Bacteria in the genera Microbacterium, Agromyces, Bacillus, Paenibacillus, and Methylophaga are reported to produce IAA (Bharti et al., 2016). IAA produced by bacteria, present in the rhizosphere enhances root growth and nutrient availability because of the increased area of fertile soil that is occupied, resulting augmented biomass of the plants resistance against diseases (Ji et al., 2019).
Endophytic microbes including bacteria are exploited extensively for the production of novel biologically active compounds such as insecticidal, cytotoxic, and antimicrobial compounds (Kim et al., 2012). The antimicrobial activity of the endophytic bacteria are very important and therefore, these characteristics of the endophytic bacteria have very significant role in medicine and agriculture. The secondary metabolites, secreted by bacteria have important application in agriculture (Gouda et al., 2018).
In the present study, we have isolated five endophytic bacteria from Calotropis plant. The isolates were screened for idole-3-acetic acid (IAA) production. Further one strain producing high concentration of IAA was selected for further study. Antimicrobial activities of the selected isolate was tested against fungi and bacteria such as Salmonella enteritidis, Bacillus cereus, Staphylococcus aureus, Escherichia coli. The overall the endophytic isolate was characterized for IAA production, plant growth promotion, and antimicrobial activity. However, endophytic bacteria should be explored further for their potential benefits in agriculture (Muthukrishnan et al., 2015). Further standardized testing and formulation is needed to discover new endophytes.
MATERIALS AND METHODS
Isolation of bacterial endophytes: Calotropis plant were collected and cut off into small pieces and all the pieces were washed for 10 minutes in running tap water to remove the debris, soil particles. After washing, with tape water, the samples were washed with deionized water and out on sterilized tissue paper. Furthermore, plant samples were dipped in 75% ethanol for 2 minutes followed by dipping in 1% of sodium hypochlorite (NaClO) for 1 minutes again. The samples were finally rinsed with deionized water for 5 times. Eventually the samples were incised through aseptic surgical blade and were placed on LB agar plates. The plants were incubated at 37°C to get the colonies bacteria.
Growth conditions of bacterial culture
In order to grow the endophytic bacteria, LB broth was made containing 10 g yeast extract (difco) 10 g/L; NaCl 10 g/L, peptone, 5 g/L and difco agar 15 g/L. The pH 7was adjusted using 0.01M HCl and/or 0.01M NaOH and the media was autoclaved. The autovalve medium was poured into plants and let the media solidified. The plants were incubated at 37°C for overnight to check the contamination. Eventually the samples were incised through aseptic surgical blade and were placed on LB agar plates. The plates were incubated at 37C for 48 h and visible bacterial colonies were detected.
Calorimetric analysis of bacterial IAA
A test tube of 5 mL LB broth, supplemented with 0.2 g/L of l-tryptophan (Trp) was inoculated with isolates. The culture media was incubated at 30°C on shaker 200 rpm for 72 h. after 72 h, the centrifugation for 12 min was carried out at 10,000 ×g for and supernatant were obtained. One milliliter of culture supernatant and 2 mL Salkowski’s reagent (Ullah et al., 2013) were mixed and were incubated at room temperature for 30 min in dark. Addition of Salkowski’s reagent and incubation of supernatant generated red color which was measured by spectrophotometer at 535 nm. The concentration of indole-3-acetic acid was estimated in comparison with standard curve made up of IAA (Sigma-Aldrich).
Antibacterial activity fungal Endophytes
The isolate was subjected for antibacterial activity using disc fission dual culture technique. In the first step, pathogenic bacterial suspension (48 h old) was poured on LB agar plate. The isolate, from the culture plate was picked and stabbed on LB plate having pathogenic bacteria. The plate was incubated for 48 h at 37°C. Calculation of the antibacterial activity done by measuring the inhibition zone, produced by endophytic bacteria against pathogenic bacteria.
Antifungal activity endophytes
The isolate was subjected for antifungal activity using disc fission dual culture technique. In the first step, pathogenic fungi was grown on potato dextrose agar plates. After 48 h of incubation at 32, the isolate, from the culture plate was picked and stabbed on BDA plate having pathogenic fungi. The plate was incubated for 72 h at 32°C. Calculation of the antibacterial activity done by measuring the inhibition zone, produced by endophytic bacteria against pathogenic fungi.
Extraction of DNA for PCR analysis
Bacterial cultured grown at 37C for 24 h was used extraction of genomic DNA. The culture was then centrifuged at 10,000 rpm and pellet was used added within 1 mL of extraction buffer containing; 50 mM Tris-HCl (pH 8.0), 50 mM EDTA, 2% cetrimide, 1% SDS, 0.7 M NaCl, and 50 µL β-mercaptoethanol. The mixture was incubated at 65°C for 1 h and then mixed with equal volume of chloroform:isoamyl alcohol (24:1). The DNA was precipitated using isopropanol and pellet was washed with 70% ethanol pellet was suspended with 100 µL TE buffer. The polymerase chain reaction (PCR) was used to amplify the DNA through universal primers targeting the 16S rDNA, 27F (5′-AGA GTT TGA TC(AC) TGG CTC AG-3′) and 1492R (5′-CGG (CT)TA CCT TGT TAC GAC TT-3′). The PCR was run with conditions: 98°C; 5 min, 97°C; 1 min, 54°C; 30 s, 72°; 1 min, and a final 72°C for 5 min and total 30 cycles were run.
Plant growth promoting assessment of bacteria
Effects of bacterial endophyte on Brassica plants was determined to estimate the value of bacterial endophytes plant growth promotion. Brassica seeds were dipped in 75% ethanol for 2 minutes followed by dipping in 1% of sodium hypochlorite (NaClO) for 1 minutes again. The seeds were finally rinsed with deionized water for 5 times and the placed on wet autoclaved filter paper on the petri-plates. The seed were kept in moist by adding distilled water after interval of 12 h. the germinated seeds planted in pots filled with autoclaved soil. The pots were placed at 28°C in controlled environment of temperature and humidity (60% relative humidity) in growth chamber. The alternate day night duration was 8 h/16 h respectively with 1600 lx of light for plant growth. Bacterial isolate was cultured in LB broth and was incubated 72 h on shaking incubator at 37°C (160 rpm). The culture was centrifuged at 10,000 rpm at 4°C for 5 min and cell pellets were collected and then suspended in 0.8% saline water (sterile). Plants were inoculated with various concentration of Cd from 0 to 30 mg/mL with concentration difference of 10 mg/mL at the time of seeding.
RESULTS DISCUSSION
Bacteria isolation
Total five entophytes were purified from the mixed culture. The isolates were primarily purified by picking a single colony from mixed culture and streaking on fresh LB agar plate. Furthermore, the isolates were identified through their morphological characteristics, e.g., color, structure, texture, shape and size of the colonies. The isolates were encoded as IU100, IU102, IU103, IU104 and IU105. All the five isolates were obtained from plant were screen for IAA production through Salkowski’s test.
Characterization of IAA production potential
Production of IAA was assessed in all the five bacterial isolates through Salkowski’s test. Results of all the five isolates were uneven and color production (indication) of presence of IAA was week in four isolates. The color development in four e.g., IU100, IU102, IU104 and IU105 was not encouraging even after 30 min of incubation in dark. However, IU103, produced reddish color after 30 min of incubation. The results (Fig 1) showed that isolates IU100, IU102, IU104 and IU105 produced negligible amount of IAA which was non-significant. However, IU103 produced significantly high concentration of IAA in culture media. Analysis of the present data revealed that endophytic bacteria isolated from Silybum marianum had significantly enhanced the root and shoot length in heavy metal stress conditions (Bharti et al., 2016; Timmusk et al., 2017).
They concluded that endophytes play a key role in the growth of plants under heavy metal. Similarly it proved vital for the increase in fresh and dry weights of the selected plants. The endophytes including fungi and bacteria have been of great interest as inoculants in agriculture to improve plant growth in many crops (Chauvin et al., 2017; Timmusk et al., 2017). Endophytic bacteria enhance plant growth by production of phytohormones such IAA, which play a central role in cell enlargement, root initiation, and cell division (Khan, 2019). Among the tested endophytic isolates, IU103 was producer of IAA. The bacterial isolates might be involved in plant growth promotion due to the IAA production the enhance plant growth by cell enlargement, root initiation, and cell division (McShan et al., 2015) (Ramesh et al., 2015). It has been speculated that production of IAA by bacteria could enhance the legume-Rhizobium symbiosis and uptake of nutrients thus increasing root length and subsequently enhance plant development (Ullah et al., 2015).
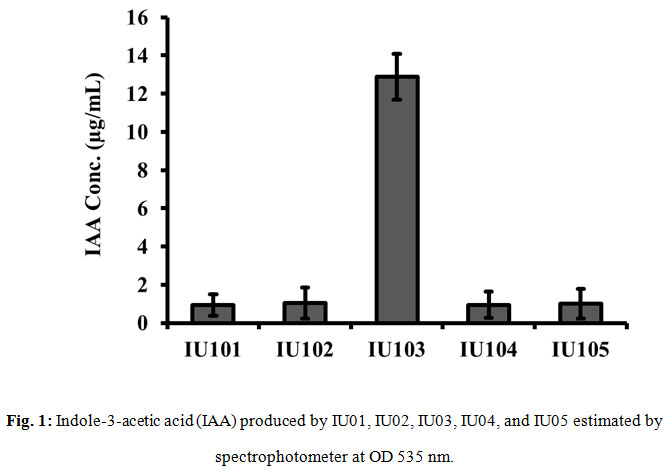 |
Figure 1: Indole-3-acetic acid (IAA) produced by IU01, IU02, IU03, IU04, and IU05 estimated by spectrophotometer at OD 535 nm |
Molecular identification of selected bacterial strains
The purified PCR products of 16S rDNA from the IU103, was sequenced, revealing nucleotide sequence lengths. The sequences was aligned with sequences identified by BLAST search of the NCBI database. The selected sequences showed the highest query coverage and sequence homology with IU103. Results of the BLAST sequence search (Fig. 2) indicated the endophyte isolate IU103 showed maximum similarity with Bacillus sp. therefore, it was named as Bacillus sp. strain IU103.
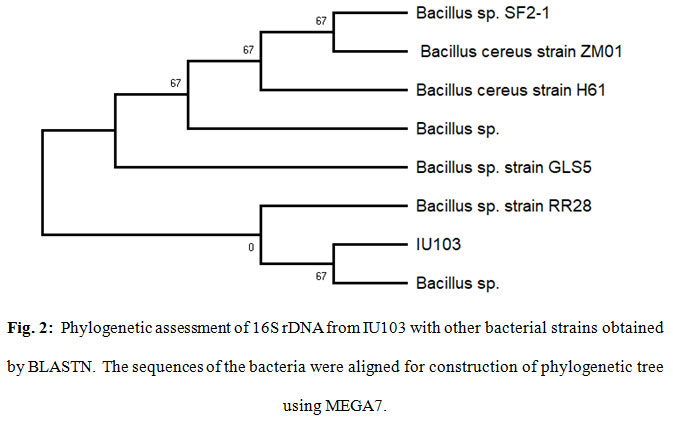 |
Figure 2: Phylogenetic assessment of 16S rDNA from IU103 with other bacterial strains obtained by BLASTN. The sequences of the bacteria were aligned for construction of phylogenetic tree using MEGA7. |
Plant bioassay of IU103
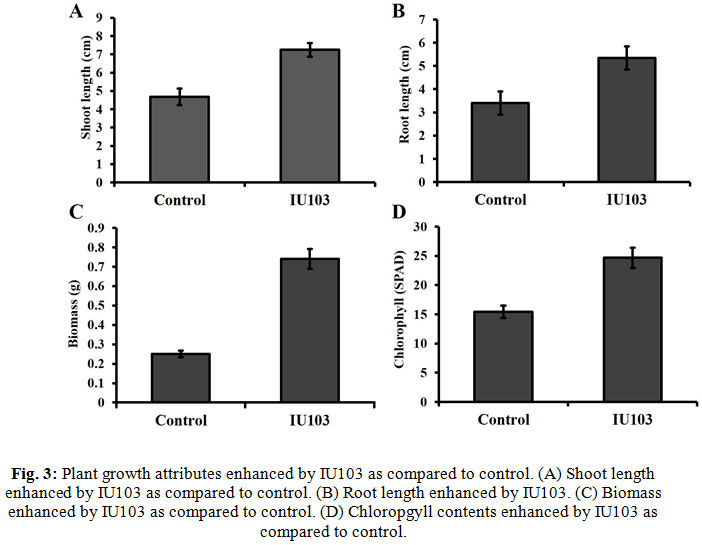
Effect of IU103 on plant growth attributes of were determined using Brassica plants. Results (Fig. 3A and 3B) showed that inoculated with the IU103 significantly promoted plant growth attributes such as shoot and lengths root length as compared to control. Chlorophyll contents of the plants were an important indicator of photosynthesis, which is necessary for the survival of the plant. Inoculation of IU103 significantly increased the chlorophyll contents of plants and increased plant biomass as compared to control (Fig. 3C and 3D). In short, overall results revealed that inoculation of IU103 significantly affected plant growth, development and application of IU103 was effective, and consistently improved plant growth attributes, e.g., shoot length, root length, chlorophyll content, and biomass as compared to control.
The ability of isolates to inhibit the growth of different pathogens is implication of the secondary metabolites secreted by endophytic bacteria (Ullah et al., 2019). It is also evident from the present study that some endophytes isolated can be developed as potential biocontrol agents (Ramesh et al., 2015; Ullah et al., 2019). Therefore, further studies are necessary to assess the ability of the isolates to confer protection against pathogens. The overall study revealed that the IU103 was effective endophyte, producing high concentration of phytohormone IAA. The IAA production could be responsible for the plant growth promotion. In addition, IU103 also had antibacterial and antifungal activities which also support the plant growth promotion my killing pathogens.
Antibacterial Activity
Antibacterial activity of IU103 was assessed against Gram-positive and Gram-negative bacteria. IU103 was screened for their antibacterial capability against four common Gram-positive bacteria, such as. Similarly, IU103 was also assessed for its antibacterial activities against Gram-negative bacteria. The results revealed that (Table 1) antibacterial compounds were produced by IU103, which were equally effective against Gram-negative and Gram-positive bacteria. Formation of clear zones around the IU103 colonies in culture plates were indication of antibacterial activities of the IU103. E. coli DH5α was used as negative control and tetracycline was used as positive control.
Antimicrobial activities have been detected in numerous endophytic bacteria and fungi, which have very important for the plant growth promoting activities because pathogenic microbes such as bacteria and fungi are killed by and hence the plant are protected from their hazardous effects (Ullah et al., 2013, Xia et al., 2016). Moreover, antimicrobial activities of the endophytic microbe revealed that elimination of pathogenic microbe around the plants results pant growth development (Xia et al. 2016). In the present study, among the five bacterial endophytic strains IU103 was found effective against all the tested pathogens.
Table 1: Antibacterial activity of endophytic bacteria IU103.
| Treatment | Antibacterial activity of IU103 | ||||
| E. coli | S. typhi | B. subtilis | S. aureus | P. vulgaris | |
| IU103 | + ve | + ve | + ve | + ve | + ve |
| Positive Control | + ve | + ve | + ve | + ve | + ve |
| Negative control | – ve | – ve | – ve | – ve | – ve |
Antifungal Activity
The inhibitory effect of IU103 was tested against the fungal phytopathogens. Antifungal activity of IU103 was assessed different fungi. IU103 was screened for their Antifungal capability against different species of fungi (Table 2). The results showed that the antifungal compounds were produced by IU103, which were effective against different species of fungi. Formation of clear zones around the IU103 colonies in culture plates were indication of antifungal activities of the IU103. E. coli DH5α was used as negative control and tetracycline was used as positive control.
Results of these study revealed that the IU103 showed excellent anti bacterial and antifungal activities. Previously a number of studies have reported antimicrobial activities of plant endophytic bacteria (McShan et al., 2015). Ullah et al., (2015) reported that endophytic bacteria isolated from Solanum sp. Showed antibacterial activities against Gram-negative bacteria (Ullah et al., 2019).
Table 2: Antifungal activity of endophytic bacteria IU103.
| Treatment | Antifungal activity of IU103 | ||||
| A. niger | A. avamori | T. konningi | F. oxysporium | P. fumicalsuri | |
| IU103 | + ve | + ve | + ve | + ve | + ve |
| Positive Control | + ve | + ve | + ve | + ve | + ve |
| Negative control | – ve | – ve | – ve | – ve | – ve |
ACKNOWLEDGMENT
The author would like to thanks Dr. Ihsan Ullah from biology department for their kindness.
FUNDING
Nil
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest to disclose.
REFERENCES
Berg, G., Hallmann, J., (2006). Control of plant pathogenic fungi with bacterial endophytes. Microbial root endophytes. Springer, pp. 53-69.
Bharti, N., Pandey, S.S., Barnawal, D., Patel, V.K., Kalra, A., (2016). Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Scientific Reports., 6, 34768.
Chauvin, J., Judée, F., Yousfi, M., Vicendo, P., Merbahi, N., (2017). Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Scientific Reports., 7, 4562.
El-Tarabily, K.A., Sivasithamparam, K., (2006). Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience., 47, 25-35.
Gouda, S., Kerry, R.G., Das, G., Paramithiotis, S., Shin, H.S., Patra, J.K., (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research., 206, 131-140.
Hardoim, P.R., van Overbeek, L.S., van Elsas, J.D., (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends in microbiology., 16, 463-471.
Jeger, M.J., Spence, N.J., (2001). Biotic interactions in plant-pathogen associations. CABI.
Ji, S.-H., Kim, J.-S., Lee, C.-H., Seo, H.-S., Chun, S.-C., Oh, J., Choi, E.-H., Park, G., (2019). Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Scientific Reports., 9, 1044.
Khan, M.A., (2019). Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis v., 77, pp. 9-21-2019 v.2077 no.2011.
Kim, Y.-C., Glick, B.R., Bashan, Y., Ryu, C.-M., (2012). Enhancement of Plant Drought Tolerance by Microbes. In: Aroca, R. (Ed.), Plant Responses to Drought Stress: From Morphological to Molecular Features. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 383-413.
McShan, D., Zhang, Y., Deng, H., Ray, P.C., Yu, H., (2015). Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. Journal of Environmental Science and Health., Part C 33, 369-384.
Muthukrishnan, S., Bhakya, S., Senthil Kumar, T., Rao, M.V., (2015). Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Ceropegia thwaitesii – An endemic species. Industrial Crops and Products., 63, 119-124.
Ramesh, P.S., Kokila, T., Geetha, D., (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 142, 339-343.
Saharan, B., (2011). Plant growth promoting rhizobacteria: a critical review. Life Sciences and Medicine Research., 21,1-30.
Sharma, S.B., Sayyed, R.Z., Trivedi, M.H., Gobi, T.A., (2013). Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2, 1.
Sturz, A., Christie, B., Nowak, J., (2000). Bacterial endophytes: potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences., 19, 1-30.
Timmusk, S., Behers, L., Muthoni, J., Muraya, A., Aronsson, A.-C., (2017). Perspectives and Challenges of Microbial Application for Crop Improvement. Front Plant Sci., 8, 49-49.
Ullah, I., Al-Johny, B.O., Al-Ghamdi, K.M.S., Al-Zahrani, H.A.A., Anwar, Y., Firoz, A., Al-Kenani, N., Almatry, M.A.A., (2019). Endophytic bacteria isolated from Solanum nigrum L., alleviate cadmium (Cd) stress response by their antioxidant potentials, including SOD synthesis by sodA gene. Ecotoxicology and environmental safety., 174, 197-207.
Ullah, I., Khan, A.L., Ali, L., Khan, A.R., Waqas, M., Hussain, J., Lee, I.J., Shin, J.H., (2015). Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M01021. Journal of microbiology (Seoul, Korea)., 53, 127-133.
Ullah, I., Khan, A.R., Park, G.-S., Lim, J.-H., Waqas, M., Lee, I.-J., Shin, J.-H., (2013). Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Science and Biotechnology., 22, 25-31.
Xia, Z.-K., Ma, Q.-H., Li, S.-Y., Zhang, D.-Q., Cong, L., Tian, Y.-L., Yang, R.-Y., (2016). The antifungal effect of silver nanoparticles on Trichosporon asahii. Journal of Microbiology, Immunology and Infection., 49, 182-188.