1Candidate of Veterinary Sciences, Associate Professor, Researcher ASRIVEA – Branch of Tyumen Scientific Centre SB RAS,
FSBEI HE Northern Trans-Ural SAU Russia
2ASRIVEA – Branch of Tyumen Scientific Centre SB RAS, FSBEI HE Northern Trans-Ural SAU Russia
3Candidate of Veterinary Sciences, Researcher ASRIVEA – Branch of Tyumen Scientific Centre SB RAS Russia
Article Publishing History
Received: 10/10/2020
Accepted After Revision: 20/12/2020
Thelaziasis of cattle is a helminthic disease caused by parasitizing of nematodes of a suborder of Spirurata, the Thelaziidae family. The primary purpose of this study is to elaborately analyze the seasonal dynamics of thelaziasis in cattle, terms of its invasive thelaziasis and the specification of the clinical implication of disease in the cattle in the Northern Trans-Ural region. The researches were conducted in 2002-2016, surveyed 27122 heads of cattle belonging to the agricultural enterprises and citizens. It can be concluded that under the conditions of the Northern Trans-Ural region, clinical signs of thelaziasis are shown from the first decade of May (EI of 3.39%). Besides, by the end of the month, this indicator increases more than twice (EI of 7.04%). Moreover, in the summers, the number of clinically sick thelaziasis animals are the highest. Clinical implication of thelaziasis is characterized by the development of the catarral exudative inflammation which is quickly changing on, in the beginning, is purulent – catarral, then there is a loss of transparency of cornea, fusion of tissues of eye and formation of erosion and ulcers on it. Due to the lack of treatment, there is a perforation of an iris of the eye or shape of a white or red cataract that may well result in the loss of sight and premature rejection of a sick animal.
Cattle, Thelaziasis, Seasonal Dynamics, Clinical Implication.
Glazunova L. A, Glazunov Y. V, Ergashev A. A. Seasonal Dynamics of Cattle Thelaziasis in Northern Trans-Ural Region and Pathogenetic Mechanisms if its Clinical Manifestations. Biosc.Biotech.Res.Comm. 2020;13(4).
Glazunova L. A, Glazunov Y. V, Ergashev A. A. Seasonal Dynamics of Cattle Thelaziasis in Northern Trans-Ural Region and Pathogenetic Mechanisms if its Clinical Manifestations. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/3os9T9r”>https://bit.ly/3os9T9r</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Considerable changes in the political climate around the world are reflected in an economic situation and our country. Sanctions and conditional isolation of Russia resulted in developing the different industries in Russia. The special attention is focused on the development of the agro-industrial complex due to which there is an import substitution of food products and implementation of the program of food security. Recently providing the population with fowl and pork reached absolute measures, there is not enough only beef meat (De León et al., 2020).
Cattle breeding – rather expensive and technologically complex branch of agriculture but to replace beef meat which contains full-fledged protein a large amount of iron at the same time rather dietary, today in our country it is not possible. Although the development of cattle breeding is under close attention and control of the state, it is not possible to provide the reason for that the population beef fully. During the summer period the most of cattle pasture on the remote pastures, mostly it is relevant for animals of the meat direction that spend on pastures about half a year (Kennedy and MacKinnon, 1994; Zubairova and Ataev, 2010; Bespalova and Grigorjeva 2015; Khedri et al., 2016; Bespalova et al., 2016; Chowdhury et al., 2018). Except for traumatic damages, the pasturable type of contents is dangerous to animals by an invasive parasite and continuous influence of insects and ticks (Stolbova et al., 2014). One of the widespread summer diseases in the Northern Trans-Ural region is thelaziasis (Glazunova et al., 2018; Glazunova and Glazunov, 2018; Luo et al., 2020).
Thelaziasis of cattle and the helminthic disease are mostly caused by parasitizing of nematodes of a suborder of Spirurata. (Sivajothi and Reddy, 2018; Munang’andu et al., 2011; Naem, 2007). Helminthes parasitize in channels of the lacrimal gland, the nasolacrimal channel, under the third century and in a conjunctival sac. Without treatment of this disease leads to a decrease in additional weights and milk yield of milk, and in the started cases to loss of sight and, as a result, to premature rejection (Safiullin, 1997). The infectiousness of cattle in some farms reaches 60-80% (Bespalova et al., 2016; Khedri et al., 2016; Otranto and Traversa, 2005). Cases of parasite invasion of specific parasites of cattle of Thelazia gulosa and Tskrjabiniare known at the person (Nath et al., 2008; Otranto and Dutto, 2008; Djungu et al., 2014; Krishnachary et al., 2014; Samardžić et al., 2015; Bradbury et al., 2018).
Thelaziasis this seasonal disease as intermediate owners of thelazia are zoophilous flies. In the Northern Trans-Ural region, the pasture of animals is begun since the end of April of the beginning of May that coincides with the start of activity of zoophilous flies – intermediate owners of thelazia which become more active in the first from the second decade of April (dependence on meteoconditions) (Glazunova et al., 2018; Glazunova and Glazunov, 2018; Deak et al., 2020). Animals, having come to pastures, are infested in 14-28 days after the beginning of the attack on them of flies that is caused by the biology of the activator which metamorphosis passes an intermediate phase in an organism of these dipterous insects (Glazunova et al., 2018; Glazunova and Glazunov, 2018; Sivajothi and Reddy, 2018; Luo et al., 2020). The purpose of us researches was detailed studying of seasonal dynamics of thelaziasis at cattle and terms of invasive of cattle thelazia in the Northern Trans-Ural region as well as the specification of the clinical implication of disease at the cattle in the region.
METHODOLOGY
To study the distribution of thelaziasis among cattle, a clinical examination was performed involving animals belonging to 35 agricultural enterprises and the cattle from personal subsidiary farms from 2002 to 2016. During the period, 27122 heads of cattle were clinically examined. For reliability of the received results, only the cattle, which was not exposed to insecticidal processing, was examined. Some microscopic examination of washouts from a conjunctival cavity was conducted for confirmation of the diagnosis (De León et al., 2020). Data processing was carried out with the application of an indicator of the extensiveness of an invasion (EI). The obtained results were processed statistically (Prabhakar et al., 2015), taking into account average sizes, their mistakes and level of reliability (P) on Student on the computer with the use of the Microsoft Excel and Biostat program.
Thelaziasis: Thelazia parasite is a small white parasite that is about 1 to 2 cm in size. The skin of this parasite has prominent transverse lines that these protrusions or the same contrast of the cream cover cause lesions in the eye and its conjunctival tissue. As mentioned, this parasite causes infection in the eyes of ruminants and has been reported more in cattle. The parasite is found in the lacrimal duct of cattle and rarely in sheep, goats and buffaloes. This parasite has an indirect life cycle, i.e. it has an intermediate host, and without the presence of an intermediate host, the evolutionary process of the parasite does not take place. The parasite hosts of this parasite are species of flies that are swallowed by flies after 15 to 20 days of infancy when eating the first stage of Thelasia viable worms. This pathogenic baby, which is in the third stage, migrates to the oral appendages in the body of the fly and is placed in the conjunctival sac around the ruminant eye when feeding the fly. This parasite causes injuries and swelling of the conjunctival tissue and lacrimal duct by contrasting its covering, which causes tears to fall and in severe infections causes the cornea to become cloudy and sore.
RESULTS
As a result of the conducted researches, it is established that the cattle which is grazed on pasture or having a range during the day is exposed to an invasive thelaziasis. The extensiveness of thelaziasis invasion varied within 2.37-36.17% and averaged 10.79±0.98% (Glazunova et al., 2018). For clarification of seasonal dynamics of an invasivethelaziasis, we performed clinical examination of animals during all calendar year (table 1).
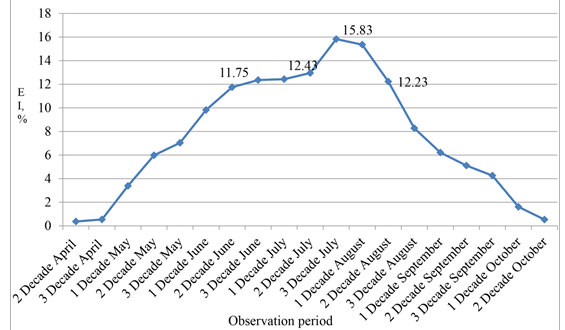
It is noticed that for the entire period of observation (from 2002 to 2016) the extensiveness of thelaziasis invasion before the beginning of a pasturable season (the first decade of May) and after its termination (the second decade of October) was minimum and made less than 1%. Changes in an epizootic situation happened practically right after an exit to a pasture. So, showing activity, zoophilous flies irritate a mucous membrane of eyes and lead to dacryagogue. At inspection of animals in May it is established that their quantity with clinical signs of thelaziasis increases by the end of the month. During this period, it is not possible to confirm the existence of thelaziasis invasion how larvae are not washed away from a conjunctival sac yet. So, if in the first decade of May of animals in the herd with the clinic of thelaziasis there were 3.39%, then by the end of the month this indicator increases more than, twice and made 7.04%.
Allowed to confirm incidence thelaziasis further observation of animals when eventually they had bright clinical signs of disease and at the microscopy of washouts from a conjunctival sac found larvae of thelazia. In summer, the number of clinically sick thelaziasis animals is the highest. So, at the end of June, the extensiveness of an invasion was 12.36%. The number of sick animals increased and reached the maximum during the period from the third decade of July to the first decade of August with indicators of 15-83 and 15.36% respectively. In the second decade of August the number of the diseased still was at the high level – 12.23%. Then also by the end of September the number of animals with clinical signs of thelaziasis less than 5% systematically decreased (Figure 1).
Table 1. Dynamics of manifestation of clinical signs of thelaziasis at cattle during the period from 2002 to 2016.
| Research month | Quantity of the examined animals | Animals with clinical signs of thelaziasis | EI, % |
| April | |||
| 2 decade | 809 | 3 | 0,37 |
| 3 decade | 1304 | 7 | 0,54 |
| May | |||
| 1 decade | 118 | 4 | 3,39 |
| 2 decade | 5085 | 304 | 5,98 |
| 3 decade | 270 | 19 | 7,04 |
| June | |||
| 1 decade | 2118 | 208 | 9,82 |
| 2 decade | 2833 | 333 | 11,75 |
| 3 decade | 1278 | 158 | 12,36 |
| July | |||
| 1 decade | 2599 | 323 | 12,43 |
| 2 decade | 3491 | 452 | 12,95 |
| 3 decade | 4366 | 691 | 15,83 |
| August | |||
| 1 decade | 1074 | 165 | 15,36 |
| 2 decade | 564 | 69 | 12,23 |
| 3 decade | 326 | 27 | 8,28 |
| September | |||
| 1 decade | 306 | 19 | 6,21 |
| 2 decade | 176 | 9 | 5,11 |
| 3 decade | 94 | 4 | 4,26 |
| October | |||
| 1 decade | 124 | 2 | 1,61 |
| 2 decade | 187 | 1 | 0,53 |
| Total: | 27122 | 2798 | 10,04±1,16 |
Figure 1: Seasonal dynamics of the clinical implication of thelaziasis at cattle (2002-2016)
DISCUSSION
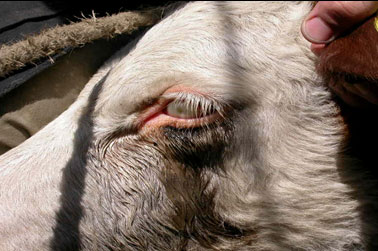
On the whole, the seasonal dynamics of cattle thelaziasis in Northern Trans-Ural region and pathogenetic mechanisms of its clinical Manifestations was fully analyzed. Thus, the high incidence of thelaziasis (higher than 10%) is fixed during the period from the second decade of June to the second decade of August. At the same time, it is necessary to consider that animals are infested much earlier than begin to show a disease clinically. It is noticed that during the winter period animals have no bright inflammatory reaction at thelaziasis, i.e. they have in a latent form and often are not exposed to preventive deworming therefore the cattle which is often infested by thelazia, coming to pasture, is an invasion source for other animals. So, allocations from eyes attract (physiological) arthropods, and they actively eat near an eye. During the food of a fly, the larva gets to the alimentary system of an insect, migrates, and there passes the development cycle. At repeated contact of the infested fly with an animal in 2-4 weeks’ live larvae independently creep out of her proboscis and get into a conjunctival sac. The hit of larvae of thelazia in a conjunctival sac is followed by alternative processes in organs of sight, caused by the allergic and mechanical influence of a parasite (Luo et al., 2020). Clinically it is shown by hypostasis a century and photophobia (Figure 2).
Figure 2: The century, photophobia and dacryagogue swelled
The developing exudation at conjunctivitis defines the appearance of plentiful dacryagogue that in turn has protective value thanks to what inoculation of a parasite is possible. Besides, the development of local inflammation (conjunctivitis) has an immunosuppressant effect. It reduces the level of local nonspecific protection of tissues of the eye that contributes to the development of opportunistic microflora. The created vascular reaction is characterized by the formation of the catarral exudative inflammation characteristic of the conjunctiva, which is quickly changing on is purulent – catarral (Bespalova et al., 2016; De León et al., 2020).
At a progression of an inflammatory reaction, the cornea which being front transparent department of the external capsule of an eye globe is involved in the process, it is subject to the influence of all adverse environmental factors. Features of the building and metabolism of a cornea (anastomosing of the regional looped network of vessels of a cornea and an innervation, lack of own vessels, low level of exchange processes) explain its fast involvement in the pathological process and specifics of its current, characterized by loss of transparency of the cornea. Usually, this process develops promptly, and in 7-10 days after the first clinical signs of conjunctivitis inflammatory process passes to a cornea (Sivajothi and Reddy, 2018; Deak et al., 2020).
Development of larvae of thelazia in a conjunctival sac, after the third century and in the nasolacrimal channel supports secondary alteration, is followed by hyperplastic changes in cornea and transition of inflammation to an eye iris of the eye that is characterized by the development of iridocyclitis. Against the background of a progression of inflammatory reaction and its deepening in eye tissue as well as the reproduction of pyogenic microflora of response of an eye, the globe is various. In most cases at exudative keratoconjunctivitis and iridocyclitis under the influence of proteolytic enzymes of purulent exudate, there is a fusion of tissues of eye and formation of erosion and welcomes, and a perforation of an iris of the eye that comes to an end with the loss of sight (Chowdhury et al., 2018).
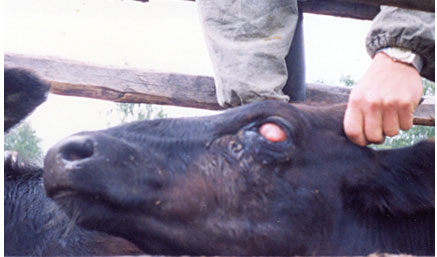
In the conclusion of the pathological process, during proliferation, on the cornea, the white or red cataract (see Figure 3 and 4) is formed. The white cataract is characterized by the fusion of a cornea and underlying tissues of an eye proteolytic enzymes and coagulation of proteins. The red cataract is formed at the expense of active vascularization of the damaged centre. In both cases, the injured eye loses the function. Much rarer, on the place of the inflammatory reaction, the new growth which growth is caused by an invasive of cattle thelazia during the summer period and development by long inflammatory response as well as the influence of solar radiation develops. Neoplastic changes most often are registered at cattle of Heleford breed that is promoted by a characteristic colour of the cattle. It is known that at the white-headed cattle of a tumour of eyes develop much more often than at animals with intensive pigmentation (Glazunova et al., 2018).
Considering pathogenesis of thelaziasis and its seasonal dynamics studying of the ecology of zoophilous flies – intermediate owners of thelazia is necessary for the development of a reasonable system of prevention of thelaziasis at cattle. Besides, considering pathogenetic bases of a current of thelaziasis for therapy, it is necessary to apply not only antiparasitic but also antibacterial, protective and immune performance-enhancing drugs which allow to adjust damages of a cornea and an iris of the eye of an eye and to prevent loss of sight as a result of turbidity of a cornea and formation on it of hems, perforations of an iris of the eye of an eye and development of new growths (see Figures 5 and 6).
Figure 3: A white cataract, as a result of the fusion of tissues of the eye
Figure 4: A red cataract, as a result of vascularization of the damaged centre
Figure 5: A hem on a cornea, after a recovery fromthelaziasis
Figure 6: A cornea perforation after a recovery fromthelaziasis
CONCLUSION
Thelaziasis is a worm disease stemmed from the Thelazia nematode, which is transmitted by flies of the infected family. In the current study, it was attempted to analyze the seasonal dynamics of thelaziasis at cattle and terms of invasive of cattle Thelazia in the Northern Trans-Ural region and the specification of the clinical implication of disease at the cattle in the area.
It can be concluded that in summer, quantity clinically sick thelaziasis animals the highest. The maximum values fixed during the period from the third decade of July to the first decade of August (% EI 15.83 and 15.36 respectively). By the end of September, the number of animals with clinical signs of thelaziasis made less than 5%. During the stall period, the extensiveness of thelaziasis invasion was minimum and was less than 1%. The clinical implication of thelaziasis is characterized by the development of the catarral exudative inflammation, which is quickly changing on, in the beginning, is purulent – catarral. There is a loss of transparency of cornea, fusion of tissues of eye and formation of erosion and ulcers on it. In lack of treatment, there is a perforation of an iris of the eye or appearance of a white or red cataract that comes to an end with the loss of sight and premature rejection of a sick animal.
ACKNOWLEDGEMENTS
The article was prepared with the financial support of the Program of the Basic Research of the Russian Academy of Sciences, registration number AAAA A18 118020690240-3 Control of an epizootic situation and forecasts of development of possible outbreaks of parasitic diseases of animals.
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Scientific Centre SB RAS, FSBEI HE Northern Trans-Ural SAU Russia.
REFERENCES
Bespalova NS, Grigorjeva NA. (2015). Thelazia spp. Infection in cattle in the Russian Federation. In16th Scientific Conference on the Theory and practice of the struggle against parasitic diseases 19-20 May 2015, Moscow, Russia (pp. 37-38). All-Russian KI Skryabin Scientific Research Institute of Parasitology of Animals and Plants.
Bespalova NS, Grigoryeva NA, Vozgorkova EO. (2016). Thelazia rhodesi infection in cattle in the Voronezh Region. In17th Scientific Conference on the Theory and practice of the struggle against parasitic diseases, 17-18 May 2016, Moscow, Russia (pp. 65-67). All-Russian KI Skryabin Scientific Research Institute of Fundamental and Applied Parasitology of Animals and Plants.
Bradbury RS, Breen KV, Bonura EM, Hoyt JW, Bishop HS. (2018). Case report: conjunctival infestation with Thelazia gulosa: a novel agent of human thelaziasis in the United States. The American Journal of tropical medicine and hygiene. (4):1171-4.
Chowdhury R, Gogoi M, Sarma A, Sharma A. (2018). Ocular thelaziasis: A case report from Assam, India. Tropical Parasitology. Jul;8(2):94.
Deak G, Ionică A. M, Oros NV, Gherman CM, & Mihalca AD. (2020). Thelazia rhodesi in a dairy farm in Romania and successful treatment using eprinomectin. Parasitology International, 80, 102183.
De León AAP, Mitchell RD, & Watson DW. (2020). Ectoparasites of cattle. Veterinary Clinics: Food Animal Practice, 36(1), 173-185.
Djungu DF, Retnani EB, Ridwan Y. (2014). Thelazia rhodesii infection on cattle in Kupang District. Tropical biomedicine;31(4):844-52.
Glazunova LA, Glazunov YV, Ergashev AA. (2018). Ecological-Epizootical Situation On Telasiosis Among Large Cattle In Northern Ural Region. Research Journal of Pharmaceutical, Biological and Chemical Sciences.;9(4):1687-93.
Glazunova LA, Glazunov YV. (2018). Abyphipr-perspective insecticide on the basis of fipronil and abomectin for combating zoophilnium flies-intermediate bells of belts. InInternational scientific and practical conference” Agro-SMART-Smart solutions for agriculture”(Agro-SMART) 2018 Dec. Atlantis Press.
Kennedy MJ, MacKinnon JD. (1994). Site segregation of Thelazia skrjabini and Thelazia gulosa (Nematoda: Thelazioidea) in the eyes of cattle. The Journal of parasitology. 1:501-4.
Khedri J, Radfar MH, Borji H, Azizzadeh M.(2016). Epidemiological Survey of Bovine Thelaziosis in Southeastern of Iran. Iranian Journal of parasitology. 11(2):221.
Khedri J, Radfar MH, Borji H, Azizzadeh M. (2016). Epidemiological Survey of Bovine Thelaziosis in Southeastern of Iran. Iranian Journal of parasitology. 11(2):221.
Krishnachary PS, Shankarappa VG, Rajarathnam R, Shanthappa M. (2014). Human ocular thelaziasis in Karnataka. Indian Journal of ophthalmology.62(7):822.
Luo ., Xiang N, Liu R, Wang W, Li Y, & Qi X (2020). Phthiriasis palpebrarum, thelaziasis, and ophthalmomyiasis. International Journal of Infectious Diseases.
Munang’andu HM, Chembensofu M, Siamudaala VM, Munyeme M, Matandiko W. (2011). Thelazia rhodesii in the African Buffalo, Syncerus caffer, in Zambia. The Korean Journal of Parasitology. 49(1):91.
Naem S. (2007). Fine structure of body surface of Thelazia skrjabini (Nematoda: Spirurida, Thelaziidae). Parasitology research.100(2):305-10.
Nath R, Narain K, Saikia L, Pujari BS, Thakuria B, Mahanta J. (2008). Ocular thelaziasis in Assam: A report of two cases. Indian Journal of Pathology and Microbiology. 51(1):146.
Otranto D, Dutto M. (2008). Human thelaziasis, Europe. Emerging infectious diseases. 14(4):647.
Otranto D, Traversa D. (2005). Thelazia eyeworm: an original endo-and ecto-parasitic nematode. Trends in parasitology. 21(1):1-4.
Prabhakar S, Vijaykumar G, Mahesh B. (2015). Human ocular thelaziasis: A case report from Karnataka. Indian Journal of medical microbiology. 33(2):324.
Safiullin RT. (1997). Distribution and economic damage from the main helminth infections of ruminants. Veterinary Medicine. (6):28-32.
Samardžić K, Tomić Paradžik Ma, Janjetović Ž, Živičnjak T, Vuković Arar Že, Martinković F, Miletić-Medved Ma (2015). The first case of ocular thelaziasis in Croatia. Acta Medica Croatica.69(5):475-9.
Sivajothi S, Reddy BS. (2018). A review on parasitic castration in veterinary parasitology. Journal of Entomology and Zoology Studies.6(1):635-9.
Stolbova OA, Glazunov AA, Nikonov AA, Glazunov V, Skosyrskih LN. (2014). Insects and mites–parasites of cattle in Northern TRANS-Ural region. Fundamental research. (11-12):2650-5.
Zubairova MM, Ataev AM. (2010). Fauna and distribution of nematodes from the suborders Spirurata and Filariata parasitizing cattle in Dagestan, from the perspective of vertical zoning. Parazitologiia. 44(6):525-30.