1Faculty of Sciences, Biology Department, King Abdel Aziz University, Saudi Arabia.
2Department of Botany and Microbiology, Kafrelsheikh University, Kafr El Sheikh, Egypt.
Corresponding author email: mmmohammad@Kau.edu.sa
Article Publishing History
Received: 14/02/2022
Accepted After Revision: 23/04/2022
Saudi Arabia flora has many medicinal plants which are traditionally used in inhibition of many human pathogens. Echinops macrochaetus (Camel thistle) and Xanthium spinosum (Spiny cocklebur) from the Asteraceae family and Lemongrass (Oymbopogon citrates) from the Gramineae family are one of the local plants which are quite popular but less studied scientfically. Hence in the present work, they were analysed for their medicinal efficacies , for which they were collected and extracted using either hot water or organic solvents (n-butanol, diethyl ether andethyl acetate). The antibacterial activities of the previous plants extracts were detected and the methanolic extract of the three tested plants gave excellent inhibition of the tested bacterial pathogen, Escherichia coli while the aqueous extracts recorded the lowest bacterial inhibition.
The susceptibility of the tested bacteria to the three methanolic plant extracts and control antibiotic was tested using agar well diffusion method. The methanolic extract of Echinops recorded excellent antibacterial activity while Xanthium and Oymbopogon recorded moderate activities against the Gram negative Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Proteus mirabilis in addition to the Gram positive Staphylococcus aureus and Enterococcus faecalis. The mean antibacterial activities (bacterial index) was maximum for the methanol extract of Echinops (21.8 mm) followed by Xanthium (13.8 mm) and Oymbopogon (12.5 mm). Moreover, In vitro MTT test and Neutral Red assays were used to detect any antitumor activities of the three tested plant extracts.
Echinops extract showed excellent antitumor activity against two cell lines, MCF-7 (breast cancer) and HepG2 (hepatocellular carcinoma) with no toxicity (recorded using Artimia salina for the assay). Also, using fluorescein diacetate microdilution method, minimal inhibitory concentrations (MICs) and the Fractional Inhibitory Concentrations of the methanolic extract of Echinops, ciprofloxacin and their mixture were calculated. FIC index ranged from 0.89-1.15 that means there is an additive effect between Echinops extract and ciprofloxacin. In conclusion, the results show that Echinops methanolic extract single or mixed with the antibiotic ciprofloxacin demonstrated excellent inhibitory activities for all tested urinary tract pathogenic bacteria.
Antibacterial, Antibiotics, Echinops, Oymbopogon, Xanthium,
Alshamrani R.I, Magda M Aly M.M. Screening of Some Local Traditional plants for their Antitumor and Antibacterial Activities Against the Global Emergence of Multi-Drug Resistant Bacteria. Biosc.Biotech.Res.Comm. 2022;15(2).
Alshamrani R.I, Magda M Aly M.M. Screening of Some Local Traditional plants for their Antitumor and Antibacterial Activities Against the Global Emergence of Multi-Drug Resistant Bacteria. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3yXzJul“>https://bit.ly/3yXzJul</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The efficacy of the current antibiotics is limited or not effective and pharmacological industries are in constant need of new products to control the harmful effects of dangerous microbes. New active antibiotics from medicinal plants are necessary and urgently needed to cure variety of diseases and contribute in improvement of human health care system. For a long period, medicinal plants are consumed as natural medicine and plants have been considered as the best sources of antibacterial, antifungal, antitumor and antioxidant drugs. Also phyto-materials can be safely used for stimulation of the immune system which degrades the effects of dangerous pathogens, (Carew and Patterson 1970; Jaleel et al. 2007; Aly et al. 2013; El Sayed and Aly 2014; Chassagne et al. 2021).
According to World Health Organization, herbal plants are used for thousands of years safely as traditional treatments compared to synthetic materials which had unsafe effects on human health. Almost all medical plants had therapeutic importance and human health care due to the presence of many active alkaloids in different plants (Santos et al. 1995; Mordmuang et al. 2019; Wink 2020; Chassagne et al. 2021).
Counts of the therapeutic products based plants were enhanced every year because they are easily used, common, wild available, had low prices and suitable to the poor people as antibacterial agents. Many plants extracts recorded excellent antibacterial activities against the multidrug resistant bacteria which cause many dangerous diseases compared to commercial antibacterial agents (Ba-Hamdan et al. 2013; NLM 2020; Chassagne et al. 2021).
Asteraceae is a big family of the flowering plant that had more than 1000 genera and about 30000 species, were described in Africa, Mediterranean area and Asia. Most of them are annuals, perennials, shrubs, or small trees. Genus Echinops L, was classified in the sub-class Asteridae, order Asterales and family Asteraceae which had about 130 plant species, found mainly in Southern of Europe and Asia in addition to Central and North of Africa. Plants of the genus Echinops characterized an erect perennial herb or shrub with long stem which grow up to 1.2 m with massive root, elliptical segmented leaves and white or bright blue corolla. Out of 120 species of the genus Echinops, E. kebericho Mesfin, E. buhaitensis Mesfin, E. ellenbeckii O.
Hoffm, E. kebericho and E. longisetus A. Rich were recorded in few localities in Ethiopia. Eighty two species were recorded in few localities in Europe and 15 species were found in Iraq while in Saudi Arabia, ten species were found. In Saudi Arabia, Echinops L. is represented by 10 species: E. abuzinadianus Chaudhary, E. erinaceus Kit-Tan, E. glaberrimus DC, E. hystrichoides Kit-Tan, E. macrochaetus Fresen, E. mandavillei Kit-Tan, E. polyceras Boiss, E. sheilae Kit-Tan, E. viscosus DC., and E. yemenicus Kit-Tan. Of these species, E. abuzinadianus, E. mandavillei, and E. sheilae are endemic to Saudi Arabia (Singh and Kumar 2001; Shukla 3003; Sharma and Borah 2012; Poulakou et al. 2018; Thielmann et al. 2019; Chassagne et al. 2021).
Species of the genus Echinops are useful for treating migraine, many heart and respiratory diseases, hemorrhoid, diabetes, and microbial infections of the urinary tracts in addition to dangerous worm’s removal. Moreover, the effect of this plant extracts on pathogenic bacteria was poorly studied whereas E. giganteus root extract showed inhibitory activities against S. saprophyticus and E. coli while the extract of Echinops ritro was the most potent extract against three species of the genus Colletotrichum which is a famous fungal pathogen of human. Also, three isolated compounds, 5’-(3-Buten-1-ynyl)-2,2’-bithiophen, R-terthienyl and 2-[pent-1,3-diynyl]-5-[4-hydroxybut- 1-ynyl]thiophene were active against Colletotrichum sp., Fusarium oxysporum, Phomopsis viticola and P. obscurans (Desta 1993; Fokialakis et al. 2006, Parekh et al. 2007; Deyno et al. 2021).
The antimicrobial activities may due to the presence of 83 different compounds like sesquiterpenoids and monoterpenoids which were isolated from E. grijsii, E. ritro, E. kebericho and E. ellenbeckii, monoterpenoids limonene and camphor which were isolated from the volatile oils of E. ellenbeckii, E. kebericho and sesquiterpenoid and sesquiterpenoid compounds which were observed in the oil extract of E. kebericho (Hymete et al. 1991; Maurya et al. 2015; Ivana, 2015; Ameya et al. 2016, Deyno et al. 2021).
Previous studies on the Echinops genus revealed the presence of diterpenoids, thiophenes, flavonoids, lignans, steroids, sesquiterpenes, and polyacetylenes but the studies on E. macrochaetus are limited (Tene et al. 2004; Hymete et al. 2005b; Yadava and Singh 2006; Abdallah et al. 2013; Ibrahim et al. 2016; Zaynab et al. 2018; Zhao et al. 2019; Wu et al. 2020; Chassagne et al. 2021).
Cancer remains one of the most serious illnesses in the world and finding anticancer treatments is still a priority of studies worldwide. In cancer treatment, chemotherapy is the major option but had many limitations while natural products may be used as anticancer agents with minimal side effects and without ethical approval. About 50-60% of cancer patients in USA use plants secondary metabolites in chemotherapy like curcumin, genistein, polyphenols, resveratrol, sulforaphane, isothiocyanates, silymarin, diallyl sulfide lycopene, rosmarinic acid, apigenin from parsley, and gingerol (Nobiliet al. 2009; Ahmed et al. 2013; Wang et al. 2014; Aro et al. 2019; Belcher et al. 2020; Chassagne et al. 2021).
For cancer treatment, two sesquiterpene glycosides I and II from the aerial parts of Echinops macrochetus extract were studied against MCF-7, HepG2, and HCT-116 tumor cells using sulforhodamine B assay method and the two Compounds showed potent cytotoxic activities towards all tested cell lines with ICs50 of 2.1, 2.9, and 3.6 µM for product I and 1.9, 3.3, and 2.3 µM, for product II, respectively. Also, the dichloromethane extract of E. macrochetus was inhibitor for acetyl cholin esterasey. Similarly, Echinops giganteus extract showed antitumor activities and can be used to treat cancer cells (Kuete et al. 2013; Saeed et al. 2015; Zamzami et al. 2019; Chassagne et al. 2021). This study has been aimed to evaluate in vitro antibacterial and antitumor activities of some plants, locally collected for evaluation of their synergistic effects along with an antibiotic in urinary tract bacterial inhibition.
MATERIAL AND METHODS
The tested pathogenic bacteria were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae (clinical isolate), Proteus mirabilis (clinical isolate), Staphylococcus aureus (ATCC 29213) and Enterococcus faecalis (clinical isolate). All bacteria were obtained from King Abdulaziz University Hospital, Jeddah, Saudi Arabia and used as test organisms. The bacteria were grown and preserved according to standard guidelines (CDC 2019; Chassagne et al. 2021).
The aerial parts (stalk and leaves) of Echinops macrochaetus, Oymbopogon citrates and Xanthium spinosum were collected in May 2020 from various areas of Taif city, Saudi Arabia. The plants were identified by a taxonomist at the Department of Biology, Faculty of Science, KAU, Saudi Arabia, in addition to their morphological features were examined, voucher samples are recorded (Collenette 1999). In sterile plastic bags, the collected plants were preserved at 4◦C until extracted with hot water, methanol, n-Butanol, Ethyl acetate and Diethyl ether (10 g/100 ml, w/v) for 10 hrs. The solvent was collected, dried and re-dissolved in 2 ml DMSO to detect the antibacterial activities of the obtained extract using Agar well diffusion method (Holder and Boyce 1994; El Sayed and Aly 2014).
Ciprofloxacin (Sigma-Aldrich, St. Louis, Missouri, USA) was used as control antibiotic and the minimal inhibitory concentrations (MICs) of the tested plant extracts or the control antibiotic were recorded for some bacterial pathogens in 96 well ELISA trays, using a colorimetric fluorescein diacetate method (Chand et al. 1994, Aly and Gumgumjee 2011). Fractional inhibitory concentration (FIC) was calculated using the formula found below (Petersen et al. 2006; Chassagne et al. 2021).
FIC index = MIC of extract in combination/MIC of extract alone + MIC of antibiotics in combination/MIC of antibiotics alone.
The combination defined synergy if ∑FIC ≤ 0.5, additively if 0.5 < ∑FIC ≤ 1, indifference if 1 < ∑FIC ≤ 4 and antagonism as ∑FIC > 4
Using brine shrimp lethality test, the cytotoxicity of the tested plant extracts in DMSO was determined at varying concentrations using brine shrimp larvae as test organism. After 8 hrs, the average of survived larvae at each concentration was determined and LD50 was calculated (Meyer et al. 1982; Aly and Gumgumji 2011). The antitumor activity of the three plant extracts against MCF-7 (breast cancer) and Hep G2 (hepatocellular carcinoma), tumor cell lines was studied using In vitro MTT and Neutral Red assays. Under sterile conditions and in 96-well plates, the MTT Test on two cell lines was evaluated (Betancur-Galvis et al. 1999).
Cells (6×103 cells/well) in 100 µl of the culture medium were grown for 24 hrs and treated with different concentrations of the tested plant extracts for 72 hrs. Then, cells were collected and treated with MTT solution (10 µl) and the viability of the cell was calculated by the absorption at A550 nm after dissolving the formazan crystals, culture medium plus MTT was used as control. Also, in 96-well plates, Neutral Red Uptake Assay, described by Betancur-Galvis et al. (1999), was used to detect in vitro cytotoxicity of three plants extracts on the two selected cell lines (Hep G2 and MCF-7).
Cells were grown in 0.1 ml/well of the culture medium with different concentrations of the tested extract for 48 hrs, and then the collected cells were suspended in culture medium (0.1 ml containing 5×10-5g/ml neutral red) and after incubation at 37◦C for three hrs, the collected cells were washed, re-suspended in a mixture of acetic acid/ethanol/ water (1:49:50 v/v/v) and A540 nm was recorded. The plant extract concentration reduced the cell viability by 50% is recorded as IC50 for the tested extract (Chassagne et al. 2021). Three replicates for each experiment were applied, the mean values, standard deviations and analysis of variance using ANOVA were recorded to detect any significant difference at P ≤p.5.
RESULTS AND DISCUSSION
Every minute, the number of the resistant microbes to almost used antibiotic in hospitals was increased causing dangerous problems to the hospitalized patients (Halwany et al. 2015; Zaman et al. 2015; Shriram et al. 2018; El Sayed et al. 2019). Many vascular plant extracts showed inhibition activity against some bacterial pathogens and the most studied families were Asteraceae, Lamiaceae and Fabaceae in addition to the genera Cinnamomum, Rosmarinus and Thymus while South Africa represented the most sources of the studied plants and methanol was the most used solvents but leaves were the most studied plant part (Chassagne et al. 2021).
Echinops macrochaetus (Camel thistle) and Xanthium spinosum (Spiny cocklebur) were belonging to Asteraceae family while Lemongrass (Oymbopogon citrates) was from Gramineae (Table 1). The aerial parts of the three collected plants were extracted with hot water or organic solvents to detect their antibacterial activities using E. coli as tested bacterial pathogen. The methanolic extract of Echinops macrochaetus showed the highest inhibition against E. coil (inhibition zones diameter about 20 mm while the lowest activity was 11 mm for the aqueous extract (Table 2). Also, the methanol extract of Oymbopogon and Xanthium had lower antibacterial activities compared to Echinops macrochaetus methanolic extract. Additionally, diethyl ether, ethyl acetate and n-butanol extracts exhibited lower antibacterial activities compared to their methanolic extracts. Therefore, the methanolic extract of the three tested plants were selected to determine their antibacterial activities against the six tested bacteria (Table 3).
The highest antibacterial activity was recorded for the methanolic extract of Echinops against the two Gram positive S. aureus and E. faecalis (mean inhibition zone 24 mm) while moderate activities were recorded against E. coli, K. pneumoniae, P. aeuroginosa and P. mirabilis, with inhibition zone diameter ranged from 20-22 mm. The extracts of Oymbopogon and Xanthium recorded the same inhibitory activity against K. pneumoniae and P. mirabilis with inhibition zone of 14 mm. The antibacterial index was a maximum for Echinops methanolic extract (21.8 mm), followed by Xanthium extract then finally the Oymbopogon extract which considered the less active extract. The susceptibilities of the tested bacteria to differed plant extracts were different according to their cell wall structure, morphology and presence of resistance genes.
Ciprofloxacin was used as a control antibiotic, it showed excellent activities against all the tested bacteria but the activities were more potent against Gram negative bacteria, with inhibition zone diameter ranged from 30-31 mm while lower activities were against S. aureus and E. faecalis with inhibition zone diameter ranged from 20-24 mm. Xanthium methanolic extract was more active against the Gram negative E. coli (16 mm) and showed moderate actives against K. pneumoniae, P. mirabilis and P. aeuroginosa (14 mm). The effect of Oymbopogon extract was moderate on Gram negative bacteria, especially E. coli, P. aeuroginosam, P. mirabilis and K. pneumoniae while showed weak activity against the two Gram positive bacteria, S. aureus and E. faecalis (Chassagne et al. 2021).
Urinary tract infections were caused mainly by Gram- negative bacteria, E. coli, Klebsiella, Proteus, Enterobacter, Pseudomonas and Serratia in addition to some low appearance Gram-positive isolates. Inhibition of these pathogens is difficult in some cases due to low effectively, limitation and resistant to the present antibiotics (El Sayed and Aly, 2014). Thus, looking for new plant extracts, safe and potent for bacterial inhibition without any harmful on the host cells describe the needed characters for the therapeutic agent. Thus, screening of local less studied plants is important and Echinops macrochaetus is one of these less studied local plants in Saudi Arabia (Chassagne et al. 2021).
Table 1. The Scientific names of the collected traditional plants, families and the used parts.
| Used part | Family | Scientific name | Common name |
| Aerial parts | Asteraceae | Echinops macrochaetus | Camel thistle |
| Aerial parts | Gramineae | Oymbopogon citrates | Lemon grass |
| Aerial parts | Asteraceae | Xanthium spinosum | Spiny cocklebur |
Table 2. Diameters of the inhibition zone of the aqueous and organic extracts
of the tested plants using E. coil as test organism.
| Type of the extract |
Used plant |
||||
| n-Butanol | Ethyl acetate | Diethyl ether | Methanol
extract |
Aqueous extract (HW) | |
| 14 ±1.01a* | 16 ±0.19d* | 17 ±1.16d* | 20 ±1.41cd* | 11 ±0.04 | Echinops macrochaetus |
| 10±1.02ab* | 11 ±0.06a* | 11±0.09a* | 12 ± 0.20a* | 08 ±0.70 | Oymbopogon citrates |
| 11 ±1.10ab* | 11 ±0.05a* | 10 ±0.08a* | 15 ±1.00d* | 08 ±0.10 | Xanthium spinosum |
*: significant results compared to aqueous extract at p ≤ 0.05, HW: hot water
Table 3. The diameter of the inhibition zones (mm) of the methanolic extract of the tested plants
using different pathogenic bacteria and compared to the antibiotic, Ciprofloxacin (positive control).
| Diameter o the inhibition zone (mm) | Pathogenic bacteria | |||
| Ciprofloxacin (control, 5µg/ml) | Xanthium | Oymbopogon | Echinops | |
| 31 ± 2.00 | 16 ±1.01* | 13 ± 0.11* | 20 ±1.44* | E. coli |
| 30 ± 1.05 | 14±1.51* | 14 ±1.05* | 22 ±0.51* | K. pneumoniae |
| 30 ± 0.09 | 14 ±0.41* | 14 ±0.09* | 20 ±1.14* | P. mirabilis |
| 31 ±0.29 | 14 ±0.45* | 12±0.06* | 21 ±1.06* | P. aeuroginosa |
| 20 ±0.25 | 13± 0.44* | 11±0.08* | 24 ±1.04 | E. faecalis |
| 24±1.33 | 13 ±1.45* | 11 ±0.09* | 24 ±1.90 | S. aureus |
| 27.7 | 13.8 | 12.5 | 21.8 | Antibacterial index+ |
+Activity index was calculated as the mean value of net zones of inhibition (mm) against the pathogenic bacteria,
* Significant difference compared to Ciprofloxacin at p ≤0.05
The genus Echinops L. (Asteraceae) was represented in Saudi Arabia with 10 species and was poorly understood. Moreover, Echinops macrochetus was recorded in Saudi Arabia but studies concerning its morphological characters, chemical competitions and biological activities were very rare and lacking. Morphological variations were affected by geographical area and environmental conditions and are very useful for taxonomically separating and distinguishing species which need sufficient experience. Root, leaves, inflorsence, corella, ovary and fruit morphological characters and diameters were applied to distinguish between the different species (Hymete et al. 2005a; Al-Joboury et al. 2021).
In Saudi Arabia folkloric medicine, different plants with therapeutic activities were traditionally applied for treatment of many bacterial pathogens and some important diseases. This study focused on collection and studying genus Echinops from local sites and identifies this plant according to plant identification keys. This plant was traditionally used as a fumigant against typhus fever, treatment of migraine, reduction of humans stomach ache, and intestinal diseases and it had strong nematicidal and molluscicidal activities, LD50 = 0.057 mg/ml (Abate and Ayehu 1993; Hymete et al. 2005; Chirayath et al. 2019; Viswanathan et al. 2019; Chassagne et al. 2021).
Aqueous and organic solvents were used in this study to select the strongest plant extract and our results proved that methanol extract is the most effective solvent for the active plant ingredients. Similarly, lemongrass, lantana and olive leaves methanol extracts had good inhibitory activity against some bacterial isolates (Aly et al. 2013). Extraction method, used plant part, type of the plant, the used solvents and the tested microorganisms, all are factors affected the antimicrobial activities. Growth inhibition of Enterococcus, Escherichia and Klebsiella by some plant extracts owing to the presence of some active metabolites like flavonoid, volatile essential oils; alkaloids; lectins, polypeptides and phenolics compounds were reported before (Chassagne et al. 2021).
The antitumor activity of the three methanolic extracts was determined using two different techniques (MTT and Neutral Red assays) and two cell lines (Hep G2 and MCF-7). Echinops macrochaetus extract showed excellent activity against MCF-7 cells and moderate activity against Hep G2 while no activity was recorded for the extracts of Xanthium and Oymbopogon. No toxicity was found for all the tested extracts except the extract of Xanthium which showed moderate toxicity, LD50= 50 µg/ml (Table 4). A recent study carried by Zamzami et al. (2019) discovered two new rare sesquiterpene glycosides, macrochaetosides A and B; originating from the aerial parts of E. macrochaetus. Their structures were discovered using different spectroscopic data while the active phytochemical agents in E. ritro were thiophenes, quinoline alkaloids, flavonoids, and sesquiterpenes, as well as fatty acids and alkanes (Bitewand Hymete 2019, Sabo and Knezevic 2019; Chassagne et al. 2021).
The minimal inhibitory concentrations were calculated using Microdilution method using fluorescein diacetate method. Moreover, the minimal inhibitory concentrations (MICs) of the methanolic extract of Echinops, Ciprofloxacin and their combination were determined and FIC index was calculated (Table 5). The obtained MICs of the tested methanol extracts were higher than the MIC of the tested antibiotics which was in the range of 0.12-0.14 µg/ml while the MICs of the extract of Echinops ranged from 0.5-0.75 µg/ml. The calculated MICs for the mixture of both ciprofloxacin and Echinops extract were in the range of 0.09-0.12 µg/ml. The inhibition activity of the combination (Fractional inhibitory concentration index, FIC) was calculated for each tested bacterial pathogen. They were ranged from 0.89-1.15. The interaction between plant extract and the tested antibiotics was either synergistic, additive where (0.5 < ∑FIC ≤ 1) or indifference (1 < ∑FIC ≤ 4) (Chassagne et al. 2021).
Acute and fatal infections of the urinary tract in patients with complicated cystitis are caused mainly by K. pneumoniae, P. mirabilis, P. aeruginosa, S. saprophyticus and E. coli which were strongly attached to the human bladder epithelium. Unfortunately, they are the most frequent causative agents of many dangerous diseases if they are untreated or not properly treated. E. coli (70-90%) followed by Klebsiella, Proteus and Pseudomonas identified as the most important urinary bacterial pathogens while S. saprophyticus, E. faecalis and S. agalactiae were less isolated Gram positive bacteria. Unfortunately, these bacterial infections were generally resistant to more than two classes of already used antibiotics, thus new search for novel agents from plants which playing an important role in improving human health is recommended by many authors (Aly and Gumgumgi 2011; Aly et al. 2013; MNPS 2020; Chassagne et al. 2021).
Furthermore, positive interaction was recorded between plant extract and antibiotic against some bacteria was recorded before. Inhibitory activity of both Ampicillin and garlic extract was excellent against S. aureus. Studying and purification of the active plant compounds was carried out and their mode of action is one or more of the suggested protocols, essential inhibition of some important enzyme, degradation of cell wall and cell membranes, prevention of DNA and protein synthesis (Aly et al. 2013; Ali et al. 2015; MNPS 2020; Al-Joboury et al. 2021).
Table 4. Antitumor activity (LD50, µg/ml) of the methanolic extracts of the three tested methanolic plant extracts against hepatocellular carcinoma ( Hep G2) and breast cancer (MCF-7) and their toxicity ( LD50, µg/ml) against Artemia salina.
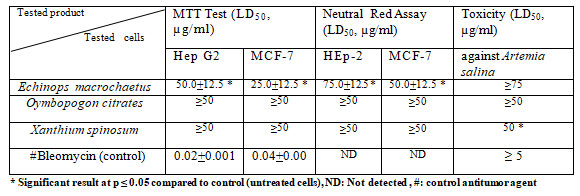
* Significant result at p ≤ 0.05 compared to control (untreated cells), ND: Not detected , #: control antitumor agent
Table 5. Minimal inhibitory concentrations (µg/ml) of Echinops methanolic extract,
Ciprofloxacin and their combination using Fluorescein diacetate protocol.
|
Tested bacteria |
Minimal inhibitory concentration ( µg/ml) | ||||
| Echinops | Ciproflox-acin | Echinops+ Ciprofloxacin | FIC index | Effect | |
| E. coli | 0.50± 0.12 | 0.12 ± 0.1 | 0.09 ± 0.0 | 0.89± 0.2 | Additive* |
| K. pneumoniae | 0.50± 0.12 | 0.12 ± 0.1 | 0. 09 ±0.0 | 0.93± 0.5 | Additive |
| P.mirabilis | 0.50 ± 0.12 | 0.14 ± 0.1 | 0.10 ±0.0 | 0.91± 0.1 | Additive |
| P. aeuroginosa | 0.50 ± 0.25 | 0.14 ± 0.1 | 0.10 ±0.0 | 0.91± 0.0 | Additive |
| E. faecalis | 0.75± 0.9 | 0.14 ± 0.1 | 0.12 ±0.0 | 1.15± 0.4 | Indepen-dent |
| S. aureus | 0.62± 0.12 | 0.17 ± 0.1 | 0.12± 0.0 | 0.94± 0.2 | Additive |
| *Additive :∑FIC less than 1, Independent :∑FIC more than 1 | |||||
Previous chemical investigation of Echinops species indicated the presence many active components including flavonoids compounds which were investigated in these plants (Al-Joboury, 2021). Six flavonoids and phenolic acid components varied in their containment were isolated by high pressure liquid chromatography from the three genera E. armatus, E. nitens and E. viscosus and all these secondary metabolites played a big role in the different recorded biological activities (Algabr et al. 2005, Cvetanovic et al. 2019, Dettweiler et al. 2020; Al-Joboury et al. 2021).
The chemical constituents of the species E. kebericho confirmed the presence of sesquiterpene lactones, β-sitosterol, stigmasterol, campesterol, β-amyrene, acetylenic thiophene lupeol and ursolic acid while tricyclic sesquiterpenes, monoterpenoids, sesquiterpenoid and sesquiterpene lactones were reported from E. grijsii, E. ritro, E. ellenbeckii and E. giganteus. Also, simple quinoline alkaloids, echinorine, 7-hydroxy-echinozolinone and echinazolinone were identified from over 14 members of the genus which act as either antibacterial or antitumor agents (Tadesse and Abegaz 1990; Afify and Hymete 1997; Weyersthal et al. 1998; Al-Joboury et al. 2021).
CONCLUSION
The results of this study confirmed the importance of some local plant extracts as a promising treatment for multidrug resistant bacteria which destroy almost used antibiotics. These plants can be used safely by local people to control some bacteria that cause many diseases. Furthermore, the dried powdered of Echinops macrochaetus, extracted with methanol was very effective against more than five different bacterial pathogens and had antitumor activities against two cancer cell lines with no toxicity. Thus, it may be used to develop new antibacterial or antitumor drugs, mainly against resistant bacteria pathogens. Combination of the previous plant extract with some antibiotics enhanced the antibacterial activity.
Conflict of Interests: Authors declare no conflict of interests to disclose.
Funding: This research did not receive any specific grant from funding agencies in the public.
REFERENCES
Abate, D and Ayehu A (1993). Medicinal Plants and Enigmatic Health Practices of Northern Ethiopia. Birhanena Selam: Addis Ababa, Ethiopia, 37– 44.
Abdallah, HM, Ezzat, SM, El Dine, RS et al. (2013). Protective effect of Echinops galalensis against CCl4-induced injury on the human hepatoma cell line (Huh7). Phytochem. Lett., 6, 73–78.
Afify MS and Hymete A (1997). Volatile constituents of the roots of Echinops kebericho Mesfin. Mansoura J. Pharmac. Sci., 13: 59-69.
Ahmed, M, Khan, MI, Khan, MR et al. (2013). Role of medicinal plants in oxidative stress and cancer. Sci. Rep., 2: 641.
Aleksic Sabo, V and Knezevic, P (2019). Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: a review. Ind. Crop. Prod. 132, 413–429.
Algabr, M, Hammoud, L, Aissaoui, H et al. (2015). Flavonoids from Pulicari ajaubertii (Asteraceae) from Yemen. RJPBCS, 6(6): 1237-1240.
Ali, MA, Al-Hemaid, FM, Lee, J et al. (2015). Unraveling systematic inventory of Echinops (Asteraceae) with special reference to nrDNA ITS sequence-based molecular typing of Echinops abuzinadianus, Genetics and Molecular Research 14 (4): 11752-11762
Al-Joboury, KR (2021). Review with checklist of Fabaceae in the herbarium of Iraq natural history museum. GSC Biological and Pharmaceutical Sciences.; 14(03): 137–142.
Aly, MM and Gumgumjee, NM (2011). Antimicrobial efficacy of Rheum palmatum, Curcuma longa and Alpinia officinarum extracts against some pathogenic microorganisms. Africian J. Biotechnology, Vol. 10 (56), pp. 12058-12063.
Aly, MM, Al-Ghamdi M, Bafeel SO et al. (2013). Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Lavandula dentata and Lectranthus tenuiflorus, Collected From Al Baha Region, Saudi. Arabia. Life Sci J., 10(4): 3302-3309
Ameya, G, Gure A and Dessalegn, E (2016). Antimicrobial activity of Echinops kebericho against human pathogenic bacteria and fungi. Afr J Tradit Complement Altern Med., 13(6):199-203.
Aro, AO, Dzoyem, JP, Awouafack, MD et al. (2019). Fractions and isolated compounds from Oxyanthus speciosus subsp. stenocarpus (Rubiaceae) have promising antimycobacterial and intracellular activity. BMC Complement. Med. Ther., 19:108.
Ba-Hamdan, A H A, Aly, M.M , Bafeel SO (2015). Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Ocimum basilicum, Collected from Jeddah Region, Saudi Arabia. Journal of Microbiology Research, 4(6A): 1-9.
Belcher, MS, Mahinthakumar, J, and Keasling, JD (2020). New frontiers: harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol., 65, 88–93
Betancur-Galvis, LA, Saez, J, Granados, H et al. (1999). Antitumor and Antiviral Activity of Colombian Medicinal Plant Extracts. Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 94(4): 531-535.
Bitew, H and Hymete A (2019). The Genus Echinops: Phytochemistry and Biological Activities: A Review
Carew, DP and Patterson, BD (1970). The effect of antibiotics on the growth of Catharanthus roseustissue cultures. Lloydia., 33:275–277.
CDC (2019). Antibiotic resistance threats in the United States, Atlanta, GA: U.S. Department of Health and Human Services.
Chand, S, Lusunzi, I, Veal, DA et al. (1994). Rapid screening of the antimicrobial activity of extracts and natural products. The Journal of Antibiotics, 57 (12) 1209-12014
Chassagne, F, Samarakoon, T, Porras, G et al. (2021). Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Frontiers in Pharmacology, Vol.11, 13,374.
Chirayath, RB, Viswanathan, A, Jayakumar, R et al. (2019). Development of Mangifera indica leaf extract incorporated carbopol hydrogel and its antibacterial efficacy against Staphylococcus aureus. Colloids Surf. B Biointerfaces, 178, 377–384.
Collenette, S (1999). Wild Flowers of Saudi Arabia. King of Saudi Arabia: National Commission for Wild Life Conservation and Development (NCWCD) and Sheila collenette. King Fahd National Library, pp. 188.
Holder, IA and Boyce, ST (1994). Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns, 20(5):426-9.
Cvetanovic, A, Zekovi C, Zengin, G et al. (2019). Multidirectional approaches on autofermented chamomile ligulate flowers: antioxidant, antimicrobial, cytotoxic and enzyme inhibitory effects. South Afr. J. Bot., 120, 112–118.
Desta, B. (1993). Ethiopian traditional herbal drugs. Antimicrobial activity of 63 medicinal-plants. J. Ethnopharmacol. 39, 129-139.
Dettweiler, M, Melander, R J, Porras, G et al. (2020). A clerodane diterpene from Callicarpa americana resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. ACS Infect. Dis. 6, 1667–1673.
Deyno, S, Mtewa AG, Hope D et al. (2021). Antibacterial Activities of Echinops kebericho Mesfin Tuber Extracts and Isolation of the Most Active Compound, Dehydrocostus Lactone. Frontiers in Pharmacology, vol 11, https://www.frontiersin.org/article/10.3389/fphar. 2020.608672
Dlamini, NP and Solomon, S (2019). Optimization of blending ratios of jam from Swazi indigenous fruits tincozi (Syzygium cordatum), tineyi (Phyllogeiton zeyheri) and umfomfo (Cephalanthus natalensis oliv.) using mixture design. Cogent. Food Agric., 5, 1684864.
El Sayed, HA and Aly MM (2014). Antibacterial Activities of Six Medicinal Plants Used Traditionally by Saudi People to Treat Common Diseases. British Biotechnology Journal 4(4): 499-510.
El Sayed HE, Fallatah NSA, Jastaniah SD et al. (2019). In vitro inhibitory effects of three traditional and medicinal plants on some human pathogenic bacteria. IOSR Journal of Pharmacy, Volume 9, Issue 1 Version. I, PP. 70-76
Fokialakis, N, Cantrell, CL, Duke SO et al. (2006). Antifungal Activity of Thiophenes from Echinops ritro. J. Agric. Food Chem., 54, 1651-1655 1651
Gao, X, Han, J, Dai, H, et al. (2009). Study on optimizing the technological condition of ethanol percolating extraction for Goupi patch. Zhongguo Yaoshi. ;12(10):1395–1397.
Halwani, MA, Tashkandy, Nl, Aly, MM et al. (2015). Incidence of Antibiotic Resistance Bacteria in Jeddah’s Ministry of Health Hospitals, Saudi Arabia. Advances in Microbiology, 2015, 5, 780-786.
Hymete, A and Kidane, A (1991). Screening for anthelmintic activity in two Echinops spp. Ethiop. Pharm., J. 9, 67–71.
Hymete, A, Rohloff, J, Iversen, TH et al. (2007). Volatile constituents of the roots of Echinops kebericho Mesfin. Flavour and Fragrance J., 22:35-38.
Hymete, A, Iversen, TH, Rohloff, J et al. (2005a). Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine, 12, 675–679.
Hymete, A, Rohloff, J, Kjosen, H et al. (2005b). Acetylenic thiophenes from the roots of Echinops ellenbeckii from Ethiopia. Nat. Prod. Res., 19, 755-761.
Hymete, A, Rohloff, J, and Iversen, TH (2004). Chemical constituents of volatile fractions from Echinops ellenbeckii O. Hoffm. J. Essent. Oil. Bear Pl., 7, 9-15.
Ibrahim, SRM, Abdallah, HM, Mohamed, GA et al. (2016). Integracides H-J: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia, 112, 161–167.
Ivana, T (2015). Phytochemische und antimikrobielle Untersuchung an Echinops kebericho Mesfin. Master’s thesis. Graz (Austria): Karl-Franzens-Universität Graz.
Jaleel, CA, Manivannan, P and Sankar, B (2007). Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Biointerfaces, 60:201-206.
Jiang, M, Zhao, S, Yang, S et al. (2020). An essential herbal medicine licorice: a review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol., 249, 112439.
Kuete, V, Sandjo LP, Wiench B et al. (2013). Cytotoxicity andmodes of action of four Cameroonian dietary spices ethnomedically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperatacyl indrica and Piper capense, Journal of Ethnopharmacology, 149 (1):245–253.
Maurya, SK, Kushwaha, AK and Seth, A (2015). Ethnomedicinal review of Usnakantaka (Echinops echinatus Roxb). Pharmacogn. Rev., 9, 149–154.
MNPS (2020). Medicinal plant names services (MNPS). Available at: https://mpns. science.kew.org/mpns-portal.
Mordmuang, A, Brouillette, E, Voravuthikunchai, SP et al. (2019). Evaluation of a Rhodomyrtus tomentosa ethanolic extract for its therapeutic potential on Staphylococcus aureus infections using in vitro and in vivo models of mastitis. Vet. Res. 50, 49.
NLM (2020). National library of medicine (US). Available from: http:// clinicaltrials.gov (Cited 24 February, 2021).
Nobili, S, Lippi, D, Witort, E et al. (2009). Natural compounds for cancer treatment and prevention. Pharmacol. Res., 59, 365-378.
Parekh, J, Nair, R and Chanda, S (2005) Preliminary screening of some folkloric plants from Western India for potential antimicrobial activity. Indian J. Pharmacol., 37:408–409.
Petersen, PJ, Labthavikul, P, Jones, CH et al. (2006). In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemoth., 57:573–576
Poulakou, G, Lagou, S, Karageorgopoulos, DE et al. (2018). New treatments of multidrug-resistant Gram-negative ventilator-associated pneumonia. Ann Transl Med.; 6(20):423–423.
Saeed, M, Jacob, S, Sandjo, LP et al. (2015). Cytotoxicity of the sesquiterpene lactones neoambrosin and damsin from Ambrosia maritime against multidrug-resistant cancer cells. Front. Pharmacol., 6, 267.
Santos, PRV, Oliveira, ACX, Tomassini, TCB (1995). Controlemicrobiógico de produtosfitoterápicos. Rev. Farm. Bioquím., 31, 35-3
Sarma, GC and Borah, RL (2012). Systematic survey of Asteraceae of Dibrugarh district of Assam India. Indian J. Plant Sci., 1, 4–39.
Shriram, V, Khare, T, Bhagwat, R et al. (2018). Inhibiting bacterial drug efflux pumps via phytotherapeutics to combat threatening antimicrobial resistance. Front Microbiol., 9:1-18.
Shukla, YN (2003). Chemical, botanical and pharmacological studies on the genus Echinops: aeview. J. Med. Aromat., Plant Sci., 25, 720-732.
Singh, V and Kumar, S (2001). Asteraceae of Sikkim. Deep Publications, New Delhi, India, pp. 209.
Tadesse, M and Abegaz, BA (1990). Revision of the genus Echinops (Compositae-Craude) in Ethiopia, with notes on phytogeography and chemistry. Proceedings of the 12th plenary meeting of the association for the taxonomic study of the flora of tropical Africa, 605–629.
Tene, M, Tane, P, Sondengam, BL et al. (2004). Lignans from the roots of Echinops giganteus. Phytochemistry, 65: 2101–2105.
Thielmann, J, Muranyi, P, and Kazman, P (2019). Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 5, e01860.
Viswanathan, V, Pharande, R, Bannalikar, A et al. (2019). Inhalable liposomes of Glycyrrhiza glabra extract for use in tuberculosis: formulation, in vitro characterization, in vivo lung deposition, and in vivo pharmacodynamic studies. Drug Dev. Ind. Pharm., 45, 11–20.
Wang, H, OoKhor, T, Zhengyuen, S et al. (2014). Plants against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med Chem.,12(10): 1281-1305.
Weyersthal, P, Marchall, H, Seelmann, I et al. (1998). Volatile constituents of the roots of Echinops kebericho Mesfin. Eur. J. Org.Chem.,6: 1205-1212.
Wink, M (2020). Evolution of the angiosperms and Co-evolution of secondary metabolites, especially of alkaloids. Co-evolution of secondary metabolites. Editors: JM Mérillon and KG Ramawat (Cham, Switzerland: Springer International Publishing), 151–174.
Wu, C, Lee, SL, Taylor, C et al. (2020). Scientific and regulatory approach to botanical drug development: a U.S. FDA perspective. J. Nat. Prod., 83, 552–562.
Yadava, RN and Singh, SK (2006). New anti-inflammatory active flavanone glycoside from the Echinops echinatusRoxb. Indian J. Chem. B., 45:1004-1008.
Zaman, RM, Aly, MM and Helmi, NR (2015). Antimicrobial susceptibility pattern of Gram-negative bacilli isolated from a Teaching Hospital in Jeddah, Saudi Arabia. Afri. J. Biotech., Vol. 9(41):2145-2158.
Zamzami, T, Abdallah, H, Shehata, I et al. (2019). Macrochaetosides A and B, new rare sesquiterpene glycosides from Echinops macrochaetus and their cytotoxic activity. Phytochemistry Letters, Vol 30: 212-219.
Zaynab, M, Fatima, M, Abbas, S, et al. (2018). Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 124, 198–202.
Zhao, LY, Liu, HX, Wang, L et al. (2019). Rhodomyrtosone B, a membrane-targeting anti-MRSA natural acylgphloroglucinol from Rhodomyrtus tomentosa. J.


