1Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
3King Fahad Medical Research Center, Microbiology Lab, P.O. Box 80216, King Abdulaziz University, Jeddah,
Saudi Arabia,
4Department of Hematology , King Abdulaziz University, P.O.Box 80215, Jeddah, Saudi Arabia
Corresponding author email: munagull@hotmail.com
Article Publishing History
Received: 07/07/2020
Accepted After Revision: 09/09/2020
Antibiotic resistance is a problem that continues to challenge the healthcare sector in a large part of the world. It is very important to control that problem, so the discovery of new active compounds and antibiotic has focused on screening bacteria for new growth inhibitory compounds. This study aimed to investigate the antibacterial, antioxidant potential and phytochemical composition of extracts of Moringa oleifera Lam leaves in methanol, ethyl acetate and hexane against clinical resistance bacterial isolates. A total of 8 bacterial isolates were selected to analyze the antibacterial and antioxidant potential of various Moringa oleifera leaf extracts. Antimicrobial susceptibility test was performed by Kirby-Bauer disc diffusion technique and MIC and MBC values were also recorded. Antioxidant potential was determined based on the free radical scavenging activity of 2, 2- diphenyl-1- picrylhydrazyl (DPPH) assay. Finally, qualitative and quantitative analyses of phytochemical constituents of Moringa oleifera leaf extracts were performed by HPLC.
Result showed that ethyl acetate extract demonstrated higher antibacterial activity against Bacillus subtilus with zone of inhibition 28 ± 8.2 mm, followed Streptococcus viridans (21.67 ± 5.86 mm). These extracts were not active against E. coli, Klebsiella pneumonia and Salmonella group B. Hexane extract showed antibacterial activity against all tested bacteria. The extracts showed strong antioxidant activity with 50% efficient concentration (EC50) values of 117.94 and 150.96 μg/ml for the methanol and ethyl acetate extracts respectively. The highest phenolic content was observed in methanolic leaf with 140.1 9 ± 0. 0.71 (mg GAE/g) while flavonoid leaf extract was found 98.67±2.10 (mg QE /g) respectively. In addition, different phenolic and flavonoid compounds were also determined individually. This study concludes that Moringa oleifera Lam. leaf extracts have significant antimicrobial and antioxidant properties which authenticate its potential as cure against a wide variety of infectious diseases.
Moringa Leaves; Pathogenic Bacteria; Antioxidants; Phytochemicals; Alkaloids; Flavonoids
Khouj W. W, Gull M, Rahimuddin S. A, Alshamrani R, Najjar A. A, Al-Hejin A. M, Zaher G. F. Screening of Antimicrobial and Antioxidant Activities of Moringa oleifera Lam Leaf Extracts Against Multidrug Resistant Pathogenic Bacteria. Biosc.Biotech.Res.Comm. 2020;13(3).
Khouj W. W, Gull M, Rahimuddin S. A, Alshamrani R, Najjar A. A, Al-Hejin A. M, Zaher G. F. Screening of Antimicrobial and Antioxidant Activities of Moringa oleifera Lam Leaf Extracts Against Multidrug Resistant Pathogenic Bacteria. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/31rhX1u
Copyright © Khouj et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Infectious diseases have appeared as one of the major threats to human health around the world and become the major cause of morbidity and mortality. The research of this line has been fruitful and provided medical science with many of the frontline antibiotics in clinical use (Ilanko, et al., 2019). However, antibiotic resistance becomes a problem that continues to challenge the health care sector in a large part of the world. The rise of untreatable bacterial diseases with more resistance to antibiotics and the cause of increasing evolution of multi-drug resistant (MDR) bacteria that remains a wildly unresolved problem and big charged to health services (Valle Jr, et al., 2015; Maillard et al., 2020; Tufa, et al., 2020). The discovery of new active compounds against new targets is very important to control the problem that most pathogenic organisms are becoming resistant to antibiotics. Natural antibacterial and antioxidants compounds produced by plants are becoming a big interest in recent research (Dalukdeniya, et al., 2016). They used as safe therapeutics for a wide range of various diseases in medicinal applications (Busani, et al., 2012; Thirumalai, et al., 2018; Adamczak et al., 2020).
Moringa oleifera leaves are a well-known source of natural antibacterial and antioxidants. For controlling the pathogenic bacteria, Moringa oleifera Lam. has become promising natural antimicrobial agents with potential applications in pharmaceutical industry (Reetu, et al., 2020). The extracts of Moringa oleifera Lam. can be used to discover antibacterial agent for developing new pharmaceuticals to control studied human pathogenic bacteria responsible for the severe illness. Moringa oleifera leaves are providing protection against infections and degenerative diseases by inhibiting and scavenging free radicals (Ashour, et al., 2020).
Phytochemical analyses have shown that its leaves are particularly rich in vitamins especially A, D and C. Also, they are containing essential amino acids, antioxidants, flavonoids, and a lot of minerals that are essential for growth and development (Gopalakrishnan, et al., 2016; Su and Chen, 2020). The extracts from Moringa oleifera exhibit multiple nutraceutical or pharmacological functions including anti-inflammatory, antioxidant, anti-cancer, hepatoprotective, neuroprotective, hypoglycemic, and blood lipid-reducing functions (Kou, et al., 2018; Shourbela, et al., 2020). Recent trials revealed that Moringa oleifera leaves might contribute to prevent obesity as well as obesity-related complications (Mabrouki, et al., 2020). Considering these facts, the present research work was designed to explore the antimicrobial and antioxidant activities of Moringa Oleifera Lam. leaf extracts.
The present study also evaluates the occurrence of natural antimicrobial and bioactive compounds in Moringa Oleifera Lam. leaf extracts and characterize it to be used as the alternative therapeutic agent. There are only a few elaborative studies on the bioactive constituents of M. oleifera leaves and their effect on multidrug resistance bacteria. This study aims to bridge the gap. Moreover, much of the evidence remains anecdotal as there has been diminutive concrete scientific studies done to hold authentic claims about Moringa oleifera indicating the need of more exploration of this plant (Fahal, et al., 2018; Suresh, et al., 2020).
MATERIAL AND METHODS
Plant Material Collection: Fresh leaves of Moringa oleifera Lam. were collected from Saudi Arabia locally and identified in the laboratory by standard flora identification method and confirmed by plant data base https://www.rbg.vic.gov.au/science/herbarium-and-resources/online-databases. The taxonomic identification of this plant also performed by comparison with existing herbarium in Biology department of King Abdulaziz University, Jeddah Saudi Arabia.
Plant Extracts Preparation: The collected Moringa oleifera leaves were directly washed to remove debris and allowed to dry under shade. The dried leaves were grounded by blender to fine powder and 790 g was obtained. For the extract preparation, plant material extracted with hexane, ethyl acetate and methanol (72 h each) using a Soxhlet extractor. Extracts were then filtered with Centrifuge at 4000 G for 5 min to remove any debris and concentrated using a rotary evaporator under vacuum at approximately 40°C. The dried extracts lyophilized in lypholyser and stored in airtight tubes at 4°C for further use.
Pathogenic Bacteria Used for Susceptibility Test: A total of 8 bacterial species were tested including four Gram positive bacteria (Bacillus subtilus, Staphylocococcus aureus, Streptococcus viridans and Methicillin resistance Staphylocococcus aureus) and four Gram negative bacteria (E. coli, Klebsiella pneumoniae, Salmonella group B, and Shigella sonnei) were obtained from Microbiology Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. These species were originally isolated from the clinical samples and identified based on standard phenotypic tests according to Bergey’s manual of systematic bacteriology.
Determination of Antibacterial Activity: Antibacterial activity of the hexane, ethyl acetate and methanol extracts of the plant was studied by standard paper disc diffusion method. Active cultures of eight bacteria were prepared by transferring a sterile loop swap of culture to 5 ml of nutrient broth and incubated at 37 °C for 24 h. The turbidity was adjusted equivalent to 0.5 McFarland units by spectrophotometry at 600 nm. Final cell concentrations were adjusted to 105 CFUmL-1 with reference to the McFarland turbidometry (Burt and Reinders, 2003). The positive control was antibiotic kanamycin (25μg/ml) for antibacterial activity. The susceptibilities of the isolated pathogens were determined by the modified Kirby-Bauer disc diffusion method (Bauer, et al., 1966) with Muller Hinton agar plates (MHA, Merck, Germany). Aliquots of inoculums were spread over the surface of agar plates with a sterile cotton swap.
To test the antimicrobial activity all extracts were dissolved in DMSO to make a final concentration of 400 μl /ml. 20 μl of each extract soaked separately into sterile discs and dried in open air. Solvents were evaporated and then the discs were placed on bacterial cultures. These discs placed on Muller Hinton agar plates, previously swabbed with the bacterial inoculums. The plates were left at room temperature for 1 hour and then the petri dishes were subsequently incubated for 24 h at 37°C. Each experiment did in triplicate and mean values were taken. Antimicrobial activity measured in the diameter (mm) of the clear inhibitory zone formed around the disc.
Determination of Minimum Inhibitory Concentrations (MIC): The minimum inhibitory concentration (MIC) of the extracts was determined for most sensitive bacterial species. A 16-hour culture was diluted with a sterile physiologic saline solution (0.9% (w/v) sodium chloride) with reference to the 0.5 McFarland turbidometry to achievement the inoculum approximately equal to 105 CFUmL-1 (Burt and Reinders, 2003). In the tube dilution assay, standard bacterial suspension and different concentration of extracts (5, 10, 20, 40, 80 and 160 mg/mL) were added to tubes containing 1 mL Muller Hinton broth. These tubes were incubated at 37°C for 24 hours. The first tube of the series with no sign of visible growth was considered as the MIC. This process has been done three times (Mahboobi, et al., 2006).
Determination of Minimum Bactericidal Concentrations (MBC): To determine the MBC, for each set of test tubes in the MIC assay, a loop full of broth was collected from the tubes without any visible growth and cultured at 37°C for 18 – 24 hours. The highest dilution that yields no colony formation on solid medium was considered as MBC (Motamedi, et al., 2009).
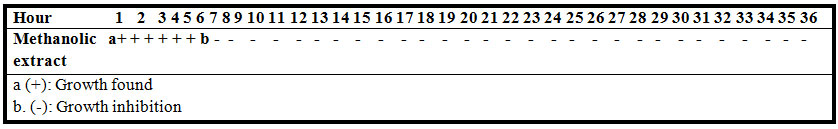
Time-Kill Kinetic Study: The time-kill kinetics was studied by culturing one standard loop of the suspension from the tube possessing MBC on MHA from 0 to 36 hours. This was performed at the first hours of intervals for the first 18-hour study, and then at 2-hour intervals for the later 18 hours (Mahboobi, et al., 2006).
Antioxidant Activity: The antioxidant activity of the hexane, ethyl acetate, and methanol leaf extracts of Moringa oleifera determined based on the free radical scavenging activity of 2, 2- diphenyl-1- picrylhydrazyl (DPPH) according to the method described by Brand-Williams, et al., (1995). One hundred and fifty µl of DPPH solution (4.3mg/3.3ml methanol) was added to 3ml methanol and absorbance was taken immediately at 517nm for control reading. 50µl of various concentrations (25, 60, 120 and 240 μg/ml) of each extract was taken and the volume was made uniformly to150µl using methanol. Each of the samples was then further diluted with methanol up to 3ml and to each 150µl DPPH was added (to exclude color factor of sample we performed sample blank to each concentration that contains No DPPH, only methanol added, then we subtract the reacted sample with DPPH from blank sample). Absorbance was taken after 15 min. at 517nm using methanol as blank on spectrometer. The % scavenging was calculated as: % scavenging = [Absorbance of control – Absorbance of test sample/Absorbance of control] X 100
Phytochemical Screening: Preliminary phytochemical screening was performed by using standard tests. Test for alkaloids was done using Dragendroff’s test. To 1ml of extract, 1ml of water and 1ml of Aq. NaOH was added. Yellowish brown precipitate was obtained which confirmed the presence of Glycosides. Presence of flavonoids was confirmed by adding 1ml of 10% lead acetate to 1ml of the extract with the appearance of yellowish green precipitate. A froth was obtained by boiling 1ml of extract with 1ml of distilled water which confirmed the presence of saponins. Few drops of 0.1% Fecl3 was added to 1ml extract. A brownish green precipitate was formed confirming the presence of tannins. A brown precipitate was obtained on adding 1ml of CHCl3, 2ml conc. H2SO4 to 1ml of extract indicating the presence of terpenoids (Evans, 2000). To 1ml of extract was added 1ml of 40% NaOH and 2 drops of 1% CuSO4. A pink color was obtained indicating the presence of proteins. Benedict’s test was performed with 1ml extract. A slight red precipitate was obtained showing the presence of carbohydrates.
Estimation of Total Phenolic Contents: The total phenolic content of the extracts was determined using the method described by Kim, et al., (2003) with modification. To start the analysis, 1 ml of the extract (0.1 mg/ml) was mixed with 0.2 ml of Folin-Ciocalteu’s phenol reagent. After 5 min, 1 ml of 7.6% Na2CO3 solution was added to the mixture followed by the addition of 2 ml of deionised distilled water. The mixture was stirred and allowed to stand for 90 minutes. The mixtures (in triplicate) were incubated at 40oC for 30 min and the absorbance was read at 760 nm. The total phenolic content was determined from extrapolation of calibration curve which was made using gallic acid solution and expressed as milligrams of gallic acid equivalents (GAE) per gram of the dry weight.
High Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds:
High performance liquid chromatography analysis with UV detection was performed for the estimation of the phenolic compounds in the plant extracts. The shaded dried plant material (200 g) was crushed to make it coarse powder. The coarse powder (20 g) was grinded with 25 ml distilled water of 2 N-HCl. The grinded plant extracts were heated in water bath using air condenser at 100°C for 1 h. The plant extracts were filtered using Whatman filter paper No.1. By using a separating funnel, the filtrate was extracted with diethyl ether. The layer of diethyl ether was washed and separated with distilled water and dried over sodium sulphate (anhydrous). The final evaporated extract was obtained using rotary vacuum evaporator at 25°C. The collected extract re-dissolved in HPLC grade ethanol (5 ml), prior to the injection into HPLC column. The samples were filtered through 0.22 μm organic filter (Millipore) before use (Joshi, 2011).
Analysis of Individual Phenolic Acids by HPLC: The reverse phase high performance liquid chromatography (RP-HPLC) analysis was performed for the estimation of phenolic acids compounds like gallic acid, parahydroxy benzoic acid, vanillic acid, syringic acid and ferulic acid. During study, used HPLC apparatus was HPLC-Beckman model-322 equipped with 100A model pump, 210 injector, 420 controller, mixer and BD-40 recorder. C18 column (ultrasphere) with specification of 5um (25 cm x 4.6 mm length). Mobile phase was setup strictly with this ratio, methanol: water (1% acetic acid in 20: 80 v/v). Prior to use in HPLC, mobile phase was degased. Flow rate was maintained at 1ml min-1 with chart speed 1cm min-1. UV detector was fixed with max 280 nm λ, aufs Attenuation (0.02) and isocratic mode. For individual phenolic compound, the detector response was calibrated and measured with standard phenolic acids strictly as described by Tandon, et al., (2001). All standard phenolic compounds were procured from Sigma-Aldrich chemical company, USA.
Statistical Analysis: The parameters tested in triplicate and the values expressed as the mean ± standard deviation (SD). Statistical analysis was performed with analysis of variance (ANOVA) test and independent sample t-test using the Mega Stat Excel (version 10.3, Butler University) and all columns versus control and the P value < .05 considered as significant.
RESULT AND DISCUSSION
Moringa oleifera Lam. is reported to have an antimicrobial effect on pathogenic bacteria. The antimicrobial properties of Moringa oleifera have been attributed to different parts of the plant, such as the leaves, seeds, pods and stems (Tirado-Torres, et al., 2019) which are known for their antibacterial activity and are counted as rich source of antimicrobial agents (Abadallah and Ali, 2019).
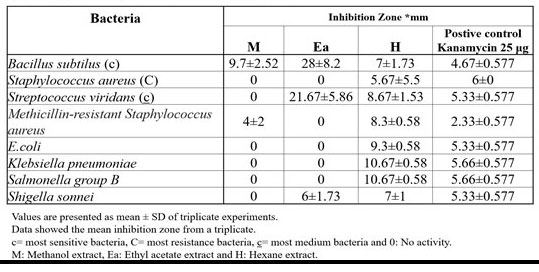
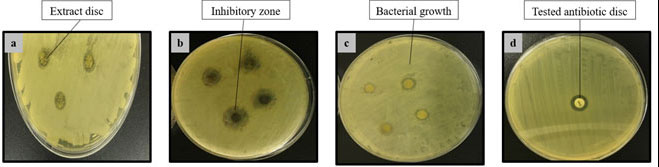
The disc diffusion method of antimicrobial susceptibility testing was performed to determine the antibacterial activities of the plant against multidrug resistance pathogenic bacteria through an in vitro assessment of finding sensitivity or resistance to an antimicrobial agent (Figure 1). The extracts of Moringa oleifera tested showed varying degree of inhibitory activity against the tested pathogens (Table 1).
Comparison of all plant extracts data for their antibacterial potentials reveals that ethyl acetate extract showed the highest antibacterial activity by zone size (28 ± 8.2 mm) while the methanol extract exhibited least activity by zone size of (4 ± 2 mm). In the study of Raj, et al., (2011) used different extracts of Moringa oleifera Lam. root that tested for antimicrobial activities against some pathogenic bacteria by disc diffusion method and the result showed high antibacterial activity against Pseudomonas aeruginosa (18.2 ± 0.2 mm) by ethyl acetate extract.
On the other hand, methanol and ethyl acetate extracts were not active against E. coli, Klebsiella pneumonia and Salmonella group B. Hexane extract showed antibacterial activity against all tested bacteria. The results were compared with results obtained using standard antibiotics, kanamycin (25 µg/disc that served as reference for inhibition zone diameter.
Table 1. The antimicrobial activity (zone of inhibitionO of the tested plant extracts the tested antibiotic (standard) against pathogenic bacteria by disc diffusion method.
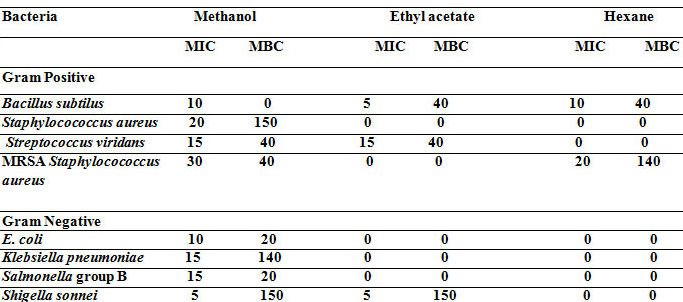
Table 1a. Antibacterial activity (MIC and MBC, mg. -1) of the plant extracts of Moringa oleifera against pathogenic bacteria
Table 1b. The Time-Kill kinetic of methanolic extract of Moringa oleifera at 20 mg/ml concentration against E. coil
The results of MIC and MBC of hexane, ethyl acetate and methanolic extracts for 8 bacterial species are shown in Table 1a and Time-kill kinetic of methanolic extract of Moringa oleifera was 6 hours (Table 1b).
Figure 1: Inhibition zones shown by Moringa oleifera extracts (a) Methanol extract against Bacillus subtilus, (b) Ethyl acetate extract against Streptococcus viridans, (c) Hexane extract against E. coli, (d) Tested antibiotic Kanamycin as positive control
Like this study, Ojiako (2014) showed that ethyl acetate possesses the highest zone of inhibition 10 mm in Staphylococcus aureus and Salmonella typhi followed by E. coli 8 mm, Candida albican 4 mm, and Mucor 2 mm.
Among all tested bacteria Bacillus subtilus was more sensitive and showed higher inhibition zone (28 ± 8.2 mm) with ethyl acetate extract, while Staphylocococcus aureus was most resistance bacteria, showed no activity with methanol and ethyl acetate extracts and less zone of inhibition (5.67 ± 5.5 mm) with hexane extract. Hexane extract showed antibacterial activity against all tested bacteria. The higher zone of inhibition (10.67 ± 0.58) was showed against both Klebsiella pneumoniae and Salmonella group B and lowest zone of inhibition (5.67 ± 5.5 mm) against Staphylocococcus aureus (Table 1). Streptococcus viridans, Methicillin resistance Staphylocococcus aureus and Shigella sonnei showed antibacterial activity with two different extracts. Comparing among these bacteria, Streptococcus viridans and Shigella sonnei showed inhibition zone (21.67 ± 5.86 mm and 6 ± 1 mm) respectively with ethyl acetate extract, while Methicillin resistance staphylocococcus aureus showed less inhibition zone (4 ± 2 mm) with methanol extract. So, Streptococcus viridans was the most medium sensitive bacteria.
Against Bacillus subtilus, ethyl acetate extract showed the highest antibacterial activity (28 ± 8.2 mm), while the least activity was (9.7 ± 2.52 mm and 7 ± 1.73 mm) showed by methanol and hexane extracts, respectively. Hexane extract was less sensitive against Staphylocococcus aureus (5.67 ± 5.5 mm) and no activity showed with methanol and ethyl acetate activity. Also, the ethyl acetate showed antibacterial activity against Streptococcus viridans (21.67 ± 5.86 mm) and less activity with hexane extract (8.67 ± 1.53 mm) but no activity with methanol extract. Methanol extract was most resistance against Methicillin resistance Staphylocococcus aureus (4 ± 2 mm) and no activity with ethyl acetate extract, while the hexane extract showed antibacterial activity with zone size 8.3 ± 0.58 mm. Methanol and ethyl extracts showed no activity against E. coli, but the hexane extract showed antibacterial potential against E. coli (9.3 ± 0.58 mm). Klebsiella pneumoniae and Salmonella group B showed no activity with methanol and ethyl acetate extracts but high activity in hexane extract with same zone size 10.67 ± 0.58 mm. Ethyl aetate and hexane extract showed activity against Shigella sonnei (6 ± 1.73 mm and 7 ± 1 mm) respectively but showed no activity with methanol extract. Similar results regarding antimicrobial activity of Moringa leaves were also reported by Singh, et al., 2013.
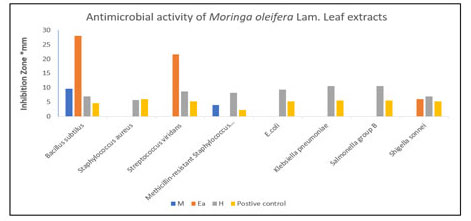
The antimicrobial potential of the experimental plant extracts was evaluated by their zone of inhibition against various pathogens and the results (zone of inhibition) were compared with activity of standard, Kanamycin (25 μg). The Moringa oleifera leaf ethyl acetate extract was found most effective comparing to the standard Kanamycin 25 μg against Bacillus subtilus (28 ± 8.2 mm), Streptococcus viridans (21.67 ± 5.86 mm) and Shigella sonnei (6 ± 1.73 mm). The inhibition zone produced by hexane extracts were more effective than standard against all tested bacteria except for Staphylocococcus aureus (5.67 ± 5.5 mm) which was most resistance bacteria. Methanol extract showed most effective comparing to standard against Bacillus subtilus and Methicillin resistance Staphylocococcus aureus (28 ± 8.2 mm and 4 ± 2 mm) respectively (Figure 2).
Figure 2: Comparison of antimicrobial potential of the MOL extracts tested by inhibition zone (mm) using Disc Diffusion method. M: Methanol extract, Ea: Ethyl acetate extract, H: Hexane extract and Kanamycin as positive control
In the study of Yee (2019), different parts of Moringa oleifera were tested the antibacterial activity of five extracts (PE, EtOAc, MeOH, 95% EtOH and H2O) and were investigated on 5 strains of bacteria which include Bacillus Subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus pumalis and Escherichia coli by agar disc diffusion method. Among these five crude extracts, EtOAc (ethyl acetate) extract showed the inhibition zone diameters in the ranged of 35-45mm has highest antimicrobial activity than the other extracts. These results support the data of present investigation.
Further the resistance and susceptibility testing of our study was quite similar with the results of Abadallah and Ali (2019) showed that ethanol extract Moringa oleifera leaf demonstrated higher antibacterial activity with average zone of inhibition of 12.49 mm than aqueous extracts (8.00 mm). Based on the susceptibility of the organisms to the extracts, Shigella spp was found to be the highest susceptible organisms with average zone of inhibition of 12.46 mm, followed Staphylococcus aureus (11.47 mm), Salmonella typhi (10.81 mm), E. coli (10.81 mm) while low average zone of inhibition is shown by Enterococcus faecalis (9.76 mm).
Many other researchers highlighted the positive and effective antibacterial activity of aqueous extracts, chloroform extracts and methanol extracts obtained from leaves, bark and roots of Moringa oleifera (Lam) against four food borne microbial pathogens, Salmonella enteritica, Vibrio parahaemolyticus, Escherichia coli and Listeria monocytogenes. The main finding of these studies was obtained that all extraction methods showed antimicrobial activity against all tested microorganisms. Lowest and highest antibacterial activity was shown by aqueous extraction and chloroform extraction of residue obtained after aqueous extraction. Highest antibacterial activity was shown by chloroform extraction of residue obtained after aqueous extraction against Salmonella enteritica. Listeria monocytogenes was found to be the most resistant organism to all types of extracts (Dalukdeniya, et al., 2016; Chakraborty, et al., 2019).
The plant is mainly ascribed to the presence of antioxidant constituents such as phenolic acids and flavonoids. Due to the high concentrations of antioxidants present in Moringa oleifera leaves, they can be used in patients with inflammatory conditions, including cancer, hypertension, and cardiovascular diseases. The antioxidants have the maximum effect on the damage caused by free radicals only when they are ingested in combination. A combination of antioxidants found in Moringa oleifera leaves was proven to be more effective than a single antioxidant, possibly due to synergistic mechanisms and increased antioxidant cascade mechanisms (Vergara-Jimenez, et al., 2017 ; Yakoub et al., 2018). Phytochemical and antimicrobial analysis of Moringa oleifera leaf was also screening by Oladeji et al., (2020).
The secondary metabolites in Moringa oleifera leaf were extracted by maceration using chloroform, ethyl acetate and ethanol. Some important bioactive metabolites in the leaf extracts, such as steroids, saponins, tannins, flavonoids, terpernoids and phlobatannins were analyzed. The ethanolic leaf extract was observed to show the highest antimicrobial activity when compared to chloroform and ethyl acetate extracts. It also compared favorably to nystatin, streptomycin and gentamicin (standard antibiotics). The study affirmed the therapeutic potency of the plant, indicated by its high antimicrobial effects on some pathogens like Klebsiella sp, P. aeruginosa, Trichoderma sp, Aspergillus flavus, Bacillus cereus, S. pneumoniae, Candida. sp, and E. coli.
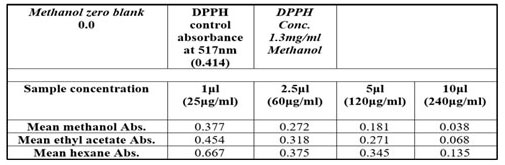
Table 2. Absorbance values of Moringa oleifera extract with different conecentration
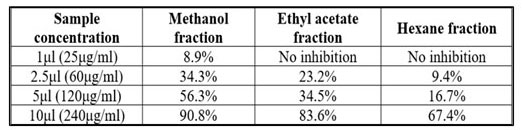
Table 3. Antioxidant activity of Moringa oleifera extracts
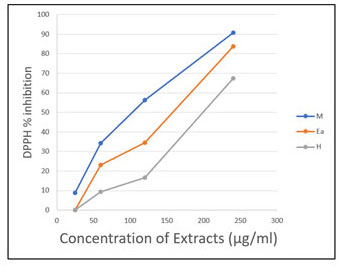
Figure 3: Antioxidant activity of Moringa oleifera extracts M: Methanol extract, Ea: Ethyl acetate extract and H: Hexane extract
Quantification of antioxidant activity using DPPH free radical scavenging showed a dose-dependent antioxidant activity for the methanol, ethyl acetate and hexane extracts (Figure 3). From the results obtained, the highest antioxidant activity of 56.3% was exhibited by the methanol extract after 15 min incubation at a concentration of 120 μg/ml. Also, ethyl acetate extract showed good scavenging activity. The extracts showed strong antioxidant activities with EC50 values of 117.94 and 150.96 μg/ml for methanol and ethyl acetate extracts respectively (Table 2).
Similar study was reported by Igbo, et al., (2015) using DPPH free radical scavenging for antioxidant activity. From the results obtained, the highest antioxidant activity of 89.1% was exhibited by the methanol extract at a concentration of 125 μg /ml. The extracts showed strong antioxidant activity with 50% efficient concentration (EC50) values of 24 and 44 μg/ml for the ethyl acetate and methanol extracts respectively (Table 3). The study of Fitriana, et al., (2016) provided that Moringa oleifera leaves possess antioxidant. The extracts have been evaluated for its antioxidant activity by 1,1- diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity assay and an improved 2,2’-azino-bis- [3-ethylbenzothiazoline sulphonate] (ABTS) radical cation decolorization assay in vitro.
The methanol extract showed the highest free radical scavenging activity with IC50 value of 49.30 μg/mL in DPPH assay and 11.73 μg/mL in ABTS assay supporting the data of present investigation (Table 2&3). Also, the experiments of Vyas, et al., (2020) was clearly indicated that Moringa oleifera leaves showed effective free radical scavenging activity which can be attributed to the presence of flavonoids and phenolics along with other compounds.
Table 4: Qualitative phytochemical analysis of Moringa oleifera leaf extracts.
| Phytochemical constituents | Extracts | ||
| Methanol | Ethyl acetate | Hexane | |
| Alkaloids | + | + | + |
| Glycosides | + | – | + |
| Flavanoids | + | + | + |
| Saponins | + | + | – |
| Tannins | + | + | + |
| Terpenoids | + | – | + |
| Proteins | + | + | + |
| Carbohydrates | + | – | + |
| Phlobatannins | – | – | + |
| – indicate absence, + indicates presence | |||
Table 5. Total phenolic (TP) and flavonoids (TF) contents of the Moringa oleifera extracts
| Extract Samples | Mean Gallic acid Equivalent (mg/g in GAE)
TPC |
Mean Quercetin Equivalent (mg/g in QE) TFC |
| Methanol | 140.1 9 ± 0. 0.7 | 98.67±2.10 |
| Ethyl acetate | 130.9 ± 0.9 | 65.77 ± 1.01 |
| n-Hexane | 119.4 + 0.5 | 32.98±2.12 |
| Values are the means ± SD of triplicate | ||
Preliminary phytochemical investigation showed the presence of diverse phytochemicals which are the bioactive components that can be of use. The result of phytochemicals in the present investigation showed that the plant contains components such as alkaloids, flavonoids, glycosides, tannins, saponins, terpenoids, proteins, carbohydrates (Rodríguez-Pérez, et al., 2015 and Udofia. et al., 2020). The phenolic compounds, particularly flavonoids, and terpenoids were abundant in these extracts (Table 4). The total phenolic and flavonoid contents of methanolic extract revealed higher values than ethyl acetate and hexane extracts. The highest phenolic content was observed in methanolic leaf with 140.1 9 ± 0. 0.71 (mg GAE/g) while flavonoid leaf extract was found 98.67±2.10 (mg QE /g) respectively. The result revealed that phenolic content of methanolic extract is higher than that of ethyl acetate and hexane extracts (Table 5).
This may be due to different polarity of the solvents used, and phenolics are mainly extracted in higher quantity especially in more polar solvents. Ethyl acetate extract also showed the good content of phenolic compounds with total phenolic content of 130.9 ± 0.9 mg GAE/g compared to the extract n-hexane that had 119.4 + 0.5 mg GAE/g, respectively (Table 5). The results are in agreement with the findings of Vongsak et al., (2013) determined the quantitative analysis of active compounds was accomplished through high-performance liquid chromatography (HPLC). The extract promoted with maximum amounts of total phenolics (13.23 g chlorogenic acid equivalents/100 g extract) and total flavonoids (6.20 g isoquercetin equivalents/100 g extract) also, exhibited high DPPH-scavenging activity (EC50 62.94 μg/ml).
Present result indicates satisfactory phenolic contents. Hence they correspond to the results obtained by Shih, et al., 2011 and Srivastava, et al., 2020) where the highest total phenolic content was found to be in a leaves extract of Moringa oleifera. Phenolic compounds have been reported to be an important class of secondary metabolites, found in medicinal plants and used tremendously as a source of anti-infection agent. Nevertheless, they help to reduce the risk of many diseases owing to their antioxidant power (Abdulkadir et al., 2015 and Zhu, et al., 2020).
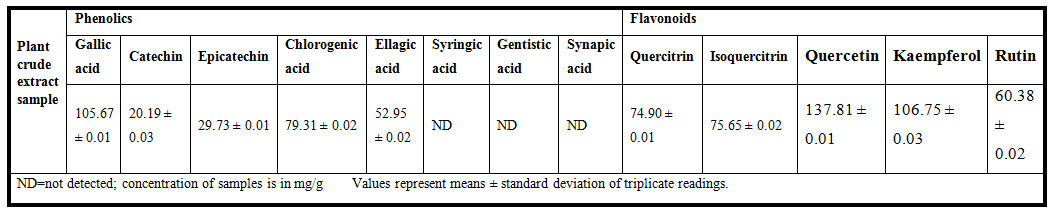
Analyses of individual phenolic and flavonoid compounds by HPLC showed the presence of diverse biochemical constitution. These compounds were identified by the comparison of their retention times and UV spectra to those of authentic standards analyzed under identical conditions. Qualitative and quantitative analyses of Moringa oleifera leaf extract depicted that gallic acid, catechin, epicatechin, chlorogenic acid, ellagic acid, quercitrin, quercetin, kaempferol and rutin were detected with different concentrations (Table 6) indicating its medicinal prospective. The medicinal uses of Moringa oleifera may be due to the antibacterial and antioxidant activities of its bioactive phytochemicals particularly phenolic compounds that showed the significance relevance of Moringa oleifera in prevention of different diseases by reducing and /or preventing free radicals. This potential may translate into prevention of chronic diseases associated with antibacterial and oxidative stress for humans who consume various parts of Moringa oleifera plant (Chhikara, et al., 2020), Also, thw work of Rocchetti, et al., (2020) has revealed great abundance of flavonoids and phenolics acids by using different Moringa oleifera leaf extracts.
Table 6. HPLC analyses of bioactive compounds of Moringa oleifera plant leaf extract
The global emergence of multidrug resistant bacterial strains is increasing, limiting the effectiveness of current drugs and treatment failure of infections. A novel approach to the prevention of antibiotic resistance of pathogenic species is the use of new compounds that are not based on existing synthetic antimicrobial agents. Based on the findings of this study it could be recommended that the extracts of these plants should be further analyzed to isolate the specific antibacterial compounds and defense mechanisms working in them. Speedy clinical trials should be carried out to explore the pharmaceutical potential of these medicinal plants in the treatment of bacterial and fungal infectious diseases.
CONCLUSION
This study found that Moringa oleifera leaf extracts have antibacterial activity against both Gram positive and negative bacteria, also revealed high potential free radical scavenging activity. The antibacterial activity showed that Staphylococcus aureus was more resistant bacteria while, Bacillus subtilus and Streptococcus viridans were found among more sensitive against all extracts. The highest antioxidant activity of 56.3% was exhibited by Moringa leaves in the methanol extract. This revealed that the leaves contain considerable concentration of antioxidants with good free radical scavenging activity. This result also indicates satisfactory phenolic and flavonoid contents in leaf extracts. It is interesting to conduct more research in depth on Moringa leaves in order that consumers benefit from them as food additive or nutraceutical and biopharmaceutical industries. This study also provides useful information about possibility of discovering new compounds with more effectiveness against multidrug resistance pathogenic bacteria.
Financial Disclosure: Financial support of this research work was partly provided by Microbiology Unit, King Fahd Research Center, King Abdulaziz University, Jeddah, Saudi Arabia and fulfilled by researchers of current study themselves also.
ACKNOWLEDGEMENTS
The authors acknowledge, Microbiology lab King Fahd center for Medical Research, King Abdulaziz University, Jeddah, Saudi Arabia for providing necessary facilities/equipment required.
Conflict Of Interest :Authors would hereby like to declare that there is no conflict of interests that could possibly arise.
REFERENCES
Abadallah, M.S. and Ali, M. (2019) Antibacterial activity of Moringa oleifera leaf extracts against bacteria isolated from patients attending general Sani Abacha specialist hospital damaturu. Journal of Allied Pharmaceutical Sciences, 1(1), pp. 61-66.
Abdulkadir, A.R., Zawawi, D.D. and Jahan, M.S. (2015) DPPH antioxidant activity, total phenolic and total flavonoid content of different part of Drumstic tree (Moringa oleifera Lam.). Journal of Chemical and Pharmaceutical Research, 7(4), pp. 1423-1428.
Adamczak, A., Zarowski,M.O. and Tomasz M. Karpinski,T.M. (2020) Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals, 13 (153), pp. 1-12.
Ashour,E.A., El-Kholy, M.S., Alagawany, M., Abd El-Hack, M.E., Mohamed, L.A., Taha,A.E., El Sheikh,A.I., Laudadio, V. and Vincenzo Tufarelli, V. (2020) Effect of dietary supplementation with Moringa oleifera leaves and/or seeds powder on production, egg characteristics, hatch ability and blood chemistry of laying Japanese quails. Sustainability, 12, 2463.
Bauer, A.W., Kirby, W.M., Sherris, J.C., Turck, M. (1966) Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology; 45 (4), pp., 493-6.
Brand-Williams, W., Cuvelier, M.E. and Berset, C.L.W.T. (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology, 28(1), pp. 25-30.
Burt, S.A and Reinders, R.D. (2003) Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Letters in Applied Microbiology, 36(3), pp. 162–7.
Busani, M., Julius, M.P. and Voster, M. (2012) Antimicrobial activities of Moringa oleifera Lam leaf extracts. African Journal of Biotechnology, 11(11), pp. 2797-2802.
Chakraborty, J., Chandravanshi, N.K., Manisha, P.D. and Dora, K.C. (2019) Antimicrobial activity of Moringa oleifera leaf extract in Pangasianodon hypophthalmus minced meat. Environment and Ecology, 37(3A), pp. 812-816.
Chhikara, N., Kaur, A., Mann, S., Garg, M.K., Sofi, S.A. and Panghal, A. (2020), “Bioactive compounds, associated health benefits and safety considerations of Moringa oleifera L. .: an updated review. Nutrition & Food Science, Publication date: 30 May, ahead-of-print No. ahead-of-print
Dalukdeniya, D. A. C. K., De Silva, K. L. S. R., and Rathnayaka, R. M. U. S. K. (2016) Antimicrobial activity of different extracts of leaves bark and roots of Moringa oleifera Lam. International Journal of Current Microbiology and Applied Sciences, 5(7), pp. 687-691.
Evans, W.C. (2002) Trease and Evans Pharmacognosy, 9th Edition published by Saunders Elsevier, pp. 553-557.
Fahal, M.E., Rani, B.M.A., Aklakur, M.D., Chanu, T.I. and Saharan, N. (2018) Qualitative and quantitative phytochemical analysis of Moringa oleifera (Lam) Pods. International Journal of Current Microbiology and Applied Sciences, 7(5), pp. 657-665.
Fitriana, W.D., Ersam, T., Shimizu, K. and Fatmawati, S. (2016) Antioxidant activity of Moringa oleifera extracts. Indonesian Journal of Chemistry, 16(3), pp. 297-301.
Gopalakrishnan, L., Doriya, K., and Kumar, D. S. (2016) Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness, 5(2), pp. 49-56.
Ilanko, P., McDonnell, P.A., van Vuuren, S. and Cock, I.E. (2019) Interactive antibacterial profile of Moringa oleifera Lam. extracts and conventional antibiotics against bacterial triggers of some autoimmune inflammatory diseases. South African Journal of Botany, 124, pp. 420-435.
Igbo, U.E., Igoli, J.O., Onyiriuka, S.O., Ejele,A.E., Ogukwe, C.E., Ayuk,A.A., Elemo,G.N., and Gray,A.I. (2015) Antitrypanosomal and antioxidant activities of Moringa oleifera Lam leaf extracts. Journal of Pharmaceutical, Chemical and Biological Sciences, 3(1), pp. 17-23.
Joshi, R.K. (2011) Qualitative analysis of phenolic constituents from leaves of Anaphalis contorta. International Journal of Applied Research in Natural Products, 1(2), pp. 23-25
Kim, D.O, Jeong, S.W and Lee, C.Y. (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81, pp. 321-326.
Kou, X., Li, B., Olayanju, J.B., Drake, J.M. and Chen, N. (2018) Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients, 10(3), pp. 343.
Mabrouki,L., Rjeibi,I., Taleb,J., and Zourgui, L. (2020) Cardiac ameliorative effect of Moringa oleifera leaf extract in high-fat diet-induced obesity in rat model. Hindawi BioMed Research International, vol 2020, article ID 6583603, pp.1-10.
Mahboobi, M, Shahcheraghi, F. and Feizabadi, M.M (2006) Bactericidal effects of essential oils from clove, lavender and geranium on multi-drug resistant isolates of Pseudomonas aeruginosa. Iranian Journal of Biotechnology, 4(2), pp. 137–140.
Maillard, J.Y., Bloomfield,S.F., Courvalin,P., Essack,S.Y., Gandra,S., Gerba,C.P., Rubino,J.R., and Elizabeth A. Scott,E.A. (2020) Reducing antibiotic prescribing and addressing the global problem of antibiotic resistance by targeted hygiene in the home and everyday life settings. American Journal of Infection Control, (Epub ahead of print), pp., 1-10.
Motamedi, H., Safary, A., Maleki, S. and Seyyednejad, S.M. (2009) Ziziphus spina-christi, a native plant from Khuzestan, Iran, as a potential source for discovery new antimicrobial agents. Asian Journal of Plant Sciences, 8(2), pp. 187
Ojiako, E.N. (2014) Phytochemical analysis and antimicrobial screening of Moringa oleifera leaves extract. International Journal of Engineering Science, 3, pp. 32-35
Oladeji, O.S., Odelade, K.A. and Oloke, J.K. (2020) Phytochemical screening and antimicrobial investigation of Moringa oleifera leaf extracts. African Journal of Science, Technology, Innovation and Development, 12(1), pp. 79-84.
Raj, A.J., Gopalakrishnan, V.K., Yadav, S.A. and Dorairaj, S. (2011) Antimicrobial activity of Moringa oleifera (Lam.) root extract. Journal of Pharmacy Research, 4(5), pp. 1426-1427.
Reetu., Bhargavi,K., Tomar,M,., and Subha.K. (2020) Moringa oleifera : a health food for animal and human consumption. Food and Scientific Reports, 1 (1), pp., 11-14.
Rocchetti, G., Pagnossa, J.P., Blasi, F., Cossignani, L., Piccoli, R.H., Zengin, G., Montesano, D., Cocconcelli, P.S. and Lucini, L. (2020) Phenolic profiling and in vitro bioactivity of Moringa oleifera leaves as affected by different extraction solvents. Food Research International, 127, article 108712, pp.1-8.
Rodríguez-Pérez, C., Quirantes-Piné, R., Fernández-Gutiérrez, A. and Segura-Carretero, A. (2015) Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Industrial Crops and Products, 66, pp. 246-254.
Shih, M.C., Chang, C.M., Kang, S.M. and Tsai, M.L. (2011) Effect of different parts (leaf, stem and stalk) and seasons (summer and winter) on the chemical compositions and antioxidant activity of Moringa oleifera. International Journal of Molecular Sciences, 12(9), pp. 6077-88.
Shourbela R.M., El-Hawarry W.N., Abd El-Latif A.M., and AboKora S.Y. (2020) Potentiality of Moringa oleifera aqueous extract as a growth modulator and anti stress in acute hypoxic Nile tilapia Oreochromis niloticus. Iranian Journal of Fisheries Sciences, 19 (1), pp. 67-84.
Singh, R.G., Negi, P.S., and Radha, C. (2013) Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. Journal of Functional Foods, 5(4), pp. 1883-1891.
Srivastava, M., Dhakad,P.K., Srivastava,B. (2020) A review on medicinal constituents and therapeutic potential of Moringa oleifera. Universal Journal of Plant Science, 8 (2), pp. 22-33.
Su, B., and Chen,X. (2020) Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Frontier in Veterinary Science., 7 (53), pp. 1-13.
Suresh, S., Chhipa, A.S., Gupta, M., Lalotra, S., Sisodia, S.S., Baksi, R. and Nivsarkar, M. (2020) Phytochemical analysis and pharmacological evaluation of methanolic leaf extract of Moringa oleifera Lam. in ovalbumin induced allergic asthma. South African Journal of Botany, 130, pp. 484-493.
Tandon, S, Sand N.K., Pant, A.K. and Ram, B. (2001) Evaluation of phenolic acids from some plants of family Asteraceae. Pestology, 25, pp. 30-31.
Thirumalai, P., Ravi, S., and Prabhu, S. (2018) Evaluation of antioxidant, anti-bacterial and in-vitro anti-inflammatory activity of Albizia richardiana seed extract. Academia Journal of Medicinal Plants, 6(7), pp. 178-185.
Tirado-Torres, D., Chan-Keb, C.A., Pérez-Balán, R.A., Ake-Canché, B., Gómez Solano, M.I., Aragón-Gastélum, J.L., Gómez-López, I., Aguirre-Crespo, F.J., López-Ramos, M.C. and Gutiérrez-Alcántara, E.J. (2019) Antimicrobial activity of Moringa oleifera against multidrug-resistant Staphylococcus aureus isolated from raw milk. Applied Ecology and Environmental Research, 17(1), pp. 587-599.
Tufa, T.B., Fuchs, A., Tufa, T.B., Stötter, Kaasch,A.J., Feld, T., Häussinger,D., Mackenzie, C.R. (2020) High rate of extended-spectrum beta-lactamase-producing gram-negative infections and associated mortality in Ethiopia: a systematic review and meta-analysis. Antimicrobial Resistance & Infection Control, 9(128) pp.1-10.
Udofia, N.E., Misonge,o.J., Mworia,M., , William, N., Apiri,M.G. (2020) Chemical composition of Moringa oleifera Lam. and Moringa stenopetala Bac. leaves from Kenya. International Journal of Plant Research, 10(1), pp. 1-10.
Valle Jr, D.L., Andrade, J.I., Puzon, J.J.M., Cabrera, E.C. and Rivera, W.L. (2015) Antibacterial activities of ethanol extracts of Philippine medicinal plants against multidrug-resistant bacteria. Asian Pacific Journal of Tropical Biomedicine, 5(7), pp. 532-540.
Vergara-Jimenez, M., Almatrafi, M.M. and Fernandez, M.L. (2017) Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants, 6(4), pp. 91.
Vongsak, B., Sithisarn, P., Mangmool, S., Thongpraditchote, S., Wongkrajang, Y. and Gritsanapan, W. (2013) Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Industrial Crops and Products, 44, pp. 566-571.
Vyas, G.D., Saxena, S., Danwe, S. and Shrivastava, A. (2020) Phytochemical screening and estimation of antioxidant potential of some selected medicinal plants from Gwalior region. International Journal of Scientific Research, 8(12), pp. 7-10.
Yakoub, A.R.B., Abdehedi, O., Jridi, M., Elfalleh, W., Nasri, M. and Ferchichi, A. (2018) Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorus olitorus l.). Industrial Crops and Products, 118, pp. 206-213.
Yee, M.M. (2019) A comparative study on antimicrobial activity and antioxidant activity on different extracts of leaf, bark, and root of Moringa oleifera Lamk (Drumstick tree). International Journal of Recent Innovations in Academic Research, 3(7), pp. 24-34.
Zhu,Y., Qinhong Yin,Q., and Yaling Yang,Y. (2020) Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules, 25 (676), pp. 1-15.