Department of Biotechnology and Zoology, Saifia College of Science, Bhopal- 462001, India.
Corresponding Author email:tasneem19.11@gmail.com
Article Publishing History
Received: 10/02/2025
Accepted After Revision: 28/03/2025
Psoralea corylifolia Linn. is an herb from the Fabaceae family, used in both Indian and Chinese traditional medicine. Commonly known as Babchi, it is a traditional medicinal plant widely utilized in Ayurveda and Unani medicine. Psoralen, a major bioactive furocoumarin found in its seeds, exhibits various pharmacological activities. In this study, High-Performance Liquid Chromatography coupled with Diode-Array Detection and Time-of-Flight Mass Spectrometry (HPLC-DAD-TOFMS) was employed to validate and quantify psoralen in P. corylifolia seed extracts. The characteristic retention time (Rt) of the psoralen standard was recorded at 3.856 min, while the crude seed extract exhibited a retention time of 3.688 min.
The ara density analysis revealed that psoralen constituted 75.27% of the crude extract. Furthermore, mass spectrometric analysis confirmed that the exact mass of psoralen in P. corylifolia (m/z 391.1) matched that of the standard psoralen ([M-H] ⁺ m/z 392.2). These findings suggest that the fully validated HPLC-DAD and HPLC-TOFMS methods can be effectively applied for the quantitative determination of psoralen in P. corylifolia. Due to its high psoralen content, P. corylifolia holds potential as a potent therapeutic agent for the treatment of hypopigmentary disorders.
Psoralea Corylifolia, Psoralen, HPLC-DAD-TOFMS, Quantitative Determination.
Husain T. Scientific Validation of Bioactive Compound Psoralen from Psoralea Corylifolia Seeds Extract Using HPLC-DAD-TOFMS Analysis. Biosc.Biotech.Res.Comm. 2025;18(1).
Husain T.Scientific Validation of Bioactive Compound Psoralen from Psoralea Corylifolia Seeds Extract Using HPLC-DAD-TOFMS Analysis. Biosc.Biotech.Res.Comm. 2024;18(1). Available from: <a href=”https://shorturl.at/dCli9“>https://shorturl.at/dCli9</a>
INTRODUCTION
Ayurvedic herbal medicines have been widely recognized for their numerous therapeutic applications, derived from ancient Indian herbal systems. In recent years, there has been a significant increase in attention and research on medicinal plants (Boparai et al., 2017). One such plant, Psoralea corylifolia L., an annual herb belonging to the Fabaceae (Leguminosae) family, is extensively distributed across subtropical and tropical regions. Commonly known as Babchi, this plant grows to a height of 30–180 cm (Alam et al., 2018) and thrives in warm environments but does not tolerate shade. It has simple, broadly elliptic leaves measuring 3.8 × 2.5–5.0 cm, with dentate margins. The seeds are oval, flattened, dark brown in color, and possess a characteristic fragrant odor. The fruit pods are ovoid-oblong, mucronate, black, and approximately 5 mm in length (Shrestha et al., 2018).
corylifolia has been of immense biological and medicinal importance for centuries, primarily due to its remarkable efficacy in treating various skin disorders, including psoriasis, leucoderma, and leprosy (Chishty and Bissu, 2016). The plant contains a diverse array of bioactive compounds, including coumarins, flavones, lipids, saponins, alkaloids, tannins, carbohydrates, monoterpenes, chalcones, resins, stigmasteroids, and flavonoids. Phytochemical studies have identified flavonoids, coumarins, and meroterpenes as the key components of P. corylifolia, with the highest concentrations found in its seeds and fruits (Zhang et al., 2016; Basera and Shah., 2020).
Among these bioactive compounds, psoralen—a linear furanocoumarin—is the principal medicinally active constituent of P. corylifolia seeds. Psoralen (C₁₁H₆O₃, m.p. 161–162°C) is known for its significant pharmacological properties, including its ability to mitigate metabolic disorders and certain cancers, as well as its therapeutic potential in treating skin conditions such as psoriasis, eczema, and vitiligo. These effects are attributed to its ability to reduce oxidative stress, inhibit low-density lipoprotein (LDL) oxidation and platelet aggregation, and promote vasodilation in blood vessels (Uikey et al., 2010; Anand David et al., 2016; Tripathi et al., 2023).
Our recent investigation further demonstrated its ability to facilitate pigment dispersion in reptilian melanophores via cholinergic receptor activation. These findings suggest that psoralen may serve as a potential therapeutic agent for managing hypopigmentary disorders such as vitiligo (Sultan and Ali., 2011). However, while previous studies have primarily focused on the biological and therapeutic significance of psoralen, there is limited literature on its qualitative and quantitative estimation, as well as its in vivo activity post-administration.
Based on these insights, the present study aims to utilize liquid chromatography-mass spectrometry (LC-MS) for the identification and characterization of psoralen in P. corylifolia seed extracts. Conventional methods such as column chromatography and high-performance liquid chromatography (HPLC) are often tedious, time-consuming, and require multiple chromatographic steps. Therefore, we also sought to develop and validate a rapid, sensitive, high-performance liquid chromatography with diode-array detection (HPLC-DAD) and time-of-flight mass spectrometry (HPLC-TOFMS) method for the efficient determination and quantification of psoralen in P. corylifolia seeds.
MATERIAL AND METHODS
Procurement of Reagent: Analytical grade methanol and ethanol were obtained from Qualigens Fine Chemicals, Mumbai, India. Acetonitrile (HPLC grade) and Water (HPLC grade) was procured from Sigma Aldrich (USA). Deionized water was purified with Milli-Q water system (Millipore, Milford, MA, USA). Standard psoralen was given as a gift sample from Dr. Syed Khalid Yousuf, Scientist, Medicinal Chemistry Division, Council of Scientific & Industrial Research-Indian Institute of Integrative Medicine (CSIR-IIIM), Srinagar. Fresh Seeds of Psoralea corylifolia were purchased and collected from Vindhya Herbals Sanjeevani Ayurveda, Bhopal, a Government of Madhya Pradesh enterprise. At the time of collection, seeds were chosen from healthy plants. The phenotypically superior samples of healthy and uninfected plants were chosen. Collected samples were stored in zip locked polyethylene bags until used.
The plant sample was identified and authenticated by Botanical Survey of India, Kolkata. The voucher specimen number – CNH/Tech.II/2022/52 was deposited at the herbarium of the Botanical Survey of India, Kolkata. Preservation of plants material was done according to the standard protocol following the literature, technical reports, and manuals.
Extraction of crude extracts of Psoralea corylifolia seeds: Extraction of crude extract from the seeds of Psoralea corylifolia has been done as per the method of Husain et al. (2016) with minor modifications. Soxhlet extraction method was performed for the preparation of crude extracts from the seeds of P. corylifolia. For the preparation of methanolic extract of P. corylifolia, fresh seeds of P. corylifolia were dried at room temperature in shade and then crushed into coarse powder. The whole or coarsely powdered plant material was extracted by 200 ml of methanol solvent for 10-12 hrs at 60°C in a Soxhlet apparatus (Khera Instruments, Pvt Ltd Delhi, India). The crude extract was filtered and evaporated to dryness on rotary evaporator (Khera Instruments, Pvt Ltd Delhi, India) and accurately weighed for further analysis.
Preparation of standard and sample solutions
Standard Solution: A standard stock solution of psoralen was prepared by dissolving 10mg of psoralen in acetonitrile and made up the volume to 10 ml in a standard volumetric flask. The solution was sonicated for 25 min. The standard solution of psoralen was further diluted as per the requirement by diluting the stock solution to obtain a concentration of 100 µg/mL.
Preparation of Sample Solution: The sample solution was prepared by weighing dried extracts (10mg), in a 10 ml volumetric flask containing acetonitrile. The solution was sonicated for 25 min, and the final volume was made with acetonitrile. Each sample solution was filtered through a 0.45 μm membrane filter into HPLC sample vial before HPLC injection. From the test sample, 1 ml was pipetted out and diluted up to 10 ml with acetonitrile for HPLC analysis. The stock solution was further diluted sufficiently to get a sample solution with a drug concentration of 100 μg/mL. 20µl of standard and sample were injected to HPLC system and then the chromatogram was recorded and the retention time of psoralen was determined in comparison with standard.
Instrumentation and conditions
HPLC Chromatographic conditions: The High-Performance Liquid Chromatography analysis was done on a chromatographic system (Waters, Massachusetts) as per the method of Shailajan et al., (2012) with slight modifications. The separation used was a thermo C 18 (250 × 4.6 mm, 5 µm), and the column temperature was maintained at 30°C. The diode array detector recorded UV spectra in the range of 200–400 nm, and the HPLC chromatogram was monitored at 247 nm. The mobile phase consisted of acetonitrile (A): water (40:60 v/v) (B). A gradient program was used as following: 0–20 min, 15–55% A; 20–30 min, 55–70% A; 30–35 min, 70–85% A, with a hold time of 10 min. The flow rate was 1 ml/min and the injection volume was 20 µL.
HPLC-TOFMS analysis: The Liquid Chromatography Mass Spectrometry (LC-MS) analysis was carried out as per the method of Tan et al., (2015) with slight modifications. The above HPLC conditions were used for HPLC-TOFMS analysis. The HPLC system was directly connected to the TOF mass spectrometer via an electrospray ionization (ESI) interface with the stream splitting ratio at 2:1. The TOFMS analysis was performed using a full scan mode and the mass range was set at m/z 10 to 3000 in a positive ion mode. The components in P. corylifolia were identified rapidly by HPLC-TOFMS according to their accurate molecular mass and molecular formulae matching against the formula database of P. corylifolia. The mass spectrometric parameters were optimized as follows: in the positive ion mode (ESI+), capillary voltage, 4500 V; nebulizer,40 psig; drying gas, 7.0 L min−1; gas temperature, 200°C; fragmentor voltage 160 V; skimmer voltage 60 V; collision cell RF 130 V. TOFMS was used for the detection and determination of accurate molecular masses of different compounds.
RESULTS AND DISCUSSION
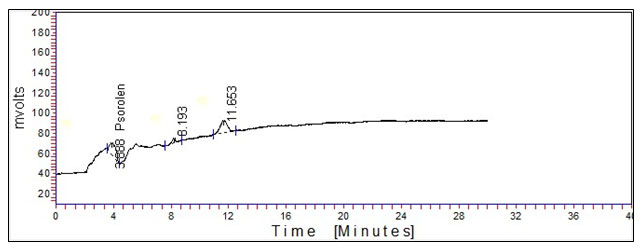
Under the optimized chromatographic conditions, psoralen standard and crude extract of the seed of Psoralea corylifolia were separated in the HPLC chromatogram. The characteristic retention time (Rt) chromatograms of the psoralen standard and crude extract of seed of Psoralea corylifolia are shown in Figures 1 and 2, respectively, in which the Rt of psoralen was obtained at 3.856 and Rt of crude extract of seed of Psoralea corylifolia was obtained at 3.688. Two unidentified peaks were also obtained in the chromatogram of the crude extract of Psoralea corylifolia seeds and the Rt of peaks are 8.193 and 11.653, respectively as shown in Figure 2.
Figure 1: Chromatogram of Psoralen standard
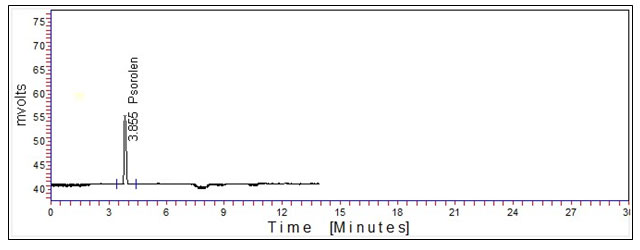
Figure 2: Chromatogram of crude extract of seed of Psoralea corylifolia.
Characterization of other compounds in Psoralea corylifolia by HPLC-TOFMS: To further confirms the unidentified peaks from the extract of Psoralea corylifolia, the crude extract was directly injected into the TOF mass spectrometer to optimize the spectrometric parameters, including capillary voltage, nebulizer gas pressure, drying gas flow rate, gas temperature and fragmentor voltage. Positive ion mode was set for the identification of compounds in Psoralea corylifolia. The total ion chromatogram (TIC) of Psoralea corylifolia extract was acquired by HPLC-ESI-TOFMS in the positive ion mode (Figure 3).
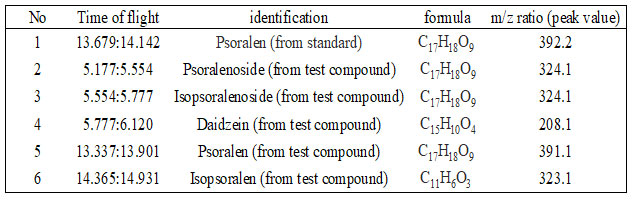
Our interest is to confirm the presence of psoralen in standard and test sample, the retention time ratio of presence of psoralen in standard was obtained at 13.679:14.142 and the retention time ratio of presence of psoralen in tested extract of P. corylifolia was obtained at 13.337:14.365. The results of mass determination accuracy for all detected peaks are summarized in Table I. The result showed that the exact mass of psoralen content of Psoralea corylifolia (m/z 391.1) was identical to that of the standard psoralen ([M-H] + m/z 392.2). Five compounds in the TIC chromatogram from TOFMS were characterized by matching against literature searched as illustrated in table 1.
Figure 3: The total ion chromatogram (TIC) of Psoralea corylifolia extract was acquired by
HPLC-ESI-TOFMS in the positive ion mode. Psoralen was detected at retention time 13.966.
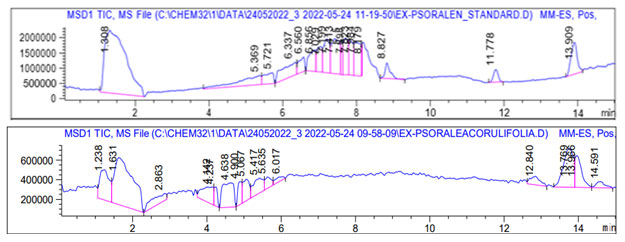
Table 1. HPLC-TOFMS Data of the Compounds in Psoralea corylifolia.
Differentiation of isomers in Psoralea corylifolia extract: Some compounds with identical molecular weights may not be distinguished only according to the accurate mass measurement capability of TOFMS, which included psoralenoside, isopsoralenoside and isopsoralen with approximate molecular weight of 324 at peaks near 5.177:5.554, 5.554:5.777 and 14.365:14.931 respectively. Since these molecular pairs cannot be distinguished by mass spectrometry without fragmentation, other analytical technique HPLC-DAD was further employed to analyze psoralen from same MWs of other isomers of Psoralea corylifolia. The HPLC-DAD data of the isomers in the positive ion mode are shown in figure 4 of crude extract of Psoralea corylifolia.
Figure 4: HPLC-DAD graph of Psoralea corylifolia in the positive ion mode
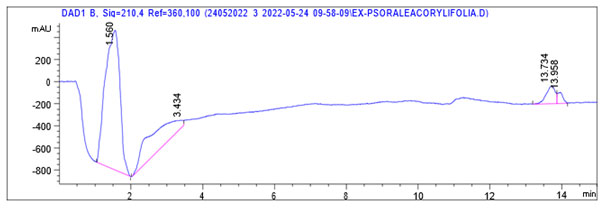
From the results of HPLC-TOFMS, it was confirmed that characteristic compound (psoralen) of standard and tested sample was identified at Rt of 13.679:14.142 and 13.337:14.365 respectively. HPLC DAD analysis employed further confirms the presence of psoralen in standard at Rt time 13.896 and the presence of psoralen in test compound at Rt 13.956 which is highly comparable which is shown figure 4 and illustrated in table 2. The quantity of psoralen in crude extract was found to be 75.27 % as calculated by dividing the peak area of psoralen by total peak area and multiplied with 99% purified standard.
Table 2: HPLC DAD data of crude extract of Psoralea corylifolia. Retention time (Rt)

Until now, no studies about the identification and quantification of psoralen in plant extracts using MS/MS detection have been described. Only Shailajan et al. (2012) and Tan et al. (2015) have reported on psoralen MS, but in the selected ion mode (SIM), which is less specific than multiple reaction mode (MRM) and high-resolution mass spectrometry identification. Our results matched the findings of Rakhmankulov and Korotkova, (2015). They pioneered the use of biologically active compounds. They reported that the seeds and roots were the richest sources of furanocoumarins (psoralen and angelicin).
Our findings are in line with the study of Shailajan et al. (2012), where they develop a reverse-phase high-performance liquid chromatography—photodiode array detector (RPHPLC—DAD) method for quantification of psoralen from P. corylifolia and its related formulations. Separation and detection of psoralen from various herbal formulations were achieved on a reversed-phase Cosmosil C18 column using acetonitrile: distilled water (40:60 v/v; flow rate: 1.0 mL/minute) and the PDA detector (247 nm).
The present findings are correlated with the findings of Luan et al. (2018), who worked and standardised the high-performance liquid chromatography-diode array detector (HPLC-DAD) fingerprint method to present the comprehensive phytochemical profile of the Psoraleae fructus. Thirteen major compounds were separated and identified by HPLC coupled with time-of-flight mass spectrometry (HPLC/TOF-MS), namely psoralenoside, isopsoralenoside, psoralen, isopsoralen, neobavaisoflavone, bavachin, corylin, bavachromene, psoralidin, isobavachalcone, bavachinin, corylifol A, and bakuchiol.
Therefore, in this work, a reliable and powerful analytical method by using a combination of HPLC-DAD and HPLC-TOFMS for rapid identification of psoralen in P. corylifolia was established. Five peaks in the HPLC chromatogram were rapidly identified by comparing their related retention times and accurate mass measurements with the standards. Using HPLC-TOFMS and literature search, apart from psoralen, four other components were characterized tentatively. Since isomers that cannot be differentiated by HPLC-TOFMS, it is feasible to apply HPLC-DAD to differentiate them based on their characteristic fragment ions. By right of this combinative technique, psoralen and four other coumarins were identified rapidly, which provided essential data for further pharmacological and clinical studies of P. corylifolia and facilitated the rapid quality control of the crude drug. The fully validated HPLC-DAD and HPLC-TOFMS method can be successfully applied for the determination of psoralen in Psoralea corylifolia, which can be used as a potent therapeutic agent for the treatment of hypopigmentary disorders.
CONCLUSION
A simple, sensitive HPLC-DAD and HPLC-TOFMS technique was demonstrated and justified for the characterization and quantitative estimation of the main coumarins psoralen in the seed extract of Psoralea corylifolia. The HPLC-TOFMS results confirmed the existence of psoralen in the crude extract of Psoralea corylifolia seeds. It is concluded that the higest amount of psoralen was found in the seed extract of of Psoralea corylifolia.
ACKNOWLEDGEMENT
The authors are thankful to the Secretary and Principal of Saifia Science College, Bhopal, India for providing the necessary facilities.
Consent for publication: Not applicable.
Availability of data and material: All the data generated and analyzed during the study are included in the main manuscript.
Competing interests: The author declares that they have no competing interests.
Funding: NA
REFERENCES
Alam, F., Khan, G.N. and Asad, M.H.H.B., 2018. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytotherapy Research, 32(4), pp.597-615.
Basera, I.A. and Shah, M.B., 2020. A validated high-performance thin-layer chromatography method for quantification of bavachin, bakuchiol, and psoralen from Psoralea corylifolia seeds. JPC–Journal of Planar Chromatography–Modern TLC, 33(3), pp.293-300.
Boparai, J.K., Singh, A., Gupta, A.K., Matreja, P.S., Khanna, P.M.L., Gupta, V. and Gautam, R., 2017. A study to determine the knowledge and level of awareness of medical undergraduates about herbal medicines and herb-drug interactions. International Journal of Basic & Clinical Pharmacology, 6(1), pp.17-24.
Chishty, S. and Bissu, M., 2016. M. A Review on Medicinal Importance of Babchi (Psoralea corylifolia). International Journal of Recent Scientific Research, 7(6), pp.11504-11512.
David, A.V.A., Arulmoli, R. and Parasuraman, S., 2016. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy reviews, 10(20), p.84.
Hussain, I., Hussain, N., Manan, A., Rashid, A., Khan, B. and Bakhsh, S., 2016. Fabrication of anti-vitiligo ointment containing Psoralea corylifolia: in vitro and in vivo characterization. Drug design, development and therapy, pp.3805-3816.
Luan, L., Shen, X., Liu, X., Wu, Y. and Tan, M., 2018. Qualitative analysis of Psoraleae fructus by HPLC‐DAD/TOF‐MS fingerprint and quantitative analysis of multiple components by single marker. Biomedical Chromatography, 32(2), p.e4059.
Rakhmankulov, U. and Korotkova, E.E., 1975. Dynamics of furocoumarine content in different parts of Psoralea drupacea,11, 98-104.
Shailajan, S., Menon, S., Singh, A., Mhatre, M., Sayed, N., Joshi, H. and Tiwari, B., 2012. Estimation of psoralen from herbal formulations containing Psoralea corylifolia using the RP-HPLC-DAD method and its application to a pharmacokinetic study. International Journal of Green Pharmacy (IJGP), 6(3).
Shrestha, S., Jadav, H.R., Bedarkar, P., Patgiri, B.J., Harisha, C.R., Chaudhari, S.Y. and Prajapati, P.K., 2018. Pharmacognostical evaluation of Psoralea corylifolia Linn. seed. Journal of Ayurveda and integrative medicine, 9(3), pp.209-212.
Sultan, T. and Ali, S.A., 2011. Psoralea corylifolia extracts stimulate cholinergic-like psoralen receptors of tadpole-tail melanophores, leading to skin darkening. Journal of Receptors and Signal Transduction, 31(1), pp.39-44.
Tan, G., Yang, T., Miao, H., Chen, H., Chai, Y. and Wu, H., 2015. Characterization of compounds in Psoralea corylifolia using high-performance liquid chromatography diode array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Journal of chromatographic science, 53(9), pp.1455-1462.
Tripathi, N., Bhardwaj, N., Kumar, S., and Jain, S.K., 2023. Phytochemical and Pharmacological Aspects of Psoralen–A Bioactive Furanocoumarin from Psoralea corylifolia Linn. Chemistry & Biodiversity, 20(11), p.e202300867.
Uikey, S.K., Yadav, A.S., Sharma, A.K., Rai, A.K., Raghuwanshi, D.K. and Badkhane, Y., 2010. The botany, chemistry, pharmacological and therapeutic application of Psoralea corylifolia L.–A review. Int J Phytomed, 2(2), pp.100-107.
Zhang, X., Zhao, W., Wang, Y., Lu, J. and Chen, X., 2016. The chemical constituents and bioactivities of Psoralea corylifolia Linn.: a review. The American journal of Chinese medicine, 44(01), pp.35-60.


