1Division of Epidemiology and Biostatistics, Department of Public Health, Faculty
of Medicine, Universitas Padjadjaran, Sumedang, Indonesia, 45363
2 TB-HIV Research Center, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia, 40161
3Internal Deparment of Hasan Sadikin Hospital, Universitas Padjadjaran, Sumedang, Indonesia, 40161
Corresponding author email: sujatmiko@unpad.ac.id
Article Publishing History
Received: 19/06/2021
Accepted After Revision: 15/08/2021
Pulmonary Tuberculosis is one of the the major infectious diseases and is among diseases with the highest mortality and morbidity in the world. Although many interventions had been done to prevent this disease, its spread of infection is still high. One of the factors that play an important role in the spread of this disease are environmental factors and contact patterns. There are only a few articles that discuss this issue in Indonesia. So, this study aims to explore the association between environmental, social contact factors, and pulmonary tuberculosis (PTB) in Bandung, Indonesia. An unmatch case-control with a 1:2 ratio was designed for the purpose of this study. Cases were defined as smear-positive pulmonary TB cases that received treatment during 2015-2016 at the four selected Public Health Centers (PHCs) in Bandung. Controls were selected from healthy neighbours of the cases.
Data were collected through home visits, interviews, and observations using a structured-questionnaire. Multivariable logistic regression was used to determine the risk factors associated with PTB. Findings from analyses on 330 respondents consisting of 113 cases and 217 controls demonstrated that the absence of Cross-ventilation inside the house was associated with PTB (AOR: 1.91; 95 % CI: 1.03-3.57) as the environment factor while family history of pulmonary TB (AOR: 4.90; 95% CI: 2.30–10.75), number of household member (AOR: 2.73; 95% CI: 1.33–5.65) and time spent inside the house (AOR: 1.12; 95% CI: 1.08–1.27) were found to be the social contact factors associated with PTB. Thus, the environment and social contact-pattern are essential factors in TB transmission. Regulations regarding this factor need to be strengthened so that this disease can be controlled.
Pulmonary, Risk Factor; Social Contact Factor, Tuberculosis
Sujatmiko B, Wahyudi K, Sukandar H, Agustian D, Alisjahbana B. Role of Environment and Contact-Pattern Factors in Pulmonary Tuberculosis Patients from Bandung City, Indonesia. Biosc.Biotech.Res.Comm. 2021;14(3).
Sujatmiko B, Wahyudi K, Sukandar H, Agustian D, Alisjahbana B. Role of Environment and Contact-Pattern Factors in Pulmonary Tuberculosis Patients from Bandung City, Indonesia. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3yF7Nsi“>https://bit.ly/3yF7Nsi</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Pulmonary tuberculosis (PTB) is world’s top infectious disease killer (World Health Organization, 2020). This disease has infected a quarter of the world’s population and caused 60 million cumulative death since 2000 (World Health Organization, 2020). Although this disease was treatable and preventable with 85 % successful treated rate, but this disease is still difficult to eradicate. Indonesia, as developing country, ranks second among countries with the highest incidence of PTB (World Health Organization, 2015). It is estimated in the latest data available on TB incidence that 1 million TB cases occur annually with an incidence rate of 395 per 100,000 population per year (World Health Organization, 2017). Bandung, as one of the major cities in Indonesia, is also facing the same problem. Bandung ranks third among cities with the highest TB incidence in West Java Province and recent PTB notification rate shows 477 cases per 100,000 population (West java Provincial Health Office, 2020; Yang et al., 2021).
This condition will affect city health and productivity. Despite the enormous efforts to control this disease, the PTB in Indonesia is still rampant. In 2016, the Minister of Health of the Republic of Indonesia issued a regulation to update and complement TB management efforts in Indonesia (Ministry of Health of the Republic of Indonesia, 2016). This regulation has already included the promotive, preventive, and curative aspects for fighting PTB. However, the social contact or contact pattern is not among them despite the fact that it plays major role in TB transmission thus becomes a potential point for intervention (Lönnroth et al., 2010, 2009; Ortblad et al., 2015). How people interact with each other indeed influences the risk for being infected by PTB, with number of contacts, duration of contacts, and a history of PTB among the contacts as the most prominent risk variables. Therefore, contact pattern needs to be evaluated when dealing with PTB infections (Mossong et al., 2008; Dodd et al., 2016; Horton et al., 2020; Yang et al., 2021).
Various literature have asessed the relationship between environmental risk factors and PTB (Alisjahbana et al., 2006; Baker et al., 2008; Bam et al., 2015). However, only few studies explores the relationship between contact factors and PTB (Dodd et al., 2016; Rahayu et al., 2015; Gelaw et al., 2019; Abreu et al., 2020; Yang et al., 2021). Therefore, this study focuses on exploring and identifying the social contact factors associated with PTB. In addition, the household environmental factors such as household density, indoor and outdoor hygiene, and availability of cross-ventilation in the houseare also explored.
MATERIAL AND METHODS
Study Location: Bandung, as one of the major cities in Indonesia, is a busy and highly populated city with 2,5 million population living in an area of 167 kilometer squares. The city consists of 30 districts and 151 sub-districts. It also has 73 Public Health Centers (PHCs) to cater for the healthcare-related needs of the citizen. Each PHCs is responsible for 2 to 6 sub districts.
Sample and procedure: We conducted an unmatched case-control study in late 2016 in four selected PHCs in Bandung: Arcamanik, Babakansari, Padasuka, and Ujungberung Indah PHCs. The criteria for selecting PHCs were having a broad working area that covers two or more subdistricts; having a laboratory capability to diagnose PTB, and having a good performance in recruiting TB patients (i.e. having a high number of PTB patients in the last two years). We use the 2015-2016 TB register data for recruiting respondents. A 1: 2 ratio was used for case and control selection. Controls were selected systematically by recruiting healthy adult respondents who lived nextdoor to the case.
Social Contact Pattern: We collected data on respondents’ social contact pattern using a structured questionnaire based on their social interaction habit in the community. We used four variables to describe the social contact pattern: number of household member (i.e., total individuals living in the house excluding respondent), time spent inside the house (i.e., the average time spent in the house each day), family history of TB (i.e., a history of family member who received treatment or were currently under treatment for PTB)(Cohen, 2000; Volz et al., 2011).
Household Environmental Factors: The household environment factors consist of four variables, namely household density, indoor household hygiene, outdoor household hygiene, and availability of cross-ventilation. We measured the household density as the total household area divided by total household members. Good indoor and outdoor hygiene was confirmed when there were no dust, waste, rats, or cockroaches seen in the household (Ministry of Health of the Republic of Indonesia, 2013). Cross-ventilation availability was confirmed if there were at least two different wall-windows in the house.
Definition and Measures: We defined cases as individuals with a history of PTB or were receiving treatment due to smear-positive result for PTB and recorded in the “TB-register” at the selected PHCs. Control was defined as healthy individual neighbours to the index case who had never been diagnosed with any TB disease.
Individual characteristics: We collected subject demographic data such as age, gender, level of education (i.e., time spent in formal education measured in years), marital status (married/widowed/single), employment (government officer/private employee/unemployed), insurance ownership (yes/no). We also characterized respondents by their economic status (described by the total monthly family income in Indonesian Rupiah), daily cigarette consumption, history of Diabetes Mellitus, and environment factors that influence TB transmission (e.g. people density/person/m2, Indoor and outdoor home hygiene, and presence of cross-ventilation in the house) as the confounding factors.
Ethical Approval: All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis: We explored the association between social contact factor, environmental factor, and PTB using multiple regression analyses. We also include potential confounders, i.e. age, sex, marital status, cigarette consumption, total monthly family income, and history of diabetes, in the analyses. All statistical analyses were performed with “R” version 3.2.3 and “R studio” as the user interface (Team Rstudio, 2015). We employed the AIC (Akaike Information Criterion) as the tool to assess best fit models.
RESULTS AND DISCUSSION
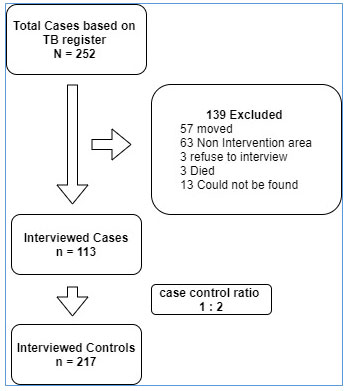
Based on the 2015-2016 TB register in four PHCs, there were 252 potential respondents as cases. About 139 eligible cases were excluded due to several reasons such as moving out of town (n=57), living outside the work area of the PHC (n=63), refused to be interviewed (n=3), died (n=3), and could not be traced (n=13). Thus, the total number of cases in this study was 113 respondents. We invited 217 respondents to participate as controls but 9 refused to be interviewed (Figure 1).
Figure 1: Study Population
Cases were younger than controls (38 ± 15.2 vs. 45.6 ± 13.5) and more likely to be male (51%). Compared to controls, cases were more likely to have lower duration of formal education (9.39 ± 3.02 vs. 9.54 ± 3.41). There were also statistical differences between cases and controls in terms of marital status, job qualification, and total monthly family income, albeit no statistical difference in Insurance Ownership variable was identified. On household density, both cases and controls had a similar household density (9.09 ± 10.05 vs. 10.34 ± 8.82) but differences were seen in Indoor household hygiene, outdoor household hygiene, and availability of cross-ventilation variables.
Respondents from the case group had higher proportions for poor indoor household hygiene , poor outdoor household hygiene , and absence of cross-ventilation inside the house. Respondents from the case and control groups had slight difference in the number of household members but the difference was statistically significant (p < 0.05). Respondents from the case group also spent longer time inside the house than those in the control group (18.06 ± 4.04 vs. 16.49 ± 4.57). A higher proportion of respondents in the case group had a family history of PTB compared to those in the control group (n=35, 31% vs. n=20, 9.2%) (Table 1).
Table 1. PTB-Associated Sociodemographic, Environmental, and Social Contact Factors in Bandung City
| Variable | Case
(n= 113) |
Control
(n= 217) |
p- value |
| Sociodemographic | |||
| Age (years), median (IQR)* | 38(25 – 48 ) | 45 (36 – 56 ) | <0.001‖ |
| Sex , n (%)* | |||
| · Female | 55(48.7) | 145(66.8) | <0.001 δ |
| · Male | 58(51.3) | 72(33.2) | |
| Duration of education (years)* | 9.39(3.02) | 9.54(3.41) | <0.001‖ |
| Marital status n(%)* | |||
|
30(26.5) | 11(5.1) | <0.001 δ |
|
74(65.5) | 174(80.2) | |
|
9(8) | 32(14.7) | |
| Job Qualification n(%) | |||
|
1(0.9) | 14(6.5) | 0.06 δ |
|
54(47.8) | 91(41.9) | |
|
58(51.3) | 112(51.6) | |
| Insurance Ownership, n(%)* | |||
|
79(69.9) | 154(71) | 0.84 δ |
|
34(30.1) | 63(29) | |
| Total monthly family income
(Thousand Rupiah) , median (IQR)* |
1,200
(500-6,800) |
1,500
(1,000-10,000) |
<0.01‖ |
| Environmental Factors | |||
| Household density ( person/m2) | 9.09(10.05) | 10.34(8.82) | 0.25‖ |
| Poor Household Indoor Hygiene* | <0.001δ | ||
|
86(76.1) | 196(90.3) | |
|
27(23.9) | 21(9.7) | |
| Poor Outdoor Household Hygiene* | <0.001δ | ||
|
84(74.3) | 188(86.6) | |
|
29(25.7) | 29(13.4) | |
| Cross-ventilation inside house * | <0.001δ | ||
|
44(39) | 114(52) | |
|
69(61) | 103(48) | |
| Social Contact Factors | |||
| Number of household members (IQR)* | 4(0-11) | 3(0-11) | <0.001‖ |
| Time spent inside house (hours) (SD)* | 18.06 (±4.04) | 16.49(±4.57) | <0.001‖ |
| Family history of pulmonary TB n(%)* | |||
|
78(69) | 197(90.8) | <0.001 δ |
|
35(31) | 20(9.2) |
Abbreviation : IQR = interquartile range ‖= Independent t test δ = chi square test
*indicates that the finding is statistically significant at the level of the confidence of 5 % (P-value < 0.05)
Table 2 presents the model of multivariable logistic regression of the study. This model has been adjusted to socioeconomic factors (such as: age, sex, marital status, total monthly family income in a month), history of cigarette smoking, and history of diabetes. We found that PTB was associated with several social contact factors including family history of pulmonary TB (AOR: 4.90; 95% CI: 2.30–10.75), number of household members (AOR: 2.73; 95% CI: 1.33–5.65), and time spent inside the house (AOR: 1.12; 95% CI: 1.08–1.27). In contrast, the only household environment risk factor associated with pulmonary TB was the absence of cross-ventilation in the house (AOR: 1.91; 95 % CI: 1.03-3.57). The model also demonstrated that history of diabetes, cigarette consumption, age and marital status are potential confounders for PTB (Table 2).
Table 2. Univariable and multivariable odds ratio’s (OR) and 95% confidence interval (95% CI) for social contact factors and environmental factors associated with PTB in Bandung City
| Variable | Crude OR | Adjusted OR | |||||
| OR | 95 % CI | OR | 95 % CI | ||||
| (Constant) | 0.190 | 0.029 | 1.144 | ||||
| Demographics | |||||||
| Age | 0.962* | 0.945 | 0.978 | 0.948* | 0.924 | 0.971 | |
| Sex (Male) | 2.123* | 1.336 | 3.389 | 3.221* | 1.479 | 7.160 | |
| Marital Status (Married) | 0.156* | 0.071 | 0.318 | 0.214* | 0.073 | 0.588 | |
| Marital Status (Divorced/Widowed) | 0.103* | 0.035 | 0.272 | 0.217* | 0.048 | 0.931 | |
| Cigarette consumption | 1.056* | 1.019 | 1.098 | 1.061* | 1.006 | 1.128 | |
| Total monthly family income (IDR) | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |
| History of Diabetes (Yes) | 4.973* | 1.933 | 14.397 | 8.976* | 2.724 | 32.402 | |
| Environmental Risk | |||||||
| People density ( person/m2) | 0.984 | 0.955 | 1.058 | 1.023 | 0.987 | 1.060 | |
| Poor Indoor Household Hygiene | 2.930* | 1.575 | 5.521 | 1.880 | 0.629 | 5.652 | |
| Poor Outdoor Household Hygiene | 2.238* | 1.257 | 3.991 | 1.883 | 0.699 | 4.970 | |
| No Cross-ventilation in house | 1.735* | 1.096 | 2.768 | 1.911* | 1.038 | 3.573 | |
| Social Contact Factors | |||||||
| Time spent inside house (hours) | 1.085* | 1.029 | 1.146 | 1.172* | 1.084 | 1.274 | |
| Number of household members | 2.351* | 1.378 | 4.017 | 2.728* | 1.332 | 5.658 | |
| Family history of PTB | 4.419* | 2.427 | 9.246 | 4.901* | 2.301 | 10.753 | |
| AIC | – | 315 | |||||
Abbreviation : IDR = Indonesian rupiah OR = Odds Ratio CI = Confident Interval AIC = Akaike Information Criterion
*indicates that the finding is statistically significant at the level of the confidence of 5 % (P-value < 0.05)
Our study shows that social contact pattern, i.e. family histroy of PTB, number of household members, and time spent inside the house, is associated with PTB in Bandung City. Family history of TB has been proven and shown to be associated with PTB in many papers. Previous studies stated that a positive PTB case can infect about 30 -40 % of theirs contacts (Gaur et al., 2017; Jindal, 2017; Lienhardt et al., 2005; Rathi et al., 2002; Sabri et al., 2019).
Therefore, the risk for getting PTB increases with the increasing number of family members who are positive for PTB as this means that there are more contacts in the house (AOR: 2.73; 95% CI: 1.33–5.65). One of the possible reasons may be related to the intensity of contact. We know that household members share the same air space and living activities, which increases the chance of disease transmission especially when there is a family member with PTB (Baker et al., 2008; Dodd et al., 2016; Qian et al., 2006).
With every additional hour spent inside the house, a 10% increase is seen in the odds of acquiring PTB (AOR = 1.172 , 95 % CI : 1.084 – 1.274). This study shows that respondents in the case group are more likely to spend more time inside the house than those in the control group. The longer people stay in their house, the more likely they will be infected with PTB bacilli. Mycobacterium tuberculosisis more likely to live in a condition with a high humidity and low light (Jindal, 2017; Sornboot et al., 2019; Taye et al., 2021). This theory could become the reason why this finding is significant. Other biological theory that can support this finding relates to the presence of cross-ventilation and history of PTB in the family (Dodd et al., 2016; Jindal, 2017; Sornboot et al., 2019; Taye et al., 2021).
There is also significant association between the absence of cross-ventilation inside the house and PTB (AOR: 1.91; 95 % CI : 1.04 – 3.57). The absence of cross-ventilation in the house almost doubles the probability to get PTB. Many studies suggested that poor ventilation is a risk factor for PTB. This study justifies and strengthen this notion (Chan and Fang, 2020; Escombe et al., 2019; Muchsin et al., 2019; Rahayu et al., 2015). Individual characteristics such as age, gender, marital status, cigarette smoking, total monthly income, and history of diabetes have been analyzed in earlier studies and are considered to be associated with PTB. Our findings also support this association (Alisjahbana et al., 2006; Andrade et al., 2019; Bam et al., 2015; Gelaw et al., 2019; Abreu et al., 2020; Yang et al., 2021).
The selection of PHCs was performed by purposive method based on several criteria, i.e : cover two or more sub districts and have laboratory facilities. In order to get normally distributed data, we use big sample and a higher ratio for case and control (1 :2). We did not assess interactions for our model because our experience is limited. However, a multicollinearity testing was performed on our model. Since our study shows that the absence of cross-ventilation is a potential factor for PTB. This emphasizes the need for the local government to implement interventions or regulations regarding healthy homes.
CONCLUSION
Based on our study, social risk factors, such as family history of PTB, number of contacts at home, time spent inside the house, and household family risk factor are associated with PTB in Bandung City. These findings show that social and environmental factors are important parts of the solution to eradicate pulmonary TB. We, therefore, recommend that the government should invest more in social interventions to eradicate PTB. Since our study demonstrated that PTB is associated with the higher number of family members in the house or a high total household contacts, it is our suggestion that the interventions should target areas with dense population as a priority area for PTB prevention.
Conflict of Interest: The authors declared no conflict of interest in this study.
Ethical Approval Number: Ethical Approval Number: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) Reference No 1164/UN6.C1.3.2/KEPK/PN/2016 Date : 01 -12 -2016.
REFERENCES
Abreu, R.G. de, Rolim, L.S., Sousa, A.I.A. de, Oliveira, M.R.F. de, (2020). Tuberculosis and diabetes: association with sociodemographic characteristics and diagnosis and treatment of tuberculosis. Brazil, 2007-2011. Rev. Bras. Epidemiol. 23, e200009.
Alisjahbana, B., Van Crevel, R., Sahiratmadja, E., Den Heijer, M., Maya, A., Istriana, E., Danusantoso, H., Ottenhoff, T.H.M., Nelwan, R.H.H., der Meer, J.W.M., (2006). Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int. J. Tuberc. Lung Dis. Vol 10, pp. 696–700.
Andrade, K.V.F. de, Nery, J.S., Araújo, G.S. de, Barreto, M.L., Pereira, S.M., (2019). Association between treatment outcome, sociodemographic characteristics and social benefits received by individuals with tuberculosis in Salvador, Bahia, Brazil, 2014-2016. Epidemiol. e Serviços Saúde Vol, 28, e2018220.
Baker, M., Das, D., Venugopal, K., Howden-Chapman, P., (2008). Tuberculosis associated with household crowding in a developed country. J. Epidemiol. Community Heal. Vol 62, pp. 715–721.
Bam, T.S., Aditama, T.Y., Chiang, C.-Y., Rubaeah, R., Suhaemi, A., (2015). Smoking cessation and smokefree environments for tuberculosis patients in Indonesia-a cohort study. BMC Public Health Vol 15, pp. 604.
Chan, P.-C., Fang, C.-T., (2020). The role of ventilation in tuberculosis control. J. Formos. Med. Assoc. https://doi.org/https://doi.org/10.1016/j.jfma.2020.11.003
Cohen, M.L., (2000). Changing patterns of infectious disease. Nature Vol 406, pp. 762–767.
Dodd, P.J., Looker, C., Plumb, I.D., Bond, V., Schaap, A., Shanaube, K., Muyoyeta, M., Vynnycky, E., Godfrey-Faussett, P., Corbett, E.L., others, (2016). Age-and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am. J. Epidemiol. Vol 183, pp. 156–166.
Escombe, A.R., Ticona, E., Chávez-Pérez, V., Espinoza, M., Moore, D.A.J., (2019). Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infect. Dis. 19, 88. https://doi.org/10.1186/s12879-019-3717-9
Gaur, P.S., Bhaskar, R., Singh, S., Saxena, P., Agnihotri, S., others, (2017). Incidence and clinical profiles of pulmonary and extra-pulmonary tuberculosis patients in North Indian population: a hospital based retrospective study. Int. J. Res. Dev. Pharm. Life Sci. Vol 6, pp. 2773–2778.
Gelaw, Y.A., Williams, G., Assefa, Y., Asressie, M., Soares Magalhães, R.J., (2019). Sociodemographic profiling of tuberculosis hotspots in Ethiopia, 2014–2017. Trans. R. Soc. Trop. Med. Hyg. Vol 113, pp. 379–391.
Horton, K.C., Hoey, A.L., Béraud, G., Corbett, E.L., White, R.G., (2020). Systematic Review and Meta-Analysis of Sex Differences in Social Contact Patterns and Implications for Tuberculosis Transmission and Control. Emerg. Infect. Dis. Vol 26, 910.
Jindal, S.K., (2017). Textbook of Pulmonary and Critical Care Medicine: Two Volume Set. Jaypee Brothers,Medical Publishers Pvt. Limited.
Lienhardt, C., Fielding, K., Sillah, J.S., Bah, B., Gustafson, P., Warndorff, D., Palayew, M., Lisse, I., Donkor, S., Diallo, S., others, (2005). Investigation of the risk factors for tuberculosis: a case–control study in three countries in West Africa. Int. J. Epidemiol. Vol 34, pp. 914–923.
Lönnroth, K., Jaramillo, E., Williams, B., Dye, C., Raviglione, M., (2010). Tuberculosis: the role of risk factors and social determinants. Equity, Soc. Determ. public Heal. Program. pp. 219-241.
Lönnroth, K., Jaramillo, E., Williams, B.G., Dye, C., Raviglione, M., (2009). Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc. Sci. Med. Vol 68, pp. 2240–2246.
Ministry of Health of the Republic of Indonesia, (2016). Regulation of the Minister of Health No. 67 on Tuberculosis Control Jakarta (Peraturan Menteri Kesehatan Nomor 67 Tentang Penanggulangan Tuberkulosis Jakarta). Jakarta Kemenkes RI.
Ministry of Health of the Republic of Indonesia, (2013). Riset kesehatan dasar (Riskesdas) 2013. Kemenkes RI. Jakarta.
Mossong, J., Hens, N., Jit, M., Beutels, P., Auranen, K., Mikolajczyk, R., Massari, M., Salmaso, S., Tomba, G.S., Wallinga, J., others, (2008). Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 5, e74.
Muchsin, M., Siregar, F.A., Sudaryati, E., (2019). The Influence of Nutritional Status and Ventilation on the Incidence of Pulmonary Tuberculosis at Langsa. Open access Maced. J. Med. Sci. Vol 7, pp. 3421–3424. https://doi.org/10.3889/oamjms.2019.436
Ortblad, K.F., Salomon, J.A., Bärnighausen, T., Atun, R., (2015). Stopping tuberculosis: a biosocial model for sustainable development. Lancet Vol 386, pp. 2354–2362.
Qian, H., Li, Y., Nielsen, P. V, Hyldgaard, C.E., Wong, T.W., Chwang, A.T.Y., (2006). Erratum: Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air. Vol 16, pp. 111-128.
Rahayu, S.R., Katsuyama, H., Demura, M., Katsuyama, M., Ota, Y., Tanii, H., Higashi, T., Semadi, N.P.D., Saijoh, K., (2015). Factors associated with tuberculosis cases in Semarang District, Indonesia: case–control study performed in the area where case detection rate was extremely low. Environ. Health Prev. Med. Vol 20, pp. 253.
Rathi, S., Akhtar, S., Rahbar, M., Azam, S., others, (2002). Prevalence and risk factors associated with tuberculin skin test positivity among household contacts of smear-positive pulmonary tuberculosis cases in Umerkot, Pakistan. Int. J. Tuberc. Lung Dis. Vol 6, 851–857.
Sabri, A., Quistrebert, J., Naji Amrani, H., Abid, A., Zegmout, A., Abderrhamani Ghorfi, I., Souhi, H., Boucaid, A., Benali, A., Abilkassem, R., others, (2019). Prevalence and risk factors for latent tuberculosis infection among healthcare workers in Morocco. PLoS One 14, e0221081.
Sornboot, J., Aekplakorn, W., Ramasoota, P., Bualert, S., Tumwasorn, S., Jiamjarasrangsi, W., (2019). Detection of airborne Mycobacterium tuberculosis complex in high-risk areas of health care facilities in Thailand. Int. J. Tuberc. Lung Dis. Vol 23, pp. 465–473.
Taye, H., Alemu, K., Mihret, A., Wood, J.L., Shkedy, Z., Berg, S. and Aseffa, A., (2021). Factors associated with localization of tuberculosis disease among patients in a high burden country: A health facility-based comparative study in Ethiopia. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases, Vol 23, p.100231.
Team Rstudio, (2015). R: A language and environment for statistical computing.
Volz, E.M., Miller, J.C., Galvani, A., Meyers, L.A., (2011). Effects of heterogeneous and clustered contact patterns on infectious disease dynamics. PLoS Comput. Biol. 7.
West java Provincial Health Office, 2020. Profil Kesehatan Provinsi Jawa Barat (2019). Bandung.
World Health Organization, (2020). Global Tuberculosis Report 2020. Geneva.
World Health Organization, (2017). Bending the curve: Ending TB in the WHO Shouth-East Asia Region.
World Health Organization, (2015). Global Tuberculosis Report 2015. France.
Yang, A., Schlichting, P., Wight, B., Anderson, W.M., Chinn, S.M., Wilber, M.Q., Miller, R.S., Beasley, J.C., Boughton, R.K., VerCauteren, K.C. and Wittemyer, G., (2021). Effects of social structure and management on risk of disease establishment in wild pigs. Journal of Animal Ecology, 90(4), pp. 820-833.


