Department of Biochemistry and Biotechnology, Annamalai
University, Chidambaram, Tamil Nadu, India
Corresponding author email: mirunasankar@gmail.com
Article Publishing History
Received: 10/01/2022
Accepted After Revision: 30/03/2022
The clinical use of capsaicin has been confined due to its low-grade solubility and frail bioavailability. So, we examined the impact of capsaicin encapsulated chitosan nanoparticles (CAP@CS-NP) on chemically induced hormone receptor-positive mammary carcinoma. The mammary tumor was induced by a single dose of 7,12-Dimethylbenz(a)anthracene (DMBA) 25mg/kg b.wt. injected subcutaneously near the mammary gland. After 7 weeks, CAP@CS-NP 4mg/kg b.wt. was administered orally to tumor-bearing rats. Furthermore, sex hormones levels, ER and PR expression, mast cell population, and molecular docking studies were carried out. Tumor-bearing rats flashed significantly hoisted levels of sex hormones, mast cell population, ER, and PR expression. Administration of CAP@CS-NP 4mg/kg b.wt. restored the levels of sex hormones, mast cell population, ER, and PR expression to near-normal levels. Additionally, molecular docking revealed good binding affinity and best glide scores. These findings suggest that nano encapsulation of CAP@CS-NP 4mg/kg b.wt. administration can regulate hormonal expression to treat hormone receptor-positive mammary carcinoma.
Breast Cancer, Capsaicin, Dmba, Estrogen Receptor, Progesterone Receptor.
Kalaiyarasi D, Manobharathi V, Mirunalini S. Restorative Effects of Capsaicin Encapsulated Chitosan Nanoparticles on Chemically-Induced Hormone Receptor-Positive Mammary Carcinoma in Sprague-Dawley Rats. Biosc.Biotech.Res.Comm. 2022;15(1).
Kalaiyarasi D, Manobharathi V, Mirunalini S. Restorative Effects of Capsaicin Encapsulated Chitosan Nanoparticles on Chemically-Induced Hormone Receptor-Positive Mammary Carcinoma in Sprague-Dawley Rats. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3L9aqIt“>https://bit.ly/3L9aqIt</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women across the globe, a pioneering cause of cancer-related fatality. According to GLOBOCAN 2020, female breast cancer comprises 11.7% of all cancer cases and 6.9% of all cancer mortality worldwide (Sung et al. 2020). Approximately two-thirds of all diagnosed breast cancers are categorized as hormone-dependent (Subramani et al. 2017). Female ovaries generate and release two groups of sex hormones, estrogen and progesterone, and their nuclear receptors are dubbed as estrogen receptors (ER) and progesterone receptors (PR).
It offers a pivotal role in forming and growing both normal and cancerous mammary epithelium (Subramani et al. 2017). Long-term and high-level exposure of these hormones has been strongly associated to an elevated peril of breast cancer. Early menarche, late menopause, late pregnancy and nulliparity are all facets that increase hormone secretion uncontrollably. Polycyclic Aromatic Hydrocarbons (PAHs) are recognized as endocrine disruptors, which intervene with the homeostasis of organisms through mimicking endogenous hormones (Zhang et al. 2016; Kerdelhue et al. 2016; Sung et al. 2020).
Synthetic PAH, DMBA is a popularly researched model chemical carcinogen for the induction of mammary tumor in rodents. Mammary tumors thus induced are hormone-dependent adenocarcinomas emerge from terminal end buds on inadequately differentiated mammary glands (Russo and Russo 1978). A recent study by Alvarado et al. evaluated the immune expression of the prognostic factors ER and PR in DMBA-induced rat mammary tumors to know the model that best suits women’s breast cancer (Alvarado et al. 2017). Current breast cancer treatments are chemotherapy, radiotherapy, hormone therapy and surgery (Sung et al. 2020).
Among these, hormonal therapy is crucial therapy to treat hormone receptor-positive breast cancer (Araki and Miyoshi 2018). Tamoxifen, anastrozole, exemestane, fulvestrant, goserelin, letrozole, leuprorelin, megestrol and toremifene are commonly used hormone therapy drugs. However, none has been proven optimal due to resistance and adverse side effects. This situation warrants the need for new anti-cancer drugs with potent and lower side effects for the treatment of mammary cancer (O’Reilly et al. 2020). Natural spices are a promising source for curing a variety of chronic ailments with their antioxidant, anti-inflammatory, antimicrobial and anti-cancer powers. It may also be used to relieve the adverse effects of cancer medications, such as fatigue, nausea, vomiting, indigestion and metallic taste (Ijpma et al. 2015; Zheng et al. 2016). Chili pepper (Capsicum annum) is a consistently consumed spice around the globe (Idrees et al. 2020). Capsaicin (CAP), the strong pungent component of chili peppers, exerts great anti-cancer effect in a vast number of malignancies (Clark and Lee 2016; Kalaiyarasi and Mirunalini 2021).
However, a major vital hindrance in the clinical practice of CAP is poor bioavailability due to its weak aqueous solubility that leads to constricted therapeutic potential and unsuccessful outcomes. The nanotechnology-based cancer therapy furnishes a promising solution to enhance the aqueous solubility and bioavailability of hydrophobic anti-cancer agents (Guo et al. 2011; Kalaiyarasi et al. 2021). Nanoparticle (NP) drug delivery systems, especially drug encapsulation with biodegradable polymeric NPs, have recently attained lots of attention due to their high cellular uptake, superior permeability and retention effect, and reduced cancer cell drug resistance (Mi et al. 2012; Tomasina et al. 2013). Chitosan (CS) is a natural-based polymer obtained from the exoskeleton of shrimps and other sea crustaceans. It is one of the rarest positively charged natural biopolymers in the world (Sogias et al. 2008; Taherian et al. 2021).
Due to its excellent physiochemical properties (non-toxic, bioadhesive, biocompatible and biodegradable) make CS a strong candidate for novel drug delivery systems, biosensors, edible films and nanofibers (Sogias et al. 2008; Taherian et al. 2021). In the current study, we aimed to investigate the anti-estrogen, and anti-progesterone effect of CAP encapsulated chitosan nanoparticles (CAP@CS-NP) on chemically induced hormone receptor-positive mammary carcinoma in female Sprague-Dawley rats.
MATERIAL AND METHODS
Capsaicin, 7,12-dimethylbenz(a)anthracene (DMBA), chitosan, sodium tripolyphosphate (TPP) were purchased from Sigma-Aldrich Co.Ltd. The primary antibodies for ER and PR were procured from Santa Cruz Biotech, USA. All other chemicals used were of analytical grade purchased from local commercial sources. CAP@CS-NP was synthesized by a novel method of ionic gelation with TPP solution (Gelling agent) and characterized by UV–visible spectroscopy, SEM analysis, FT-IR analysis and in vitro drug release (Arulmozhi et al. 2013). For in vivo experiment, 8 to10 weeks old female Sprague-Dawley rats (weight 130–150g) were purchased from Biogen Laboratory Animal Facility, Bangalore, India. Rats were maintained under controlled conditions of temperature 24 ± 2°C, humidity 50 ± 10% and photoperiod of 12 h (dark/light cycle), and bare access to a standard pellet diet and water provided in the Central Animal House, Rajah Muthiah Medical College, Annamalai University, Chidambaram, Tamil Nadu, India. This study was approved by the Institutional Animal Ethics Committee (IAEC), regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India (Reg No. 160/1999/CPCSEA and Proposal No. 1203). Total numbers of 36 rats were randomly divided into 6 groups, each containing 6 rats (n = 6).
Group I rats served as control (normal untreated rat). Group II, III, IV & V rats received a single subcutaneous injection of DMBA 25mg/kg b.wt. (near the mammary gland) at the first week of the experiment. After 7 weeks, the tumor-bearing groups III, IV & V rats were treated with CAP 8mg/kg b.wt., CAP@CS-NP 4mg/kg b.wt. and CS-NP 5mg/kg b.wt. for 21 days (thrice per week). Group VI rats received bare Free CAP@NP for 21 days (thrice per week). The doses were fixed based on previous research studies (Jung et al. 2006; Koleva et al. 2013; Anandakumar et al. 2015). The experiment was terminated at the end of the 14th week, all the rats were sacrificed. Levels of Estradiol and Progesterone in plasma were measured by using Enzyme-linked immunosorbent assay (ELISA) kits (LifeSpan BioSciences Inc, USA) as per the manufacturer’s instructions.
For immunohistochemical analysis, five micron-sized mammary tissue sections were embedded on poly-L-lysine coated glass slides. First, tissue slides were deparaffinized using xylene (5 min) and rehydrated in graded alcohol (10 min), washed in double-distilled water (5 min). Then the sections were incubated with 1% H2O2 in double distilled water at 22 oC (15 min) to quench the endogenous peroxidase activity, rinsed with Tris–HCl containing 150 mM NaCl and 1X TBS buffer at 22 oC (1 hr). After washing with 1X TBS buffer, the sections were incubated with primary antibodies ER and PR overnight at 4 oC.
Followed by incubation, the respective secondary antibodies IgG-HRP conjugates for 1 hr at 4 oC. After that, slides were washed with 1X PBS then reactivity was developed with 0.03% of diaminobenzene and H2O2. Finally, the slides were visualized under a microscope (40×). For histopathological analysis, mammary tissues were sliced, immersed in 10% neutral buffered formalin for fixation, dehydrated with graded ethanol solutions, and then embedded in paraffin. Paraffin-embedded mammary tissue sections (3–5 μm) were cut using a microtome and stained with Toluidine blue. Then, slides were observed under a light microscope (40×).
Additionally, molecular docking study was carried out using Schrödinger software (Maestro V9.5). The structure of CAP ligand molecule was retrieved from the PubChem databases (https://pubchem.ncbi.nlm.nih.gov/), and crystal structure of ER targets (PDB ID: 1X7R, 4PPS, 4ZN7, 5KCU) and PR targets (PDB ID: 1SQN, 1SR7, 3D9O, 4OAR) were retrieved from the protein databank (PDB) (http://www.rcsb.org/pdb). Ligprep and Maestro-Glide were used to prepare ligand and receptor grid for docking algorithm. Subsequently, a Glide extra precision (XP) visualizer is used to explore the interaction between the CAP ligand and active targets (ER and PR), as well as the binding distance of various amino acid residues. The data were expressed as mean ± standard deviation (SD). Statistical analysis was carried out using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). The comparisons between groups were done using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. A value of P< 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Today’s pharmaceutical research requires the discovery of secure and potent inhibitors with lower side effects, which are pivotal for cancer therapeutics. Over the past few years, we have been engaged in the creation of new nano formulated anti-cancer drugs against oral cancer and breast cancer (Arulmozhi et al. 2013; Isabella and Mirunalini 2017). In this direction, we have demonstrated the potency of CAP encapsulated chitosan nanoparticles against hormone receptor-positive (hormone-dependent) breast cancer. Hormones are signaling molecules secreted by glands in multicellular organisms and transmitted to distant organs through the circulatory system to bridle physiology and behavior (Bohra and Bhateja 2015). There are two types of hormones strongly related to hormone-dependent breast cancer (Trabert et al. 2020).
The first is estrogen, a crucial female sex hormone secreted in the ovary, placenta and adrenal cortex, promotes the growth and development of female genital organs and female secondary sex characteristics and triggers endometrial development. It is also considered one of the important etiological factors of mammary tumors. The three major endogenous estrogens with estrogenic hormonal activity are estrone (E1), estradiol (E2) and estriol (E3). Out of them, estradiol is the strongest biologically active hormone in mammary tissue and a major growth regulator for the mammary cancer subset (Russo and Russo 2006). The second hormone is progesterone, secreted by the corpus luteum in the ovary and is committed in both the menstrual cycle and early stages of pregnancy (Trabert et al. 2020). Higher circulating progesterone levels were closely linked to an escalating peril of breast cancer (Khan 2020).
Chemical carcinogen-induced cancer models in experimental rodents are a valuable resource. DMBA is a top-notch toxic which chemically promotes mammary cancer in the rat model. It renders ductal epithelial cells hyperplasia and atypical hyperplasticity and carcinogenesis of the terminal ducts. The tumor engendered by this chemical model resembles a human hormone-dependent breast tumor in terms of histology and hormone response profiles (Abba et al. 2016). In the current study, we found that DMBA-induced rats exhibit significantly elevated estradiol and progesterone levels in plasma (Group II) compared with the control rats (Group I). However, CAP 8mg/kg b.wt. (Group III) and CAP@CS-NP 4mg/kg b.wt. (Group IV) administration to tumor-bearing rats significantly altered these hormones levels to near normalcy. No changes were noted in CS-NP 5mg/kg b.wt. (Group V) treated rats when compared with DMBA induced rats (Group II). Although, no significant differences were detected in Free CAP@NP (Group VI) alone treated rats when compared to control rats (Group I) (Khan 2020).
CAP@CS-NP 4mg/kg b.wt. was found to be more efficient than CAP 8mg/kg b.wt. in reducing sex hormones levels (Table 1). Our findings agree with the previous report of El‐Aziz et al., which proved that melatonin treated rats showed near-normal levels of endocrine hormones when compared with DMBA rats (El‐Aziz et al. 2005). Hormone receptor, a receptor molecule that sticks to a pertinent hormone and is mainly expressed in the female reproductive organs of humans. ER and PR are the most routinely studied markers in mammary carcinoma (Althuis et al. 2004). IHC examination of DMBA-induced tumor-bearing rats (Group II) (B) were displayed increased expression of ER and PR when compared to control rats (Group I) (A), confirming the origins of hormone positive-receptor mammary carcinoma. Contrastingly, administration of CAP 8mg/kg b.wt. (Group III) (C) and CAP@CS-NP 4mg/kg b.wt. (Group IV) (D) dramatically reduced the expression of ER and PR when compared with DMBA induced rats (Group II) (B). No alterations in the ER and PR expression of CS-NP 5mg/kg b.wt. (Group V) (E) treated rats when compared with DMBA induced rats (Group II) (B) (Khan 2020).
Even though no modifications were noticed in Free CAP@NP (Group VI) (F) alone treated rats when compared to control rats (Group I) (A). Notably, CAP@CS-NP 4mg/kg b.wt. was shown to be more proficient than CAP 8mg/kg b.wt. in inhibiting abnormal hormone expression (Figure 1 (A–F) and Figure 2 (A–F)). Moreover, our result was also collaborating with previous studies of Isabella et al., who discovered that oral supplementation of DIM@CS-NP in tumor-bearing rats reduced the expression of ER and PR status (Isabella et al. 2018). Mast cells (MCs) represent a controversial constituent of the stromal compartment of breast cancer and a potent proangiogenic factor that promotes tumor development. The populations of MCs were favorably correlated with ER and PR expression (Glajcar et al. 2017; Aponte-Lopez et al. 2018; Khan 2020).
Our histopathological report clearly shows the excessive mast cell population in the mammary tissues of DMBA induced rats (Group II) (B) when compared with the control rats (Group I) (A). Conversely, CAP 8mg/kg b.wt. (Group III) (C) and CAP@CS-NP 4mg/kg b.wt. (Group IV) (D) administration to tumor-bearing rats greatly diminished the levels of a mast cell population when compared with DMBA induced rats (Group II) (B). No conversions were specified in CS-NP 5mg/kg b.wt. (Group V) (E) treated rats when compared with DMBA induced rats (Group II) (B). However, no differences were spotted in Free CAP@NP (Group VI) (F) alone treated rats when compared to control rats (Group I) (A). Noteworthy, CAP@CS-NP 4mg/kg b.wt. was more suitable than CAP 8mg/kg b.wt. in alleviating the mast cell population (Figure 3 (A–F)), which is in agreement with earlier research of pathological markers in mammary tissues (Arivazhagan et al. 2014; Khan 2020).
Figure 4 and 5 shows the 2D Structure binding interactions of CAP ligand with four distinct ER and four distinct PR breast cancer targets. The binding modes of the docked CAP compound with ER and PR targets get an excellent Glide score (-9.01, -8.92, -10.21, -8.17, -8.93, -9.24, -8.77, -7.79 kcal/mol), binding energy, lipophilic evidence and a number of hydrogen bond, according to the data (Table 2). Likewise, CAP ligand hydrogen bond, amine, and functional groups have interacted with PHE 404, GLU 353, LEU 346, GLY 521, LEU 718, ARG 766, ASN 719, GLU A: 725, and ASN A: 719 amino acid residues in ER and PR targets. As a result, these interaction modes seem to be the most crucial notion in drug discovery at the preclinical stage. our findings concur with the previous report of Acharya et al. who reported that furanocoumarin phytochemicals have the best docking confirmation with ER, PR, EGFR, and mTOR (Acharya et al. 2019; Khan 2020).
Table 1. Effect of CAP and CAP@CS-NP on sex hormones in plasma of control and experimental rats
| Groups | Estradiol (pg/mL) | Progesterone (ng/mL) |
| Control (I) | 35.03±1.18 | 21.60±1.39 |
| DMBA (II) | 52.28±2.78### | 32.18±2.26### |
| DMBA+ CAP (III) | 44.29±2.18*** | 27.84±1.99** |
| DMBA+ CAP@CS-NP (IV) | 38.33±1.72*** | 23.73±1.52*** |
| DMBA+ CS-NP (V) | 50.31±2.78 | 31.15±2.26 |
| Free CAP@NP (VI) | 36.28±1.33 | 22.91±1.41 |
Values are expressed as mean ± SD for six rats in each group. Significant levels are ###P < 0.001 when compared with control group and **P < 0.01, ***P < 0.001 when compared with DMBA group.
Figure 1: Immunohistochemical analysis of ER expression in the mammary tissues of control and experimental rats. Immunohistochemical on mammary tissues of control (A) and Free CAP@NP (F) alone treated rats showed normal mammary tissue staining; Mammary tissues of DMBA induced (B) and CS-NP 5mg/kg b.wt. (E) treated rats showed increased expression of ER; Mammary tissues of CAP 8mg/kg b.wt. (C) and CAP@CS-NP 4mg/kg b.wt. (D) treated rats showed diminished expression of ER as compared to DMBA induced rats (B).
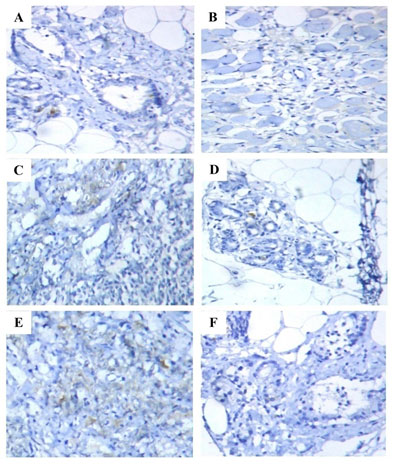
Figure 2: Immunohistochemical analysis of PR expression in the mammary tissues of control and experimental rats. Immunohistochemical on mammary tissues of control (A) and Free CAP@NP (F) alone treated rats showed normal mammary tissue staining; Mammary tissues of DMBA induced (B) and CS-NP 5mg/kg b.wt. (E) treated rats showed increased expression of PR; Mammary tissues of CAP 8mg/kg b.wt. (C) and CAP@CS-NP 4mg/kg b.wt. (D) treated rats showed diminished expression of PR as compared to DMBA induced rats (B).
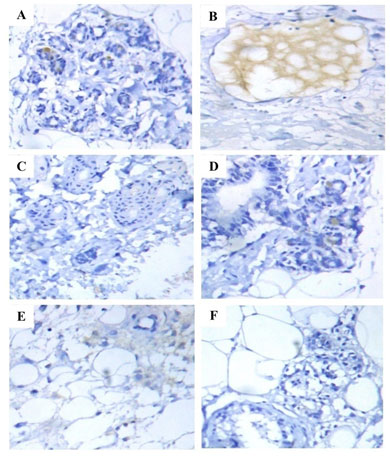
Figure 3: Histopathological analysis of mast cell population in the mammary tissues of control and experimental rats. Histopathological on mammary tissues of control (A) and Free CAP@NP (F) alone treated rats showed normal mammary tissue staining; Mammary tissues of DMBA induced (B) and CS-NP 5mg/kg b.wt. (E) treated rats showed increased levels of mast cell population; Mammary tissues of CAP 8mg/kg b.wt. (C) and CAP@CS-NP 4mg/kg b.wt. (D) treated rats showed decreased levels of the mast cell population as compared to DMBA induced rats (B).
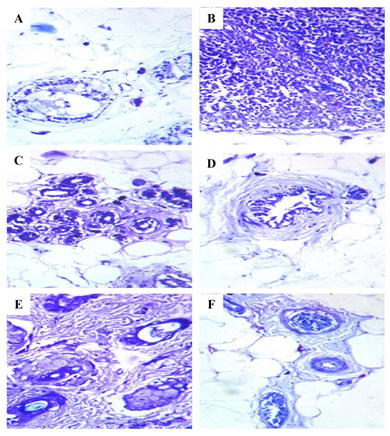
Figure 4: 2D Structure binding interactions of CAP ligand with ER targets.
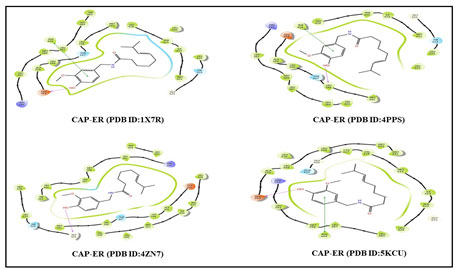
Figure 5: 2D Structure binding interactions of CAP ligand with PR targets.
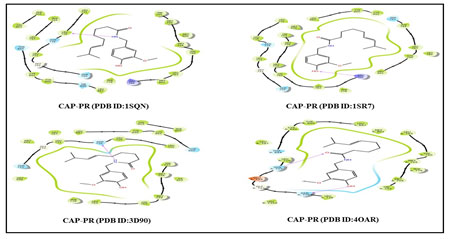
Table 2. Molecular docking result of CAP ligand with ER and PR targets
| S.No | Ligand | Receptor | PDB ID | G
Score |
Lipophilic
Evdw |
PhobEn | Hbond | Electro | Site map | Low
MW |
Rot
Penal |
| 1. | CAP | ER | 1X7R | -9.01
|
-6.21
|
-1.91
|
-0.48
|
-0.29
|
-0.4
|
-0.48
|
0.76
|
| 2. | CAP | ER | 4PPS | -8.92
|
-6.12
|
-1.63
|
-0.96
|
-0.1
|
-0.4
|
-0.48
|
0.76
|
| 3. | CAP | ER | 4ZN7 | -10.21
|
-6.41
|
-2.69
|
-1.01
|
-0.14
|
-0.24
|
-0.48
|
0.76
|
| 4. | CAP | ER | 5KCU | -8.17
|
-5.25
|
-2.11
|
-0.57
|
-0.2
|
-0.32
|
-0.48
|
0.76
|
| 5. | CAP | PR | 1SQN | -8.93
|
-6.28
|
-1.73
|
-0.78
|
-0.13
|
-0.29
|
-0.48
|
0.76
|
| 6. | CAP | PR | 1SR7 | -9.24
|
-6.55
|
-1.99
|
-0.48
|
-0.25
|
-0.25
|
-0.48
|
0.76
|
| 7. | CAP | PR | 3D90 | -8.77
|
-6.15
|
-1.14
|
-1.18
|
-0.23
|
-0.35
|
-0.48
|
0.76
|
| 8. | CAP | PR | 4OAR | -7.79
|
-4.82
|
-0.6
|
-1.74
|
-0.69
|
-0.22
|
-0.48
|
0.76
|
CONCLUSION
The findings of the present study shed light on the therapeutic prospect of nano-formulated drugs in the treatment of breast cancer. In cases of breast carcinomas, the role of sex hormones is well recognized. The antiestrogen and antiprogesterone activity of CAP@CS-NP was proven by the reduction of ER and PR expression. In addition, an alteration in the mast cell population implies anti-inflammatory activity. As a consequence, the current study clearly establishes the efficacy of CAP@CS-NP against DMBA induced hormone receptor-positive mammary carcinoma in rats. Also, the molecular docking study bolsters this research.
Conflict of Interest: Authors declare no conflict of interests to disclose.
Ethical Clearance: This study was approved by the Institutional Animal Ethics Committee (IAEC), regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India (Reg No. 160/1999/CPCSEA and Proposal No. 1203)
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Abba, M C, Zhong, Y, Lee, J et al. (2016). DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations. Oncotarget, Vol 7 No 39 Pages 64289–64299.
Acharya R, Chacko S, Bose P et al. (2019). Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer. Scientific reports, Vol 9 No 1 Pages 1-3.
Althuis, M D, Fergenbaum, J H, Garcia-Closas, M et al. (2004). Etiology of hormone receptor–defined breast cancer: a systematic review of the literature. Cancer Epidemiology and Prevention Biomarkers, Vol 13 No 10 Pages 1558-1568.
Alvarado, A, Lopes, A C, Faustino-Rocha, A I et al. (2017). Prognostic factors in MNU and DMBA-induced mammary tumors in female rats. Pathology-Research and Practice, Vol 213 No 5 Pages 441-446.
Anandakumar, P, Kamaraj, S, Jagan, S et al. (2015). The anticancer role of capsaicin in experimentally induced lung carcinogenesis. Journal of Pharmacopuncture, Vol 18 No 2 Pages 19-25.
Aponte-Lopez, A, Fuentes-Panana, E M, Cortes-Munoz, D et al. (2018). Mast cell, the neglected member of the tumor microenvironment: role in breast cancer. Journal of Immunology Research, Vol 1 Pages 1-11
Araki, K and Miyoshi, Y (2018) Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer, Vol 25 No 4 Pages 392-401.
Arivazhagan, L and Pillai, S S (2014). Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7, 12-dimethylbenz (α) anthracene-induced rat mammary carcinoma. The Journal of Nutritional Biochemistry, Vol 25 No 11 Pages 1140-1153.
Arulmozhi, V, Pandian, K and Mirunalini, S (2013). Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids and Surfaces B: Biointerfaces, Vol 110 No 1 Pages 313-320.
Bohra, A and Bhateja, S (2015). Carcinogenesis and sex hormones: a review. Endocrinology and Metabolic Syndrome, Vol 4 No 1 Pages 1-14.
Clark, R and Lee, S H (2016). Anticancer properties of capsaicin against human cancer. Anti-cancer Research, Vol 36 No 3 Pages 837-843.
El‐Aziz, M A A, Hassan, H A, Mohamed, M H et al. (2005). The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma: an animal model. International Journal of Experimental Pathology, Vol 86 No 6 Pages 383-396.
Glajcar, A, Szpor, J, Pacek, A et al. (2017). The relationship between breast cancer molecular subtypes and mast cell populations in tumor microenvironment. Virchows Archiv, Vol 470 No 5 Pages 505-511
Guo, G, Fu, S, Zhou, L et al. (2011). Preparation of curcumin loaded poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cells. Nanoscale, Vol 39 No 1 Pages 3825-3832.
Idrees, S, Hanif, M A, Ayub, M A et al. (2020). Chili pepper. In Medicinal Plants of South Asia, Vol 1 No 1 Pages113-124.
IJpma, I, Renken, R J, Ter Horst, G et al. (2015). Metallic taste in cancer patients treated with chemotherapy. Cancer Treatment Reviews, Vol 41 No 2 Pages 179-186.
Isabella, S and Mirunalini, S (2017). Protective effect of 3, 3′-Diindolylmethane encapsulated chitosan nanoparticles prop up with lipid metabolism and biotransformation enzymes against possible mammary cancer. Journal of Applied Pharmaceutical Science, Vol 7 No 03 Pages 194-201.
Isabella, S, Mirunalini, S and Pandiyan, K (2018). 3, 3′-Diindolylmethane encapsulated chitosan nanoparticles accelerates inflammatory markers, ER/PR, glycoprotein and mast cells population during chemical carcinogen induced mammary cancer in rats. Indian Journal of Clinical Biochemistry, Vol 33 No 4 Pages 397-405.
Jung, K J, Wallig M A and Singletary, K W (2006). Purple grape juice inhibits 7, 12-dimethylbenz [a] anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer letters, Vol 233 No 2 Pages 279-288.
Kalaiyarasi, D and Mirunalini, S (2021). Capsaicin (Capsicum Annuum): A ubiquitous compound with multivarient pharmaceutical properties. Research Journal of Chemistry and Environment, Vol 25 No 5 Pages 234-240.
Kalaiyarasi, D, Manobharathi, V and Mirunalini, S (2021). Development of nano drugs: A promising avenue for cancer treatment. Research Journal of Biotechnology, Vol 16 No 4 Pages 234-244.
Kerdelhue, B, Forest, C and Coumoul, X (2016). Dimethyl-Benz (a) anthracene: A mammary carcinogen and a neuroendocrine disruptor. Biochimie open, Vol 3 No 1 Pages 49-55.
Khan, S A (2020). Progesterone Exposure and Breast Cancer Risk—Addressing Barriers. JAMA network open, Vol 3 No 4 Pages 203608-203609.
Koleva Gudeva, L, Maksimova, V, Serafimovska Darkovska, M et al. (2013). The effect of different methods of extractions of capsaicin on its content in the capsicum oleoresins. Scientific Works: Food Science, Engineering and Technology, Vol 60 No 1 Pages 917-922.
Mi, Y, Liu, X, Zhao, J et al. (2012). Multimodality treatment of cancer with herceptin conjugated, thermomagnetic iron oxides and docetaxel loaded nanoparticles of biodegradable polymers. Biomaterials, Vol 33 No 30 Pages 7519-7529.
O’Reilly, M, Mellotte, G, Ryan, B et al. (2020). Gastrointestinal side effects of cancer treatments. Therapeutic Advances in Chronic Disease, Vol 11 No 1 Pages 1-7.
Russo J and Russo I H (2006). The role of estrogen in the initiation of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology, Vol 102 No 5 Pages 89-96.
Russo, I H and Russo, J (1978). Developmental stage of the rat mammary gland as determinant of its susceptibility to 7, 12-dimethylbenz [a] anthracene. Journal of the National Cancer Institute, Vol 61 No 6 Pages 1439-1449.
Sogias, I A, Williams, A C and Khutoryanskiy, V V (2008). Why is chitosan mucoadhesive?. Biomacromolecules, Vol 9 No 7 Pages 1837-1842.
Subramani, R, Nandy, S B, Pedroza D A et al. (2017). Role of growth hormone in breast cancer. Endocrinology, Vol 158 No 6 Pages 1543-1555.
Sung, H, Ferlay, J, Siegel, R L et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians, Vol 71 No 3 Pages 209-249.
Taherian, A, Esfandiari, N and Rouhani S (2021). Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnology, Vol 12 No 1 Pages 1-20.
Tomasina, J, Lheureux, S, Gauduchon, P et al. (2013). Nanocarriers for the targeted treatment of ovarian cancers. Biomaterials, Vol 34 No 4 Pages 1073-1101.
Trabert, B, Sherman, M E, Kannan, N, et al. (2020). Progesterone and breast cancer. Endocrine Reviews, Vol 41 No 2 Pages 320-344.
Zhang, Y, Dong, S, Wang, H, et al. (2016). Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environmental pollution, Vol 213 No 1 Pages 809-824.
Zheng, J, Zhou, Y, Li, Y, et al. (2016). Spices for prevention and treatment of cancers. Nutrients, Vol 8 No 8 Pages 495- 499.


