1Department of Chemistry, Panimalar Institute of Technology, Chennai, Tamil Nadu, India.
2Post Graduate& Research Department of Chemistry, Government Arts College, Ariyalur, Tamil Nadu, India.
Corresponding author email: vasanthchem84@gmail.com
Article Publishing History
Received: 30/12/2021
Accepted After Revision: 25/03/2022
Environmental and industrial problems arising from polluted water workably influence the relevance of separation of metal ions. Most of the industries in to-days world dump their wastewater into river, pond or sea which pollutes the water and increases the pollution level. Hence, it is increasingly important to purify the polluted surface water and also industrial effluents, especially for the exclusion of metal ions, by employing several physico–chemical processes. The different sources of heavy metal pollution are geological weathering, mining and industrial processing of ores leads to leaching of metals ions from waste, metal excretions from animals and run-off from agricultural fields using metallic biocides.
The CPACs were chosen because of its cheapness and easy carbonization from the abundant carbonaceous agricultural wastes/ by-products. BDST model used to predict the presentation of a column for adsorption of metal ions. The performance of the column charged with granular CAC and CR [Tulsion CXO – 9(H)] was also studied and compared. It is concluded that activated carbon prepared from mixed plants more efficient and very cheap than other types of adsorbents. Also, this study is very useful for researcher and public to get an idea about the removal of heavy metals ion in groundwater.
Activated Carbon, Biocides, Carbonization, Granular, Industrial Effluents,
Vasanthan S, Murugesan A, Kistan A, Selvam A. Removal of Heavy Metal Ions using Activated Carbon by Mixed Plants. Biosc.Biotech.Res.Comm. 2022;15(1).
Vasanthan S, Murugesan A, Kistan A, Selvam A. Removal of Heavy Metal Ions using Activated Carbon by Mixed Plants. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3hpbbjW“>https://bit.ly/3hpbbjW</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Industries such as electroplating, pickling, galvanizing, leather, metal finishing and processing, chemical etc, are also some other sources.The metal ions are highly toxic due to their bioaccumulation tendency and required affinity for the sulphydryl (-SH) groups of enzymes / proteins, thus preventing the enzymatic activity and / or disrupting the cellular structures (Bayader et al. 2018). Generally, heavy metals reason for irritation,nerve tissue damages, cardiac strain, heart diseases, disturbed metabolism, kidney malfunction, hyper-tension, ruin of central nervous and renal systems, brain damage and cancer.
Therefore, an economical way to achieve this without losing creativity and maintaining strict reset limits has become a challenge to human ingenuity and duty(Fergusson 1990; Söderholm et al. 2019).This has led to the continued refinement of existing treatment techniques and the recognition and development of promising emergent technologies like adsorption(Benjamin and Victoria 2020; Mofijur et al. 2021).
The removal of metallic ions using an economic approach still remains: as a great trouble even as some of successive structures have industrialized with adsorption strategies recently.. Activated carbon (AC) adsorption has advantages over traditional water treatment and reuse methods in terms of initial investment, simplicity of design, ease of operation and freedom of toxic substances.(Van 1983; Rahim et al. 2021). Commercial AC(CAC) in powder and granular forms have the most common adsorbent and broadlywas used but are expensive. This has contributed to the search for inexpensive adsorbents other than CAC.
References are made to the cultivation and use of beneficial adsorbents such as chitin and chitosan, silica, wood, peat, natural clay, bagasse heartwood, dyed cellulosic material, apple waste, waste AC, GAC models, fibers and polymers/resins. it’s possible. absorbent. However, most effective and completely constrained quantity of statistics is to be had on using chemically prepared activated carbons (CPACs) from agricultural wastes, by product of organic material (Poots et al. 1976; Filippi and Krukonis 1980; McKay et al. 1980; McKay et al. 1982;Poots et al. 1986; McKay and Bino1987; Pollard 1992; Maranon and Sastree 1992; Shukla et al. 1992; El-Geundi 1993; McKay 1998; Annesini and Monticelli 2000; Sivakumaraet al. 2020).
Fixed bed/column processes are commonly used for pollution control methods such as ion adsorption through ion exchange beds or carbon adsorption beds. Several models have been introduced into the industry to study data and predict outcomes for different adsorption schemes.(Tien1994; Subin Parkand Junghyun 2016). Although these models based on important mass transfer mechanisms with external films, pores and bottom diffusion have been proposed, solutions of some partial differential equations involving solids and dynamic parameters are required(Löhner et al. 2021).
Shortcut models based on pilot plant testing methodremain used mostly for confirmationrelatively than information collection, money and saving time. The bed depth facility time (BDST) model andmass transfer zone [MTZ] model (Alan 1952; WalkerL andWeatherley 1997).Adsorber performance provides simple tactics and quick predictions. The BDST model has been successfully used to describe the dye adsorption of the column.
The purpose of this present study is to analyse the capacity of CPACs such as SC and SDC to eliminate metal (Cu2+, Pb2+Cr3+ and Zn2+) ions from aqueous solution, by means of the column technique. CPAC was chosen because of its low cost due to the high volume of carbonaceous agricultural waste/by-products and its ease of carbonation. BDST model used to predict the performance of metal ion adsorption columns (Mamdouh 2006). The performance of column charged with granular CAC and CR [Tulsion CXO – 9(H)] was also studied and related(Vithanage et al. 2015; Afroza et al. 2020; Löhner et al. 2021).
In the fixed bed depth service time model, the basic principle of the strategy is to predict the effectiveness of the adsorbent material with which it can withstand the removal of a certain number of contaminants from the solution before regeneration is required (Mohamed et al. 2020).Required period of time is called the service time(t) of the bed. Hutchins projected a simple approach to fixed bed absorbers to relate the service time with the process variable quantity like, initial concentration, flow rate and adsorption capacity, by equation (1) (Arunachalam et al 2021).
t = [(NoZ) / CoV] – {(1/kaCo) ln [(Co/Cb)-1]} … (1)
where, Co = initial concentration of metal (mg dm-3) (Zümriye Aksu, JülideYener 2001)
Cb = break through adsorbate concentration (mg dm-3)
ka = BDST adsorption rate constant (dm3 mg-1 min-1)
V = velocity (cm min-1)
Z = bed height (cm)
The theoreticdeepness of adsorbent (AC) adequate to avoid the adsorbate concentration from beyondCb at t = 0, termed the bed depth (Zo, in cm) can be attained, when the service time is zero (t = 0) and given by equation (2):
Zo = (V/ ka No) ln [(Co/Cb)-1] … (2)
By determining the service time t for the formation depth Z from the experimental data, we can estimate No and ka from the slope of the graph and the values of the intersection (at t = 0), respectively. Graph of the critical formation depth equation.Reciprocalvalue of sloperemains the rate at which the adsorbent bed is consumed, and increasing this particular value by the adsorbent’s outward bulk thickness gives adsorbent utilized rate to continuous discharge waste water of acceptable quality (Elwakeel et al. 2020). BDST is written as simplified method as follows:
t = AZ + M … (3)
where, slope,
A = (No / CoV) … (4)
and intercept,
M = (1/kaCo) ln [(Co/Cb)-1] … (5)
The value of straight line presented is used to explain the working of the bed, if there is initial concentration Co,1, to a new value Co,2, Hutchins projected that new slope A2 and new intercept M2 can give by eqns.(6) and (7), similarly.
A2 = A1 (Co,1/ Co,2) … (6)
M2 = M1 (Co,1/ Co,2) ln {[Co,2/ Co,b)-1] / [Co,1/Co,b)-1]} … (7)
(McKay et al. 1998)detailed that, When the calculated data is important for changing the permeate volume flow rate in a similar adsorption system, the new slope (A2) through the unaltered segment (M2 ; M2 = M1) can be written as:
A2 = A1 (Q1/ Q2) = A1 (V1/ V2) … (8)
Apart from BDST model, MTZ model also predicts the design parameters similar to the BDST model.
MATERIAL AND METHODS
The activated carbon prepared by Sol gel method(Nurul et al. 2021). Chromium (Cr), Lead (Pb), Zinc (Zn), Copper (Cu) and were determined by spectrometric method using Atomic Absorption Spectrometer – Model: PerkinElmer-Analyst -400. The obtained results were formulated, estimated and mentioned according to the standards prescribed below ‘Indian standard drinking water specification IS 10500: 1992’ of Bureau of Indian Standards [BIS].
For the fixed bed experiments, the groundwater from the column is collected at regular intervals of time (30 – 45 min.) and the metal ions were estimated spectrometric method using Atomic Absorption Spectrometer – Model: PerkinElmer-Analyst -400(Allen and Minear 1982;Rao and Ramakrishna 1982; Jefferyet al. 1991; Lahrich et al 2019).
RESULTS AND DISCUSSION
Effect of initial concentration: The specific concentration of metal ions in the raw water is an important parameter and major determinant, but a given adsorbent capacity only absorbs a certain amount of metal ions. So, for the more concentrated solution of an incoming, the small amount of adsorbent can purify. Many experiments have been done to study the effect of changing the initial concentration on the rate of metal ion removal from solution. An increase in initial metal ion concentration increases the slope of intercept curve, decreasing the volume of influent treated earlier the adsorbent renewal and also results in an early breakthrough and exhaustion of the bed/column(Talat et al. 2018; Abdullah et al. 2019; Bounaaset al. 2021).
Activated Carbon Characterization : The activated carbon was prepared from three different plants of stems used (Neem, Mango and palm tree) (bulkdensity, porosity, porevolume, ash content,Average particlesize, iodine number etc,) and characterized by SEM (Bounaaset al. 2021). ThedataofthepreparedsamplesareshowninFig1.
Table 1. Characteristics of Activated carbon prepared from Mixed Plants
| Parameter | Observed Value | Standard Value |
| Bulk Density | 0.521gm/cm3 | 1.285 gm/cm3 |
| Porosity | 1.253 cm3g-1 | 1.365 cm3g-1 |
| Iodine Number | 1022 mgs/gm | 576.86 mgs/gm |
| Average particle size | 0.76 mm | 0.81 mm |
| Ash content | 8mg/g | 10.65 mg/g |
Figure 1: SEM images of Activated carbon prepared from three different stems of plants
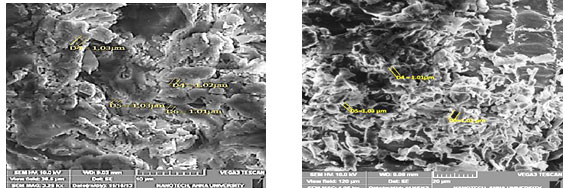
Table 2. Concentration of heavy metal in ground water samplesbefore adsorption.
| S.No | Heavy metals | BIS (IS 10500: 1991) | R-1 | R-2 | R-3 | R-4 | R-5 | R-6 | R-7 | R-8 | R-9 | R-10 |
| 1. | Chromium | 0.05 | 0.528 | 0.834 | 0.538 | 0.24 | 0.451 | 1.06 | 0.31 | 0.201 | 0.507 | 0.211 |
| 2. | Lead | 0.01 | 0.324 | 0.156 | 0.232 | 0.258 | 0.125 | 0.26 | 0.526 | 0.291 | 0.123 | 0.199 |
| 3. | Zinc | 5 – 15 | 7.32 | 9.24 | 6.45 | 12.16 | 11.84 | 8.16 | 8.28 | 4.95 | 6.9 | 4.06 |
| 4. | Copper | 0.05 – 1.5 | 4.45 | 7.94 | 5.58 | 3.42 | 6.84 | 5.88 | 4.56 | 3.28 | 6.24 | 4.48 |
Table 3. Concentration of heavy metal in ground water samples after adsorption.
| S.No | Heavy metals | BIS (IS 10500: 1991) | R-1 | R-2 | R-3 | R-4 | R-5 | R-6 | R-7 | R-8 | R-9 | R-10 |
| 1. | Chromium | 0.05 | 0.201 | 0.507 | 0.211 | 0.092 | 0.124 | 0.384 | 0.086 | 0.174 | 0.18 | 0.082 |
| 2. | Lead | 0.01 | 0.291 | 0.123 | 0.199 | 0.225 | 0.092 | 0.235 | 0.493 | 0.258 | 0.09 | 0.166 |
| 3. | Zinc | 5 – 15 | 4.95 | 6.9 | 4.06 | 9.79 | 8.84 | 5.78 | 5.89 | 2.56 | 4.51 | 1.67 |
| 4. | Copper | 0.05 – 1.5 | 3.28 | 6.77 | 4.41 | 2.22 | 5.63 | 4.71 | 3.4 | 2.11 | 5.6 | 3.24 |
Table 4. The percentageremoval of Chromium, Lead, Zinc and Copper with respect to time using
CPACDosage = 3 g/100 mL, pH = 4, Temp = 300 K
| Time in mins | % Removal of heavy metal with respect to dosage | |||
| Cr | Pb | Zn | Cu | |
| 15 | 55.85 | 51.53 | 59.56 | 56.45 |
| 30 | 59.42 | 52.53 | 62.53 | 57.86 |
| 90 | 67.54 | 61.23 | 69.85 | 63.85 |
| 120 | 71.23 | 64.78 | 71.53 | 65.42 |
Figure 2: The percentage removal of Heavy metal with respect to contact time
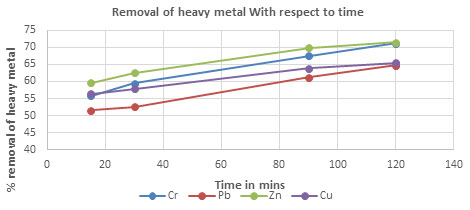
Table 5. The percentage removal of Chromium, Lead, Zinc and Copper with respect to dosage
of Activated carbonContact time 30 minutes, pH = 4, Temp = 300 K
|
Dosage amount |
% Removal of heavy metal | |||
| Cr | Pb | Zn | Cu | |
| 3g | 55.62 | 50.53 | 58.56 | 55.53 |
| 5g | 58.63 | 51.23 | 61.42 | 56.53 |
| 7g | 60.53 | 53.23 | 63.54 | 56.12 |
| 9g | 63.17 | 57.53 | 66.51 | 60.54 |
Figure 3: The percentage removal of Heavy metal with respectto dosage of adsorbent
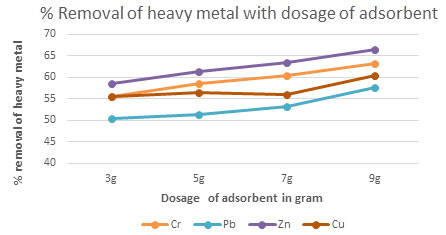
Table 6.The percentage removal of Chromium, Lead, Zinc and Copper with respect to pH using
CPACDosage = 3 g/100 mL, Contact time 30 minutes, Temp = 300 K
| pH | % Removal of heavy metal with respect to pH | |||
| Cr | Pb | Zn | Cu | |
| 1 | 51.53 | 49.53 | 48.53 | 43.53 |
| 2 | 54.56 | 53.23 | 51.53 | 48.46 |
| 3 | 60.53 | 55.45 | 53.59 | 56.53 |
| 4 | 65.53 | 57.86 | 59.53 | 62.53 |
| 5 | 60.27 | 42.53 | 50.42 | 52.42 |
| 6 | 40.23 | 37.89 | 35.53 | 42.53 |
| 7 | 35.23 | 32.21 | 29.53 | 36.35 |
| 8 | 30.26 | 27.53 | 24.53 | 27.53 |
| 9 | 25.63 | 22.53 | 22.53 | 21.53 |
Figure 4: The percentage removal of Heavy metal with respect to pH
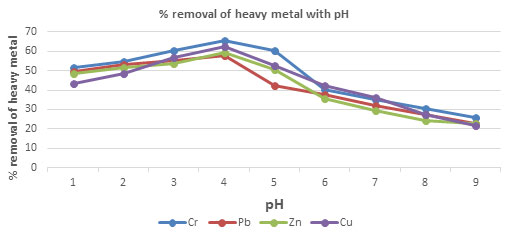
Table 7. The removal percentage of Chromium, Lead, Zinc and Copper with respect to
Temperature using CPACdosage = 3 g/100 mL, Contact time 30 minutes, pH4
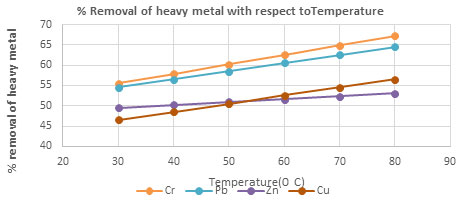
| Temperature(0 C) | % removal of heavy metal with Temperature | |||
| Cr | Pb | Zn | Cu | |
| 30 | 55.53 | 54.53 | 49.53 | 46.53 |
| 40 | 57.86 | 56.53 | 50.23 | 48.53 |
| 50 | 60.19 | 58.53 | 50.93 | 50.53 |
| 60 | 62.52 | 60.53 | 51.63 | 52.53 |
| 70 | 64.85 | 62.53 | 52.33 | 54.53 |
| 80 | 67.18 | 64.53 | 53.03 | 56.53 |
Figure 5: The percentage removal of Heavy metal with respect to temperature Heavy metal
Consequence of contact time on removal of heavy metals: The result of contact time on the exclusion of Activated carbon was evaluated for various concentrations (3 to 9 g/L), at regular time interval 15 to 80 minutes showsaincrease in metal concentration removal with increase in time and concentration of Activated carbon(Marrakchi et al. 2020).The increase in thickness of activated carbon increases the number of active sites on the surface get increases, which involved in removal of heavy metal ion in groundwater samples. It is explained that the part of degradation this metal concentration (Pb, Cr, Zn and Cu) progressively increased 3 to 9 g/L, afterward there is no removal of metal concentration.(Fig 2 & 5).
This indicates that the fluid to adsorbent mass transfer rate of the metal ion increases with the increase in initial concentration (Co). This is expected, since the concentration gradient across the film surrounding the adsorbent particle will be higher at the higher concentrations of metal ions (Felebueguet al. 2006; Dev et al. 2020). Increasing the concentration of metal ions entering the continuous stream decreases the output. This is due to high initial concentration (Co) soaking the adsorbent rapidly, thusreducing the break through time(Awan et al. 2021).
Consequence of catalyst dosage on removal of Pb, Cr, Zn and Cu: The experiments done withchanging the amount of dosage from 3 to 9 g/L for groundwater samples. The solutions kept under sunlight illumination for 15 to 80 minutes. As a result,shown remarkably greater in removal of heavy metal concentration from 40 mg/L dosage.It is well understood that the removal heavy metal concentration from groundwater activity increase with the increase in the dosage of Activated carbon(Fig 2 to 5)(Sujatha et al. 2021).
% of removal heavy metal concentration = (Absorbance at initial –Absorbance at final)/Absorbance at initial) X 100
The percentage removal of removal heavy metals was calculated using above formula and experimental data obtained from different experiment.
Effect of pH and Temperature on removal of heavy metals: The removal of heavy metal is high at pH 4, after pH 4 the percentage removal of heavy metal decrease, this is due to increase repulsion between adsorbent and heavy metal ions(Almomani et al. 2020). The removal percentage of heavy metal increase with increase time up to 80 minutes and further rising temperature decrease the percentage removal of heavy metal, this is due to desorption takes place above 800C(Jayanthi et al. 2021).
CONCLUSION
The findings of the present study show that the amount of wastewater treated before the initial breakthrough was directly proportional to the capacity of the adsorption tower and increased as the contact time increased. The effect of contact time on the amount of metal ions adsorbed by other metals also increased. Activated carbon was effectivelyproduced by sol-gel method and it was characterized by SEM. The produced Activated carbon was utilized for removal Pb, Cr, Zn and Cu metals from groundwater samples.
It is clear that the part of exclusion of heavy metals of groundwater gradually improved from 50 to 80 percent. The percentage removal of heavy metals graduallyimproved with contact time up to 120 minutes. This environmentallygood Activated carbon material used for removal of heavy metal such as Pb, Cr, Zn, Cu. It is concluded that activated carbon prepared from mixed plants more efficient and very cheap than other types of adsorbents. Also, this study is very useful for researcher and public to get an idea about the removal of heavy metals ion in groundwater.
ACKNOWLEDGEMENTS
The laboratory facilities for this study were provided by the Panimalar Institute of Technology, Tamil Nadu, India.
Conflict of Interests: Authors declare no conflict of interests to disclose.
Data Availability Statement: The database generated and/or analysed during in the current study are not publicly available due to privacy and confidentiality agreements as well as other restriction but are available from the corresponding author on responsible request.
REFERENCES
Abdullah, N. H., Shameli, K., Abdullah, E. C., et al. (2019). Solid matrices for fabrication of magnetic iron oxide nanocomposites: synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Composites Part B: Engineering Vol 162 Pages 538-568. https://doi.org/10.1016/j.compositesb.2018.12.075
Ahmed, S.F., Mofijur, M., Nuzhat, S., et al.(2021). Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater, Journal of Hazardous Materials Vol 416, Pages 125912. https://doi.org/10.1016/j.jhazmat.2021.125912
Aksu Z. andYener J. (2001). A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents, Waste Management Vol 21 No 8 Pages 695-702.
Allen,H.E, and Minear, R.A. (1982). Metallic Ions, In:Examination of Water for Pollution Control: Physical, Chemical and Radiological Examination, M.J. Suess, Pergamon, Oxford, (2), Pages 141.
Almomani, F, Bhosale, R, Khraisheh, M, et al. (2020). Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Applied Surface Science Vol 506 Pages 144924. https://doi.org/10.1016/j.apsusc.2019.144924
Annesini, M C, Gironi, F and Monticelli, B. (2000). Removal of oxygenated pollutants from wastewater by polymeric resins: data on adsorption equilibrium and kinetics in fixed beds Water Res, Vol 34 No 11Pages 2989-2996.
Ateia, M., Helbling, D.E. and Dichtel, W.R., (2020). Best Practices for Evaluating New Materials as Adsorbents for Water Treatment, ACS Materials Letters, Vol 2, No11, Pages 1532-1544. DOI: 10.1021/acsmaterialslett.0c00414
Awan, F. U. R., Keshavarz, A., Azhar, M. R, et al. (2021). Adsorption of nanoparticles on glass bead surface for enhancing proppant performance: a systematic experimental study. Journal of Molecular Liquids Vol 328 Pages 115398. https://doi.org/10.1016/j.molliq.2021.115398
BAYADER, F.A. and WESSAL, M.,(2018). Environmental Pollution with the Heavy Metal Compound Research J. Pharm. and Tech Vol 11 No 9 Pages 4035-4041.
Bounaas, M., Bouguettoucha, A., Chebli, D. et al. (2021). Role of the Wild Carob as Biosorbent and as Precursor of a New High-Surface-Area Activated Carbon for the Adsorption of Methylene Blue. Arab. J. Sci. Eng Vol 46 Pages 325–341. https://doi.org/10.1007/s13369-020-04739-5
Bounaas, M., Bouguettoucha, A., Chebli, D. et al. (2021). Role of the Wild Carob as Biosorbent and as Precursor of a New High-Surface-Area Activated Carbon for the Adsorption of Methylene Blue. Arab. J. Sci. Eng Vol 46 Pages 325–341. https://doi.org/10.1007/s13369-020-04739-5
Dev, V. V., Baburaj, G., Antony, S., et al. (2020). Zwitterion-chitosan bed for the simultaneous immobilization of Zn (II), Cd (II), Pb (II) and Cu (II) from multi-metal aqueous systems. Journal of Cleaner Production Vol 255 Pages 120309. https://doi.org/10.1016/j.jclepro.2020.120309
Dey A.A. and Rahman M.M. et al. (2020). A Novel Self-Standing Porous Composite Bed of Kaolinite-Choline Chloride for Highly Efficient Removal of Anionic Azo Dye from Aqueous Solutions J. Mater. Environ. Sci., Vol 11 No 11 Pages 1828-1838.
Driel J.V. (1983). In:Activated carbon: a fascinating material, A. Capelle and F. de Vooys (Eds.), Norit N.V, Amensfoort, The Netherlands Pages 40-57.
El-Geundi, M, S. (1993). Adsorption of Basic Dyes on Granular Activated Carbon and Natural Zeolite Ads. Sci. Technol, Vol 9 No 2 Pages 109-211.
Elwakeel, K. Z., Elgarahy, A. M., Elshoubaky, G. A., et al. (2020). Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. Journal of Environmental Health Science and Engineering Vol 18 No 1 Pages 35-50. https://doi.org/10.1007/s40201-019-00435-1
Felebuegu, A.O., Lester, J.N., Churchley, J. et al.(2006). Removal of an Endocrine Disrupting Chemical (17α-ethinyloestradiol) from Wastewater Effluent by Activated Carbon Adsorption: Effects of Activated Carbon Type and Competitive Adsorption Environmental Technology Vol 27 No 12 Pages 1343-1349.
Fergusson J E. (1990). The Heavy Elements: Chemistry, Environmental Impact and Health Effects, Pergamon Press, Oxford Pages 85-101.
Jayanthi, G., Sumathi, S., and Andal, V. (2021). Synthesis and applications of perovskite in heavy metal ions removal-a brief perspective. Materials Today: Proceedings. https://doi.org/10.1016/j.matpr.2021.06.147
Jeffery, G.H., Bassett, J,Mendham, J, et al. (1991). Vogel’s Text Book of Quantitative Chemical Analysis, ELBS, Longman, London, (5) Pages 689.
Lahrich, S, Saqrane, S, Manoun, B, et al. (2019). Voltammetric determination of trace level of cadmium in mussels and seawaters by a lacunar apatite-modified carbon electrode. Journal of Food Measurement and Characterization, Vol 13 No 3 Pages 2318-2327. https://doi.org/10.1007/s11694-019-00151-2
Löhner, R., Antil, H., Srinivasan, A. et al. (2021). High-Fidelity Simulation of Pathogen Propagation, Transmission and Mitigation in the Built Environment. Arch Computat Methods Eng. Vol 28, Pages 4237–4262. https://doi.org/10.1007/s11831-021-09606-6
Mahmud, N.A., Malik, L.A., Mazlan, N.W.,et al. (2021). Processing of Y3+-doped Ba(Ce,Zr)O3 by using the sol–gel method assisted with functionalized activated carbon as a composite anode for proton ceramic fuel cells, Materials Research Bulletin Vol 139, Pages 111277. https://doi.org/10.1016/j.materresbull.2021.111277
Maranon, E, and Sastree, H. (1992). Preconcentration and removal of trace metals from water by apple waste Bioresour. Technol Vol 38 Pages 29-39.
Marrakchi, F, Hameed, B. H and Bouaziz, M. (2020). Mesoporous and high-surface-area activated carbon from defatted olive cake by-products of olive mills for the adsorption kinetics and isotherm of methylene blue and acid blue 29. Journal of Environmental Chemical Engineering Vol 8 No 5 Pages 104199. https://doi.org/10.1016/j.jece.2020.104199
McKay G., Otterburn M.S. and Sweeney A.G. (1980). The removal of colour from effluent using various adsorbents. III. Silica: rate processes. Water Res Vol 14 No1) Pages 15-20.
McKay, G. (1998). Application of Surface Diffusion Model to the Adsorption of Dyes on Bagasse PithAdsorption Vol 4 No 3 Pages 361-372.
McKay, G., and Bino, M.J. (1987). Integration of enzyme catalysis in an extractive fermentation process, in Biocatalysis in Organic Chem, J, Tech. Biotechnol Vol 37 Pages 187 -199.
McKay, G., Blair, H.S. and Gardner J.R. (1982). Removal of coloured organic matter by adsorption onto low-cost waste materials J. Appl. Polym. Sci Vol 27 Pages 3043-3057.
MichaelsA.S.(1952). Simplified Method of Interpreting Kinetic Data in Fixed-Bed Ion Exchange industrial and Engineering chemistry Vol 44 No 8 Pages 1922-1930.
Modell, M., de Filippi, R.P. and Krukonis, V. (1980). In:Activated Carbon Adsorption of Organics from Aqueous Waste, Mc Guire, M.J, and Suffet, I.H, Vol. 1 (Ann Arbor Sci. Pub. Michigan).
Nassar, M.M.(2006).Adsorption of Fe and Mn from Ground Water onto Maize Cobs Using Batch Adsorber and Fixed Bed Column, Separation Science and Technology Vol 41 No 5 Pages 943-959.
OteneB.B. and Harry V.B. (2020). Assessment of Metal Concentrations in Water from the Downstream Segment of New Calabar River, Port Harcourt Segment of New Calabar River, Port Harcourt Research & Reviews: Journal of Ecology Vol 9 No 1 Pages 1-7.
Park S., Lee Y. and LeeJ.H. (2016). Association between energy drink intake, sleep, stress, and suicidality in Korean adolescents: energy drink uses in isolation or in combination with junk food consumption Nutrion Journal vol 15 Pages 87.
Pollard, S.J.T., Fowler, G.D., Sollars, C.J., et al. (1992). Low-cost adsorbents for waste and wastewater treatment: a review Sci. Total Environ Vol 116 Pages 31-52.
Poots, V. J, McKay, G., and Healy,J.J. (1976).The removal of acid dye from effluent using natural adsorbents: II. Wood. Wat. Res. Vol 10 Pages 1067-1070.
Poots, V. J., McKay, G., and Healy, J.J. (1986). The sorption of dyes onto peat in fixed beds I Chem E Symp Ser, No. 96, Effluent Treatment and Disposal Water Res, Pages 199 – 210.
Rao T.P. and RamakrishnaT. V. (1982). Spectrophotometric determination of trace amounts of cadmium in pure zinc materials with iodide and pyronine G Analyst Vol 107 Pages 704.
Shahrokhi-Shahraki, R., Benally, C., El-Din, M.G. et al.(2021). Efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon, Insights into the adsorption mechanisms, Chemosphere Vol 264, No 2 Pages, Part 1128455. https://doi.org/10.1016/j.chemosphere.2020.128455
Shukla S.R. and Pai R.S.(1992). Comparison of Pb(II) uptake by coir and dye loaded coir fibres in a fixed bed column Journal of Hazardous Material Vol 125 No 1 Pages 147-153.
Söderholm P., Bergquist A., and Söderholm, K. (2019). Environmental Regulation in the Pulp and Paper Industry, Impacts and Challenges. Curr Forestry Rep Vol 5 Pages 185–198. https://doi.org/10.1007/s40725-019-00097-0
Sujatha, S., and Sivarethinamohan, R. (2021). A critical review of Cr (VI) ion effect on mankind and its amputation through adsorption by activated carbon. Materials Today: Proceedings Vol 37 Pages 1158-1162. https://doi.org/10.1016/j.matpr.2020.06.351
Talat, M., Mohan, S., Dixit, V., et al. (2018). Effective removal of fluoride from water by coconut husk activated carbon in fixed bed column: Experimental and breakthrough curves analysis. Groundwater for Sustainable Development Vol 7 Pages 48-55. https://doi.org/10.1016/j.gsd.2018.03.001
Thirunavukkarasu, A., Nithya, R. and Sivashankar, R., (2021). Continuous fixed-bed biosorption process: A review, Chemical Engineering Journal Advances Vol 8 Pages 100188. https://doi.org/10.1016/j.ceja.2021.10018
Tien, C. (1994). Adsorption Calculations and Modeling, Butterworth, Heinemann, Boston.
Vithanage M. and Bhattacharya P. (2015). Fluoride in Drinking Water: Health Effects and Remediation. In: Lichtfouse E., Schwarzbauer J., Robert D. (eds) CO2 Sequestration, Biofuels and Depollution. Environmental Chemistry for a Sustainable World, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-319-11906-9_4
WalkerL G.M. and Weatherley R. (1997). Adsorption of acid dyes on to granular activated carbon in fixed beds Water Research Vol 31 No 8 Pages 2093-2101.


