Post Graduate and Research Department of Biochemistry, Sri Akilandeswari
Women’s College, Wandiwash, Tamil Nadu, India.
Corresponding author email: editorjpsr@gmail.com
Article Publishing History
Received: 25/12/2021
Accepted After Revision: 28/03/2022
The production of reactive oxygen species (ROS), which induce oxidative stress in humans, is linked to the negative effects of Chromium 6.The protective action of Cucumis melo L. fruit extracts was evaluated in this study in an animal model of hematological and biochemical parameters, which was induced by chromium VI (K2 Cr2 O7). The purpose of this study was to analyse the efficacy of Cucumis melo L. on chromium VI (K2 Cr2 O7)-induced rats. For 42 days, male albino rats (160–20 g) were given the stated oral LD50 dosage of chromium VI (K2Cr2O7) (10 mg/kg body weight).
After 42 days, chromium-induced rats were administered with two different concentrations of Cucumis melo L. and ascorbic acid (250 mg/kg, 500 mg/kg body weight) for 7 days. Following therapy, blood was drawn and analysed for a variety of biochemical markers.The results revealed that ingestion of either plant extract, ascorbic acid, or their combination on chromium 6 induced rats significantly increase the activity of Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST) and decreased the activity of Gamma Glutamyl transferase (γ-GT) was recorded.
This study has proven that fruit extract particularly its combination with ascorbic acid has a potential prophylactic effect. Cucumis melois vital for modifying the Chromium (VI) induced toxicity on male albino rats. Indeed, the recommended fruits should be consumed to the Chromium (VI) deposited harmful region since they may protect cells from environmental stress.
Biochemical Changes, Cucumis Melo, Kidney, Liver and Potassium Dichromate.
Malathi G, Vadivelu J. Prophylactic Effect of Cucumis melo on Chromium Vi-Induced Male Albino Rats. Biosc.Biotech.Res.Comm. 2022;15(1).
Malathi G, Vadivelu J. Prophylactic Effect of Cucumis melo on Chromium Vi-Induced Male Albino Rats. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3vyEust“>https://bit.ly/3vyEust</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Environmental toxin such as chromium, which is made up of chromium (0), chromium (III), and chromium (IV) significantly affects human health. According to the Environmental Protection Agency’s (EPA) list of the eight most frequent pollutant heavy metals, chromium is one of them, (Eman and Farag 2020). Chromium, a transition metal element, is found in abundance in the earth’s crust, including rocks, volcanic dust, gases, and soils, in addition to plants and animals.
The toxicity of chromium 6 is greater than that of chromium 3.The human body requires trivalent chromium to increase the action of insulin in bodily tissues for sugar, protein, and fat consumption. Furthermore, chromium may enhance cascade ranges in pig and broiler chicks. Hexavalent chromium is typically produced by industrial operations(Genchi et al. 2021).
Hexavalent chromium compound potassium dichromate is used in tanning, dyeing, pharmaceuticals, photography, alloys, dry battery production, stainless steel manufacturing, electroplating, and wood preservation. Several papers on pharmacotherapeutics, genotoxicity, and its deleterious implications are available (Czarnek et al. 2021). Few studies have attempted to relate potassium dichromate at various dose levels to hematological and biochemical alterations in rats using an adequate animal model (Ezekaibeya et al. 2020). According to clinical evidence, Cr (VI) exposure can cause serious kidney damage(Wu et al. 2020).
The major cause of cellular malfunction and death is the formation of free radicals as a result of a decline in Cr 6 levels within the cell. (Wu et al. 2020).Plants have an important part in the treatment of illnesses, as seen by their widespread use in all major medical systems.Several medicinal plants may have health-promoting pharmacological effects (Luo et al. 2020). Long-term use of natural remedies without evidence of harm to health might indicate that a chemical is safe (Silva et al. 2020).
In the field of medicine, many plants are yet to be investigated. One of these plants is Cucumis meloL. It is a Cucurbitaceae family annual climber whose fruit is mostly used as a vegetable. This plant can be found in rural and coastal areas. Researchers found that both Cucumis meloand P. dactylifera plant extracts, as well as their combination, exhibit potential hepatoprotective effects in streptozotocin-induced rats(Manchali et al. 2021).
Because of its excellent nutritional content, the fruit is typically consumed as a vegetable (Ganji et al. 2020). This fruit was high in carbohydrates, protein, fats, and vitamins. The aqueous extract of ripe fruit contained water-soluble vitamins such as ascorbic acid, phenylalanine, glutamine, and asparagine (Leon et al. 2021). The goal of the study is to see how prolonged low-dose chromium exposure affects several hematological and biochemical markers in male albino rats. Cucumis melo L. fruit was also analyzed, and all essential components were confirmed to be present in adequate amounts for active prophylactic efficacy.
MATERIAL AND METHODS
Cucumis melo L. fruits were harvested in Vandavasi, Thiruvannamalai, Tamil Nadu, India, for extract extraction. From December to January of 2019-2020 and accordingly, all plant materials were gathered. Siddha Central Research Institute, Chennai (Central Council for Research in Ayurveda and Siddha, New Delhi, Ministry of Health & Family Welfare, Government of India), Reference No: C14022001M, verified the fruits. Extraction took place at room temperature under normal circumstances.
Aqueous was used to extract around 5g of shade-dried powder from Cucumis melo L. fruits, which was then maintained in a boiling water bath for 30 minutes. The extract was filtered before being concentrated in a water bath at 100°C.For drugs and chemicals, Chromium 6 was purchased from scientific Lab Chemicals, Chennai, and was used to make the suspension in a dose of 10 mg/kg body weight for the respective groups. All other chemicals were purchased from Sisco Research Laboratory, Chennai.
Male albino rats weighing 160 to 180 g were procured from the Biomedical Research Unit and Laboratory Animal Centre, BRILAC/SDCH/SIMATS/IAEC/3-2020/049 Chennai, and were cared for accordance to CPCSEA norms under the supervision of the Animal Ethical Committee. All animals were kept in regular circumstances (25 1 °C, 12 h light/12 h dark cycle) with free access to food and water for 7 days prior to the experiment. According to Giang et al. 2021, the rats are evenly allocated into four treatment groups, each with six rats.
For 50 days, the animals in various groups were given the following treatments orally by gavages: GROUP I: The untreated rat (Normal Control). GROUP II: K2 Cr2 O7 induction (10 mg/kg body weight) was given for 42 days. GROUP III: Treatment with Ascorbic acid (500 mg/kg body weight) for 7 days after induction of K2 Cr2 O7 (10 mg/kg body weight) for 42 days. GROUP IV: K2 Cr2 O7 was induced (10 mg/kg body weight) for 42 days and then treated for 7 days with Cucumis melo L. Aqueous Extract (500 mg/kg body weight).
GROUP V: K2 Cr2 O7 was induced (10 mg/kg body weight) for 42 days and then treated for 7 days with Ascorbic acid (500 mg/kg body weight) and Cucumis melo L. aqueous extract (500 mg/kg body weight). Animals were slaughtered by cervical dislocation after anaesthesia at the end of the treatment period. The Indian Council of Medical Research rules were followed to the letter during the sacrifice. Blood and tissue were taken and utilised for biochemical assessments after the liver and kidney were removed and thoroughly cleansed in normal saline.
Freshly removed Liver and Kidney were rinsed in cold saline (0.9 percent NaCl), blotted dry, put into a cold beaker, and chopped thoroughly with scissors for tissue homogenates. Then a 0.25M sucrose solution (8 ml per gramme wet weight) was added. The homogenate was then filtered and centrifuged for 10 minutes at 5000 rpm. As previously, the particle was homogenised and resuspended. The supernatants were mixed and centrifuged for another 20 minutes at 15,000 rpm (Fatima et al. 2021).
According to Chang et al. 2021, the activities of Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), and Gamma Glutamyl transferase (-GT) were measured.The levels of urea and creatinine were analyzed using tissue homogenates.Serum creatinine and Serum urea was measured by Berthelot’s method were determined by spectrophotometric methods as described by (Rahman et al.2021).The statistical software programme version (SPSS) 7.0 was used to examine the data. The significance of differences between male albino rats was determined using the student’s t-test. All results were reported as mean SD for a total of n=6 people. At p<0.05, p<0.01, and p<0.001, differences were declared significant.
RESULTS AND DISCUSSION
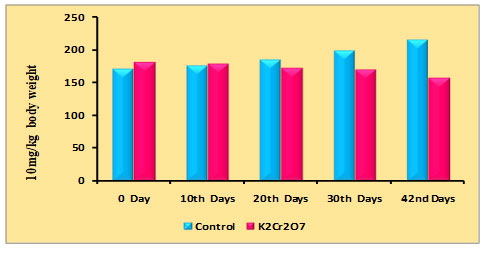
Changes in body weight of rats due to PDC on the 0th, 10th, 20th, 30th, and 42nd days were recorded and are shown in table 1 and figure 1. It may be noted that initially there was a gradual increase in body weight in the control animals starting from 0 to 42 days, whereas the administration of PDC on 10th, 20th,30th, and 42nd day a significant reduction in body weight was observed from 20th days when compared with the control and further it may be noted that on 42nd day the decrease was much more which may be due to PDC toxicity.
Table 1. Changes in body weight of rats treated with Potassium Dichromate (PDC/K2Cr2O7)
| Days | Control | K2Cr2O7 (10 mg/kg) |
| 0th Days | 170 ± 6.02 | 180 ± 6.38 |
| 10th Days | 176 ±6.23 | 178 ± 6.30 |
| 20th Days | 184 ± 6.51 | 172 ± 6.08 |
| 30th Days | 198 ± 7.01 | 169 ± 5.98 |
| 42nd Days | 215 ± 7.61 | 157 ± 5.56 |
Figure 1: Distribution of body weight rats treated with Potassium Dichromate
The current study discovered the presence of physiologically active phytocompounds in Cucumis melo, tasty oval-shaped fruit with high nutritional content that is widely consumed in many tropical nations. Many secondary metabolites, including as polyphenolics and flavonoids, are found in the fruits, and they have powerful pharmacological properties such as antibacterial, antioxidant, anti-inflammatory, and anticancer properties (Moustafa et al. 2020; Nash et al. 2020). Cucumis melo whole fruit extract has the greatest gallic acid and rutin concentration, according to Xudong et al. (2020).In agreement with this fruit,the extract is more combat to the external environmental stresses when compared to the seeds (Cunha et al. 2020).Therefore our present study infers that Cucumis melo 500 mg of fruits aqueous extract was confirmed to modify the chromium VI induced male albino rat body weight as well as their organ weight when compared to the control animal, (Cunha et al. 2020).
Table 2 and Figure 2: Changes in body weight of rats treated with PDC and Cucumis melo
| Days | Control | PDC
(10 mg/kg) |
PDC+CM (250 mg/kg) | PDC+CM (500 mg/kg) | CM (500 mg/kg) |
| 42nd days | 215 ± 7.61 | 157 ± 5.56$ | 157 ± 5.56$ | 157 ± 5.56$ | 215 ± 7.61NS |
| 49th days | 232 ± 7.61 | _ | 168 ± 5.94$ | 182 ± 6.44$ | 227 ± 8.03NS |
PDC-Potassium DiChromate; CM- Cucumis meloL.(CM)
Values represent mean± SD of six animals – @P<0.05, $P<0.01, * P<0.001 when compared to 49th days animals.
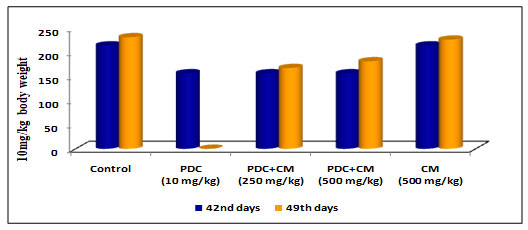
When compared to control animals, rats on PDC therapy exhibited a drop in body weight as the days of treatment progressed, and the changes in body weight were significant at the P<0.01 level. When the same rats were fed Cucumis melo L (250mg/kg body weight and 500mg/kg body weight) for 42 days, the body weight steadily increased and reached near-normal weight with control rats, which was statistically significant at the P<0.01 level.On the 49th day when the animal was treated with Cucumis melo L (500mg /kgbody weight) the increase in body weight was gradual and this was significant indicating the role of Cucumis melo L which could have helped to bring back the normal weight acting as an antidote to PDC (Table 2 and Fig 2)(Cunha et al. 2020).
Table 3 and Figure 3: Changes in body weight of rats treated with PDC and Ascorbic Acid
| Days | Control | PDC
(10 mg/kg) |
PDC+AA (250 mg/kg) | PDC+AA (500 mg/kg) | AA (500 mg/kg) |
| 42nd days | 215 ± 7.61 | 157 ± 5.56$ | 157 ± 5.56$ | 157 ± 5.56$ | 215 ± 7.61NS |
| 49th days | 232 ± 7.61 | _ | 164 ± 5.81$ | 175 ± 6.19$ | 225 ± 7.24NS |
PDC-Potassium DiChromate; AA- Ascorbic Acid
Values represent mean± SD of six animals – @P<0.05, $P<0.01, * P<0.001 when compared to 49th days animals.
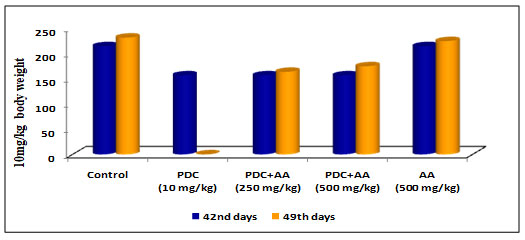
Rats on treatment with PDC showed a decrease in body weight as the days of treatment increased and the changes in the body weight were significant at P<0.01 level when compared with the control animals. After the 42ndday when the samerats werefed with ascorbic acid (250mg/kg body weight and 500mg/kg bodyweight), the bodyweight was found to increase gradually and reached near-normal weight with control rats, and the increase was statistically significant at P<0.01 level.On the 49th day when the animal was treatedwith ascorbic acid (500mg /kgbody weight), the increase in body weight was gradual and this was significant indicating the role of ascorbic acid which could have helped to bring back the normal weight acting as an antidote to PDC (Table 3 and Fig 3)(Wu et al. 2021).
Table 4. Changes in body weight of rats treated with PDC and Ascorbic Acid (AA) along with Cucumis melo L
| Days | Control | PDC
(10 mg/kg) |
PDC+AA+CM (250 mg/kg) | PDC+AA+CM (500 mg/kg) | AA+CM (500 mg/kg) |
| 42nd days | 215 ± 7.61 | 157 ± 5.56$ | 157 ± 5.56$ | 157 ± 5.56$ | 215 ± 7.61NS |
| 49th days | 232 ± 7.61 | _ | 164 ± 5.81$ | 175 ± 6.19$ | 223 ± 7.24NS |
PDC-Potassium DiChromate; CM- Cucumis meloL.(CM); AA-Ascorbic Acid
Values represent mean± SD of six animals – P<0.05, P<0.01, P<0.001 when compared to 49th days animals.
Figure 4: Body weight changes
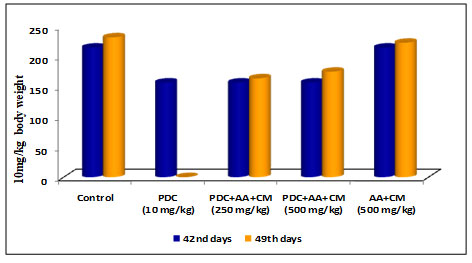
Rats after treatment with PDC for 42 days when fed with Cucumis melo L and Ascorbic acid (250 and 500 mg/kg body weight) for 7 days and (49th day) the result on 49th day showed that the bodyweight of rats showed a slight increase in 250 and 500 mg/kg body weight (Table 4 and Fig 4) when compared with the control animals [6.05± 0.53 for Liver and 1.75± 0.16 for kidney]. The increase was statistically significant at P<0.01 level respectively. The combined role of Cucumis melo L and Ascorbic acid for 500 mg/kg body weight dose was found to be more effective in bringing back the bodyweight of rats to normal than the 250 mg/kg body weight dose(Wu et al. 2021).
Table 5. Changes in organ weight of rats treated with PDC, Cucumis meloL,and ascorbic acid
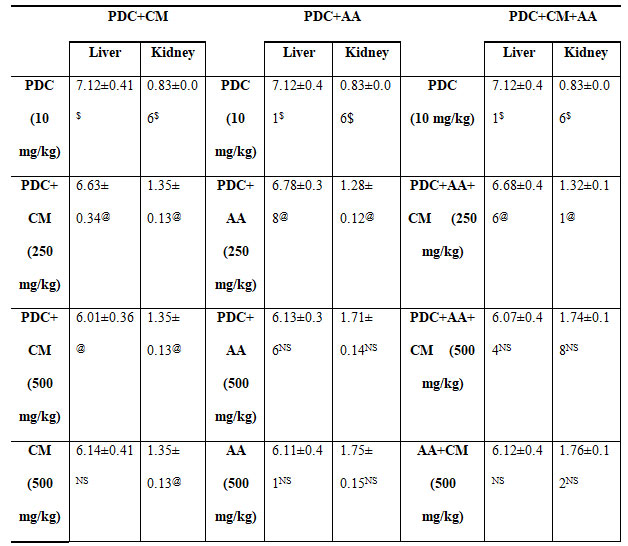
PDC-Potassium DiChromate; CM- Cucumis meloL.(CM); AA-Ascorbic Acid
Values represent mean± SD of six animals
P<0.05, P<0.01, P<0.001 when compared to 49th days animals
Chromium (VI) is a toxic metal that is widely utilized in industry. It has harmful effects on the liver, kidneys, and other organs. (A.J. Machado et al., 2021) As a result, several studies show that chromium (VI) causes considerable oxidative stress in both the liver and the kidney, although the kidney appears to be more prone and sensitive to Chromium (VI) poisoning than the liver. Because of its role in xenobiotic metabolism, the liver is particularly vulnerable to damage.Potassium dichromate’s toxicity was demonstrated at the cellular level (Wu et al. 2021). After 24 and 48 hours of treatment, cells exposed to 10 M potassium dichromate (K2Cr2O7), a dangerous Cr(VI) compound, had significantly lower viability and increased intracellular ROS generation. 2021 (Wu and colleagues) In K2Cr2O7-exposed HK2 cells, the expression levels of indicators that initiate the apoptotic cascade, such as cleaved caspase 3 and poly (ADP-ribose) polymerase, were considerably elevated. HK2 cells treated to K2Cr2O7 also demonstrated activation of intrinsic and extrinsic apoptotic markers. (Wu et al. 2021).
The effect of PDC after 42 days followed by 7 days of treatment with Cucumis melo L on two different organ weights of rats’ viz., liver and kidney are represented in table 5.When compared to the control, there is a substantial rise in liver weight and a reduction in kidney weight, both of which are significant at the P0.01 level. There was a decrease in the weight of the liver and an increase in the weight of the kidney in rats given Cucumis melo L (250 and 500 mg/kg body weight). When Cucumis melo L was given individually (500 mg/kg body weight), the findings were comparable, suggesting a change that was statistically significant at the P0.05 level.On feeding the rats with Ascorbic acid (250 and 500 mg/kg body weight) for 7 days the rats showed a significant decrease in the weight of liver and increase in the kidney and the changes were so observed significant at P<0.05 when compared with the PDC toxicity alone(Pinero et al. 2021).
Whereas animal fed with ascorbic acid (500 mg/kg body weight) above showed results which were near equivalent to control, indicating that ascorbic acid would have detoxifies the effect of PDC. Treatment with Cucumis melo L and ascorbic acid to rats already treated rats with PDC showed reduced liver weight and the weight increased in the kidney weight and these changes were significant at P<0.05 level when compared with the PDC toxicity rats. Treatment with 500 mg/kg body weight of Cucumis meloL,ascorbic acid and its combination was more significant than the 250 mg/kg of body weight when compared with the PDC toxicity rats, were 500 mg/kg body weight of Cucumis melo L and ascorbic acidcombined dose was able to detoxify the effect of PDC(Pinero et al. 2021).
Figure 5: Quantify the Liver marker enzymes such as ALT, AST, and γ-GT on ratstreated with PDC, Cucumis meloL,and ascorbic acid
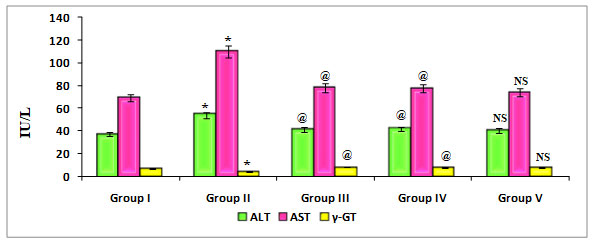
Group I: Control; Group II: K2 Cr2 O7/PDC; Group III: K2 Cr2 O7/PDC+ Ascorbic acid; Group VI: K2 Cr2 O7/PDC + Cucumis melo L; Group V: K2 Cr2 O7/PDC+ Ascorbic acid + Cucumis meloL
Values represent mean± SD of six animals
@P<0.05, $P<0.01, * P<0.001 when compared to 49th days animals
Figure 5 depicts the effect of PDC toxicity and Cucumis melo L therapy on liver marker enzymes in rats. When compared to control rats, PDC-treated rats showed a substantial drop in -GT, which was statistically significant at the P0.001 level, however there was a significant rise in the levels of ALT and AST, which was statistically significant at the P0.001 level. When compared to the PDC treated animals, those given Cucumis melo L (500 mg/kg) and Ascorbic acid (500 mg/kg) or a combination of Cucumis melo L (500 mg/kg) and Ascorbic acid (500 mg/kg) showed an increase in -GT that was statistically significant at P0.05, P0.05, and P-Non significant levels, as well as a significant decrease in the levels of ALT and AST.Cucumis melo L has an antidote effect on PDC toxicity, however it is not considerable. According to reports by Das et al. (2020), ascorbic acid has the capacity to alter gene expression, apoptosis, and other cellular activities in living systems exposed to heavy metals, such as nickel (Das et al. 2020).
Figure 6: Quantify the Kidney marker enzymes Urea on rats treated with PDC, Cucumis meloL,and ascorbic acid
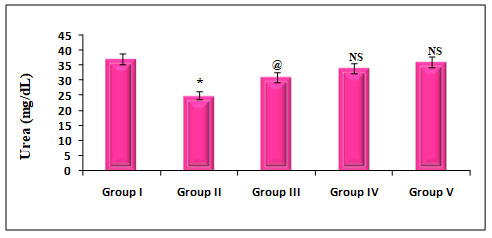
Group I: Control; Group II: K2 Cr2 O7/PDC; Group III: K2 Cr2 O7/PDC+ Ascorbic acid; Group VI: K2 Cr2 O7/PDC + Cucumis meloL; Group V: K2 Cr2 O7/PDC+ Ascorbic acid + Cucumis meloL
Values represent mean± SD of six animals
P<0.05, P<0.01, P<0.001 when compared to 49th days animals
The effect of PDC toxicity and treatment with Cucumis melo L on kidney marker enzymes in rats is shown in fig 6. PDC-treated rats showed a decrease in Urea by 32% which was statistically significant at a P<0.001 level when compared with the control animals. These animals on treatment with Cucumis melo L (500 mg/kg) and ascorbic acid (500 mg/kg) and a combination of Cucumis melo L (500 mg/kg), Ascorbic acid (500 mg/kg), showed an increase in Urea by 16%, 8% and 3% which was statistically significant at P<0.05, P-Non significant and P-Non significant level, when compared with the PDC, treated rats, indicating that the antidote effect of Cucumis melo L on PDC toxicity(Pinero et al. 2021).
Hepatocytes must maintain a delicate balance between cellular oxidants and antioxidant defences even when they are at rest (Pinero et al. 2021). Due to the generation of inflammatory cytokines, the activation of numerous signalling pathways, and cellular changes, a breakdown of this equilibrium may lead the cells to enter an inflammatory state, resulting in harm to both the cells involved and the surrounding tissues. Although the activities of AST and ALT are the most widely used and recognised enzyme indicators of liver damage, they only vary in the late stages of many liver illnesses and frequently lack sensitivity in the early stages (Asdaq et al. 2021).
Figure 7: Quantify the Kidney marker enzymes Creatinine on rats treated with PDC,Cucumis meloL,and Ascorbic acid
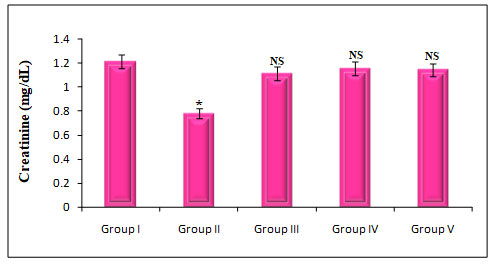
Group I: Control; Group II: K2 Cr2 O7/PDC; Group III: K2 Cr2 O7/PDC+ Ascorbic acid; Group VI: K2 Cr2 O7/PDC + Cucumis meloL; Group V: K2 Cr2 O7/PDC+ Ascorbic acid + Cucumis meloL;
Values represent mean± SD of six animals
P<0.05, P<0.01, P<0.001 when compared to 49th days animals
The effect of PDC toxicity and treatment with Cucumis meloLon kidney marker enzymes in rats is shown in fig 7. PDC-treated rats showed a decrease in Creatinine by 36% which was statistically significant at a P<0.001 level when compared with the control animals. These animals on treatment with Cucumis melo L(500 mg/kg) and ascorbic acid (500 mg/kg) and a combination of Cucumis melo L(500 mg/kg), ascorbic acid (500 mg/kg), showed an increase in creatinine by 8%, 5%, and 6% which was statistically significant when compared with the PDC treated rats, indicating that the antidote effect of Cucumis melo Lon PDC toxicity(Asdaq et al. 2021).
CONCLUSION
The findings of this study suggest that the beneficial effect of Cucumis melo Lis to alleviate liver and kidney function impairment due to the presence of their phytonutrients, which may render the toxic effect of Chromium (VI) induced liver damage via the assessment of significant increases in AST and ALT levels, which may render the toxic effect of Chromium (VI) induced liver damage via the assessment of significant increases in AST and ALT levels. Cucumis melo can also assist with damaged kidney enzymes such as urea and creatinine. In the future, it is hoped that the findings of this study may aid in the development of effective therapies for acute Cr (VI) poisoning.
Conflict of Interests: Authors declare no conflict of interests.
Ethical Clearance: Siddha Central Research Institute, Chennai (Central Council for Research in Ayurveda and Siddha, New Delhi, Ministry of Health & Family Welfare, Government of India), Reference No: C14022001M, verified the fruits.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Asdaq S, Venna S, Mohzari Y, et al. (2021). Cucumis melo Enhances Enalapril Mediated Cardioprotection in Rats with Isoprenaline Induced Myocardial Injury. Processes 9(3): 557.
Chang ML, Lin YT, Kung HN, et al. (2021). A triterpenoid-enriched extract of bitter melon leaves alleviates hepatic fibrosis by inhibiting inflammatory responses in carbon tetrachloride-treated mice. Food & Function 12(17):7805-15.
Cunha JAD, Rolim PM, Damasceno KS, et al. (2020). From seed to flour: sowing sustainability in the use of cantaloupe melon residue (Cucumis melo L. var. reticulatus). PloS one 15(1): p.e0219229.
Czarnek K and Siwicki AK. (2021). Genotoxicity of chromium (III) and cobalt (II) and interactions between them. Current Issues in Pharmacy and Medical Sciences
Das S, Reddy RC, Chadchan KS, et al. (2020). Nickel and oxidative stress: cell signaling mechanisms and protective role of vitamin C. Endocrine, Metabolic & Immune Disorders-Drug Targets. Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders 20(7): 1024-1031.
Eman S and Farag AI. (2020). Chromium-induced hepatotoxicity and potential protective effect of selenium in adult male albino rat: a histological, immunohistochemical and molecular study. The Medical Journal of Cairo University88: 187-196.
Ezekaibeya AC, Nnenna AO and Kenechukwu OC. (2020). Proximate, phytochemical, and vitamin compositions of Cucumis metuliferus (Horned Melon) rind. Journal of Complementary and Alternative Medical Research 18: 40-50.
Fatima H, Shahid M and Jamil A (2021). Therapeutic Potential of Selected Medicinal Plants Against Carrageenan Induced Inflammation in Rats. Dose-Response 19(4): 15593258211058028.
Ganji SM, Singh H, and Friedman M. (2020). Phenolic content and antioxidant activity of extracts of 12 melon (Cucumis melo) peel powders prepared from commercial melons. Journal of food science 84(7): 1943-1948.
Genchi G, Lauria G, Catalano A et al. (2021). The double face of metals: The intriguing case of chromium. Applied Sciences 11(2): 638.
Giang BL, Tuan NT, and Dung LT, et al. (2021). Alfa glucosidase inhibitory, anti-inflammatory activities, and a new furanocoumarin derivative of Ruellia tuberose. Natural product research 35(22): 4248-4255.
Leon AF, Martinez SG (2021). Grafting snake melon (Cucumis meloL. subsp. melo Var. flexuosus (L.) Naudin) in organic farming: Effects on agronomic performance; resistance to pathogens; sugar, acid, and VOC profiles; and consumer acceptance. Frontiers in plant science 12: 113.
Luo F, Li Q, Yu L, et al. (2020). High concentrations of CPPU promotes cucurbitacin B accumulation in melon (Cucumis melo var. Makuta makino) fruit by inducing transcription factor CBT. Plant Physiology and Biochemistry 154: 770-781.
Machado AJ et al. (2021). Single and combined toxicity of amino-functionalized polystyrene nanoparticles with potassium dichromate and copper sulfate on brine shrimp Artemia franciscana larvae. Environmental Science and Pollution Research 16:1-18.
Manchali S, Murthy KNC and Patil BS. (2021). Nutritional composition and health benefits of various botanical types of melon (Cucumis melo L.). Plants 10(9): 1755.
Moustafa SF, Gabr NM, Zaki JT et al. (2020). The anti-inflammatory, anti-ulcer activities and phytochemical investigation of Cucumis melo L. cv. Ismailawi fruits. Natural Product Research 8:1-5.
Nash RJ, Bartholomew B, Penkova YB et al. (2020). Iminosugar idoBR1 isolated from Cucumber Cucumis sativus reduces inflammatory activity. ACS Omega 5(26): 16263-16271.
Pinero MC, Otalora G and Collado J. (2021). Foliar application of putrescine before short‐term heat stress improves the quality of melon fruits (Cucumis melo L). Journal of the Science of Food and Agriculture 101(4): 1428-1435.
Rahman S, Jan G and Rahim HU. (2021). Phytochemical Screening and Antidiabetic, Antihyperlipidemic, and Antioxidant Effects of Leptopus cordifolius Decne. In Diabetic Mice. Frontiers in Pharmacology 12.
Silva MA, Albuquerque TG and Alves RC et al. (2020). Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods. Trends in Food Science & Technology 98: 181-189.
Wu YH, Lin JC, Wang TY et al. (2020). Hexavalent chromium intoxication induces intrinsic and extrinsic apoptosis in human renal cells. Molecular medicine reports 21(2): 851-857.
Zhang X et al. (2020). Anticancer Properties of Different Solvent Extracts of Cucumis melo L. Seeds and Whole Fruit and Their Metabolite Profiling Using HPLC and GC-MS. BioMed research international.


