1Engineering Department, University of Technology and Applied Sciences, Salalah, Oman
2Department of Biotechnology, Kumaraguru College of Technology, Coimbatore, India
Corresponding author email: sivmansel@gmail.com
Article Publishing History
Received: 24/11/2021
Accepted After Revision: 25/03/2022
This study aims to assess the applicability of organic acid pretreatment on culm of Bambusa balcooa for the production of maximum total reducing sugars. The experiments were performed by varying organic acids (Formic, acetic, pyruvic, maleic, malic, tartaric, adipic, citric and oxalic acids), concentration of oxalic acid (1-4% (w/v)), solvent to solid ratio (5-40 mL/g), pretreatment time (0-60 min) and temperature (75-135 °C) to maximize total reducing sugars (TRS) concentration using fractional factorial design based one-factor-at-a-time (OFAT) approach. Total reducing sugars concentration was estimated by using 3,5-dinitro salicylic acid (DNSA) method. Among various organic acids, oxalic acid exhibited higher reducing sugars concentration. The optimal values show that the maximum TRS of 48.33 g/L was achieved at oxalic acid concentration, solvent to solid ratio, time and temperature of 3% (w/v), 10 mL/g, 15 min and 121 °C for the pretreatment of cassava stem with oxalic acid. A low value of coefficient of variation (CV = 0.32%) showed that the optimal conditions were validated by experiments. Thus, B. balcooa could be used as a potential feedstock for the production of TRS by organic acid pretreatment
Bamboo, Fractional Factorial Design, Oxalic Acid, Pretreatment, Total Reducing Sugars.
Sivamani S, Kaveri R, Nandhini S. U. Production of Total Reducing Sugars from Bambusa balcooa through Oxalic Acid Pretreatment. Biosc.Biotech.Res.Comm. 2022;15(1).
Sivamani S, Kaveri R, Nandhini S. U. Production of Total Reducing Sugars from Bambusa balcooa
through Oxalic Acid Pretreatment. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3sSGJ8c“>https://bit.ly/3sSGJ8c</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Biofuels are major sustainable alternative to petroleum-based fuels due to current reliance on supplies from the organization of petroleum exporting countries, increased emissions of greenhouse gases in the atmosphere and depletion of oil reserves (Zahan and Kano 2018). Bioethanol produced from renewable resources emits fewer gases than fossil fuels and reduces the burden of carbon dioxide emissions to the atmosphere (Handler et al. 2016). When bioethanol is blended with petrol, the fuel mixture is oxygenated and burns more completely and reduces polluting emissions (Wu et al. 2020). Although bioethanol can be produced by chemical route, it is majorly produced through fermentation of sugar (Devi et al. 2021). The energy stored in the plants in the form of sugar is utilised for bioethanol production (Thatoi et al. 2016; Wu et al. 2020; Devi et al. 2021).
First generation biofuels are produced from starch and sugar. But it requires food crops such as sugarcane, corn, wheat, and sugar beet. Using food crops as raw material, first generation bioethanol threatens food supplies and biodiversity (Anushya et al. 2019; Sivamani et al. 2020). The alternative cheaper and polysaccharide-rich sources is required to explore as raw materials for bioethanol production to reduce the fuel-food conflicts, increase the available raw materials, and produce economically competitive with petroleum-based fuels. Thus, second generation biofuels can help solve the problems created by first generation biofuels (Vanitha et al. 2017).
Second generation biofuels utilize lignocelluloses derived feedstocks that are abundant and less utilized renewable resources. These include the residues from agriculture and forestry (sugarcane bagasse, corn stover, straw, etc.) and energy crops (Chandrasekaran and Sivamani 2018; Sivamani et al. 2020). The plant residues consist of stems, leaves and husks of non-food crops. Several countries including South Africa are currently engaged in major research projects studying the utilization of lignocellulosic materials to produce bioethanol (Bensah et al. 2015; Sivamani et al. 2018). The lignocelluloses compound is rich in cellulose and hemicellulose, which are covered by lignin (Sivamani et al. 2021).
Lignocellulosic materials are hence recalcitrant to hydrolysis (saccharification) and require several steps before they are converted to bioethanol which makes the process somewhat complex (Chandrasekaran et al. 2017). During pretreatment, plant cell walls were pretreated to break the lignocellulosic matrix. Then, hemicellulose and cellulose present in lignocellulosic materials hydrolysed to their monomers, xylose and glucose predominantly. Finally, the monomeric sugar units were fermented using ethanologenic organisms to bioethanol (Sivamani et al. 2018; Sivamani et al. 2021).
Bamboo-based residues are one of the lignocellulosic materials that can be used as a feedstock for bioethanol production due to the relatively higher growth rate of the plants, their abundancy and availability in the tropics (Alzagameem et al. 2019). Bamboo is specifically utilised as a building material where the wood plays a major role (Shen et al. 2019). Bamboo plants are found notably in South Asia, Southeast Asia and East Asia for the economic and cultural significance used for building materials, as a food source and versatile raw material (Sathishkumar et al. 2020). Bambusa balcooa is an evergreen bamboo forming a dense clump of erect, woody stems. This species is one of the most important village bamboos used for construction. The plant is widely cultivated on a small scale in Northeast India and Bangladesh and occasionally also outside this region (Banik 2015; Banik 2016; Sathishkumar et al. 2020).
Tang et al. (2021) examined the potential Bacillus velezensis LC1 for degradation of polymers in bamboo to monomeric sugar units. The, they subjected the hydrolysate to ethanolic fermentation with Saccharomyces cerevisiae and Escherichia coli KO11. The degradation efficiencies were found as 59.90, 75.44 and 23.41% for cellulose, hemicellulose and lignin, respectively, and the ethanol yield was achieved at 10.44 g/L after 96 h. Yang et al. (2019) investigated the alkaline liquid hot water pretreatment of a bamboo species, Neosinocalamus affinis, by examining the effect of temperature and alkali dosage. Bioethanol yield of 4.8 g/L was achieved by separate hydrolysis and fermentation (SHF) at 0.5% (w/v) NaOH 1t 170 °C (Sathishkumar et al. 2020).
From the analysis of literature, the various pretreatment methods such as bacterial degradation, alkaline liquid hot water, modified alkaline hydrogen peroxide, autohydrolysis, alkaline extraction, steam explosion, green liquor (mixture of Na2S and Na2CO3), ultra-high-pressure explosion, hydrothermal, and chemical (acid or alkali) treatment (Li et al. 2015; Dai et al. 2020). Organic acid pretreatment was employed for cassava stem, corncob, wheat straw, Napier grass, water hyacinth and so on (Kootstra et al. 2009; Amnuaycheewa et al. 2017; Sivamani and Baskar 2018; Qiao et al. 2019; Tantayotai et al. 2019). But only limited literature is available on organic acid pretreatment of bamboo biomass (Li et al. 2014; Sindhu et al. 2014; Sathishkumar et al. 2020). Hence, in the current study, culm from B. balcooa was evaluated as a feedstock for organic acid pretreatment by varying different organic acids, organic acid concentration, solid to liquid ratio, pretreatment time and temperature.
MATERIAL AND METHODS
Culm from Bambusa balcooa was collected from Forest College and Research Institute, Mettupalayam (Longitude 11.19’N, Latitude of 77.56’E), Coimbatore district. Sulphuric acid, sodium hydroxide, acetic acid, trichloroacetic acid, oxalic acid, citric acid, tartaric acid, adipic acid, formic acid, malic acid, maleic acid, xylose, 3,5 dinitro salicylic acid, crystalline phenol, sodium sulphite, sodium hydroxide, potassium sodium tartrate, ethanol, toluene, glacial acetic acid, sodium chlorite , acetyl bromide, perchloric acid, nitric acids were procured from Finar chemicals Ltd. and HiMedia Laboratories Pvt. Ltd. B. balcooa was characterized for lignin, cellulose, hemicellulose and ash by using standard operating procedure reported elsewhere (Chandrasekaran et al. 2017). Different organic acids such as formic acid, acetic acid, malic acid, maleic acid, adipic acid, trichloro acetic acid, lactic acid, tartaric acid, oxalic acid, and citric acid were taken separately for the pretreatment.
30 mL of 1% (w/w) organic acid concentration was added to 3 g of culm of B. balcooa. The mixture was cooked in the domestic pressure cooker at 121 °C for 15 min. Then the samples were made up to 100 mL and total reducing sugars (TRS) content was estimated following dinitrosalicyclic acid (DNSA) method (Miller 1951). Due to higher productivity of sugars, oxalic acid was selected for further pretreatment. Oxalic acid solution was prepared in different concentrations (1, 2, 3 and 4% (w/v)). 30 mL of 1% (w/v) oxalic acid was added to 3 g of the sample. Pretreatment was carried out in the domestic pressure cooker at 121 °C for 15 min. These steps were repeated for 2, 3 and 4% oxalic acid solution (Pandian et al. 2016). Then, the samples were diluted to 100 mL and TRS content was estimated following dinitrosalicyclic acid (DNSA) method.
Solid to liquid ratio was varied by varying volume of solvent from 15, 30, 60, 90 and 120 mL for 3 g of the sample. 3 g of the culm sample was mixed with 15 mL of the 3% (w/v) oxalic acid solution was added. Pretreatment was carried out in the domestic pressure cooker at 121 °C for 15 min. These steps were repeated for 30, 60, 90 and 120 mL of 3% (w/v) oxalic acid solution. Then, the samples were diluted to 100 mL and TRS content was estimated following dinitrosalicyclic acid (DNSA) method. The pretreatment time was varied from 10, 20, 30 and 40 min for pretreatment of culm from B. balcooa. 30 mL of 3% (w/v) oxalic acid solution was prepared and added to 3 g of the culm sample.
Pretreatment was carried out in the domestic pressure cooker at 121 °C for 15 min. These steps were repeated for 30, 45 and 60 min of pretreatment. Then, the samples were diluted to 100 mL and TRS content was estimated following dinitrosalicyclic acid (DNSA) method. The pretreatment temperature was varied from 75, 90, 105, 121 and 135 °C for pretreatment of culm from B. balcooa. 30 mL of 3% (w/v) oxalic acid solution was prepared and added to 3 g of the culm sample. Pretreatment was carried out in the domestic pressure cooker at 121 °C for 15 min.
These steps were repeated for 75, 90, 105 and 135 °C of pretreatment. Then, the samples were diluted to 100 mL and TRS content was estimated following dinitrosalicyclic acid (DNSA) method. The experiments were performed in triplicate under optimized conditions to validate the optimal conditions. Optimal experiment was performed by mixing 30 mL of 3% (w/v) oxalic acid solution and 3 g of the sample. Pretreatment was carried out in the domestic pressure cooker at 121 °C for 15 min. Then, the samples were diluted to 100 mL and TRS content was estimated following dinitrosalicyclic acid (DNSA) method.
RESULTS AND DISCUSSION
Biochemical characterization of B. balcooa:
Table 1. Biochemical characterization of B. balcooa
| Lignin | Hemicellulose | Cellulose | Ash | References |
| 22.0 | 24.7 | 44.4 | 2.4 | Vena et al. 2010 |
| 22.1 | 22.9 | NA | NA | Hongbin et al.,2014 |
| 24.29 | 21.60 | 37.21 | 1.41 | Li et al. 2012 |
| 27.1 | 26.5 | 40.7 | 1.2 | Tippayawong et al. 2011 |
| 20 | 23 | 48 | 2.2 | Present study |
NA – Not available
Table 1 shows the biochemical characterization of culm from B. balcooa. The results reveal that it contains 20% lignin, 48% cellulose 23% hemicellulose and 2.2% ash on a dry weight basis. The hemicellulose accounted to 23%, which is higher than hemicellulose obtained in Hongbin et al. (2014). The cellulose content (48%) is higher than Li et al. (2012) and Tippayawong et al. (2011) that has made B. balcooa suitable for ethanol production.
Organic acid pretreatment of B. balcooa: Formic acid, acetic acid, malic acid, maleic acid, adipic acid, trichloro acetic acid, lactic acid, tartaric acid, oxalic acid, and citric acid were used for the pretreatment of B. balcooa. Table 2 shows the pKa values of various oxalic acids used in this study. pKa represents dissociation constant of acid that describe the acidity of a particular molecule. It can be calculated from Henderson-Hasselbalch equation. The smaller the pKa value, strong acids have weak conjugate bases. Organic acids with single carboxylic group have one pKa value and acids with multiple carboxylic acid groups have multiple carboxylic values (Adcock 2001; Sathishkumar et al. 2020).
Table 2. pKa values of different organic acids
| Organic acid | pKa1 | pKa2 | pKa3 |
| Formic acid | 3.8 | – | – |
| Acetic acid | 4.75 | – | – |
| Pyruvic acid | 2.39 | – | – |
| Oxalic acid | 1.25 | 4.23 | – |
| Maleic acid | 2 | 6.25 | – |
| Malic acid | 3.4 | 5.11 | – |
| Tartaric acid | 2.89 | 4.4 | – |
| Adipic acid | 4.4 | 5.4 | – |
| Citric acid | 3.14 | 4.77 | 6.30 |
Figure 1 illustrates about the pretreatment of B. balcooa using various organic acids. Among nine different acids attempted, oxalic acid pretreated culm sample produced 48.3 g/L of reducing sugars. Hence, oxalic acid was selected for further experiments of pretreatment for maximum production of TRS. Li et al. (2014) reported that sulphuric acid, oxalic acid, and formic acid produced 56.46%, 56.68% and 61.64% of glucose, respectively, with sulphuric acid being generated higher amount of fermentation inhibitors than the samples pretreated with oxalic and formic acids (Sindhu et al. 2010; Sathishkumar et al. 2020).
Figure 1: Effect of various organic acids on pretreatment of B. balcooa
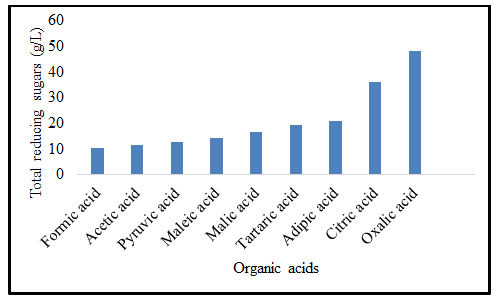
Effect of concentration of oxalic acid on pretreatment of B. balcooa: Figure 2 exhibits the impact of concentration of oxalic acid on pretreatment of B. balcooa. When the concentration of oxalic acid solution increased from 1 to 3% (w/v), the concentration of TRS increased from 18.1 to 48.3 g/L. When the oxalic acid solution concentration exceeds 3% (w/v), the concentration of TRS remained constant. The TRS concentration does not exhibit significant variation with an increase in concentration of oxalic acid solution beyond 3% (w/v) (Jeong and Lee 2016; Sathishkumar et al. 2020).
Figure 2: Effect of concentration of oxalic acid on pretreatment of B. balcooa
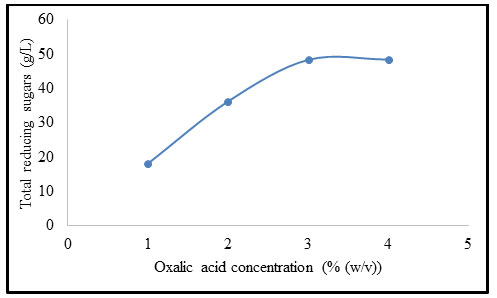
Effect of solvent to solid ratio on pretreatment of B. balcooa: Figure 3 illustrates the influence of solvent to solid ratio on pretreatment of B. balcooa. When the solvent was not added to the feed mixture, the concentration of TRS was minimum at 10.3 g/L. When the solvent to solid ratio was increased to 10 mL/g, the TRS concentration was increased to 48.2 g/L. When the oxalic acid solution concentration exceeds 10 mL/g, the concentration of TRS dropped as the volume of solvent increases or solid loading decreases. The TRS concentration exhibits declination with an increase in solvent to solid ratio beyond 10 mL/g (Song et al. 2020).
Figure 3: Effect of solvent to solid ratio on pretreatment of B. balcooa
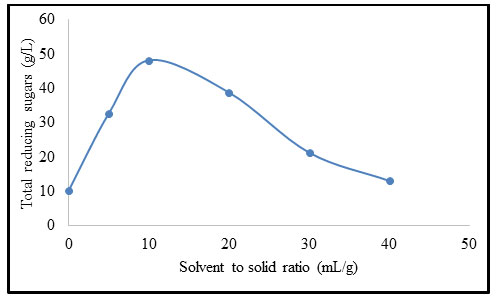
Effect of time on pretreatment of B. balcooa: Figure 4 exhibits the impact of time on pretreatment of B. balcooa. As the pretreatment progresses, the concentration of TRS increases to 48.4 g/L. When the pretreatment time exceeds 15 min, the concentration of TRS dropped as the TRS further form furfural and other similar compounds. The TRS concentration demonstrates declination with an increase in pretreatment time beyond 15 min (Yuang et al. 2019; Sathishkumar et al. 2020).
Figure 4: Effect of time on pretreatment of B. balcooa
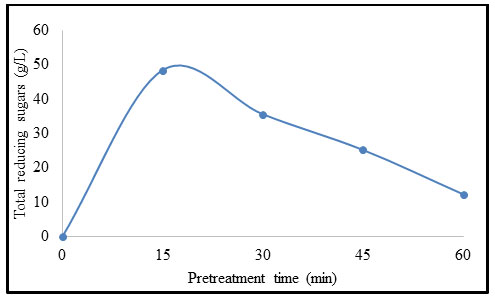
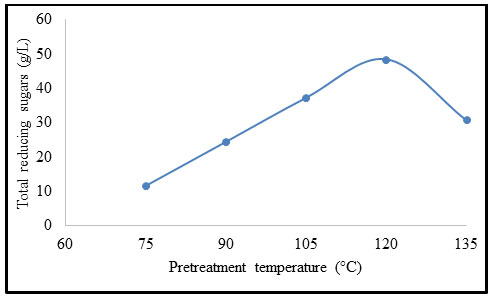
Effect of temperature on pretreatment of B. balcooa: Figure 5 exhibits the influence of temperature on pretreatment of B. balcooa. When the pretreatment temperature increased from 75 to 121 °C, the concentration of TRS increased from 11.6 to 48.5 g/L. When the temperature exceeds 135 °C, the concentration of TRS decreases to 30.8 g/L. The TRS concentration exhibits declination with an increase in temperature beyond 121 °C (Huang et al. 2020).
Figure 5: Effect of temperature on pretreatment of B. balcooa
Oxalic acid pretreatment of B. balcooa under optimized conditions: The optimal conditions were confirmed by performing experiments in triplicate under optimal conditions (Table 3). The mean±standard deviation between the TRS concentration obtained from the experiments was found to be 48.33±0.15 g/L. A low value of coefficient of variation (CV = 0.32%) showed that the optimal conditions were validated by experiments (Sathishkumar et al. 2020).
Table 3. Optimal conditions for the pretreatment of B. balcooa with oxalic acid
| Run no. | Oxalic acid concentration (% (w/v)) | Solvent to solid ratio (mL/g) | Time (min) | Temperature (°C) | TRS concentration (g/L) | Mean | Standard deviation |
| 1 | 3 | 10 | 15 | 121 | 48.3 | 48.33 | 0.15 |
| 2 | 3 | 10 | 15 | 121 | 48.5 | ||
| 3 | 3 | 10 | 15 | 121 | 48.2 |
Table 4 shows the various pretreatment methods employed for bamboo and compared the results obtained in the present study with the previous literature.
Table 4. Pretreatment methods employed for bamboo
| Pretreatment methods | Process | Outcomes | Reference |
| Modified alkaline hydrogen peroxide | Simultaneous saccharification and fermentation | 1 ton of ethanol produced per 5.6 ton of bamboo | Huang et al. (2020) |
| Autohydrolysis and alkaline extraction | Sequential two-stage pretreatment and fermentation | 0.467 g ethanol per g hydrolysate | Yuan and Wen (2017) |
| Steam explosion followed by green liquor | Simultaneous saccharification and fermentation | 20.3% ethanol yield | Gao et al. (2021) |
| Ultra-high-pressure explosion | Simultaneous saccharification and fermentation | Theoretical ethanol yield percentage of 89.7-95.1% | Jiang et al. (2016) |
| Oxalic acid pretreatment | – | 48.33 g/L | Present study |
CONCLUSION
The findings of the present study aimed to utilize B. balcooa as a potential feedstock to produce TRS by organic acid pretreatment. Oxalic acid produced maximum total reducing sugars among other carboxylic acids. The optimal values show that the maximum TRS of 48.33 g/L was achieved at oxalic acid concentration, solvent to solid ratio, time and temperature of 3% (w/v), 10 mL/g, 15 min and 121 °C for the pretreatment of cassava stem with oxalic acid. Thus, B. balcooa could be used as a potential feedstock for the production of TRS by organic acid pretreatment.
ACKNOWLEDGEMENTS
The facilities and continuous support for this study have been provided by the University of Technology and Applied Sciences, Salalah, Oman and Kumaraguru College of Technology, Coimbatore, India.
Conflict of Interests: Authors have no conflict of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
Author Contribution Information: S. Sivamani: Conceptualization, Methodology, Writing – review & editing; R. Kaveri: Methodology, Investigation, Writing – Original draft; S. Umaa Nandhini: Methodology, Investigation, Writing – Original draft;
Research Funding Information: Not available
REFERENCES
Adcock, J. L. (2001). Teaching Bronsted-Lowry acid-base theory in a direct comprehensive way. Journal of Chemical Education, 78(11), 1495.
Alzagameem, A., Bergs, M., Do, X. T., et al. (2019). Low-input crops as lignocellulosic feedstock for second-generation biorefineries and the potential of chemometrics in biomass quality control. Applied Sciences, 9(11) 2252.
Amnuaycheewa, P., Rodiahwati, W., Sanvarinda, P., et al. (2017). Effect of organic acid pretreatment on Napier grass (Pennisetum purpureum) straw biomass conversion. KMUTNB Int J Appl Sci Technol, 10(2), 107-117.
Anushya, A., Swathika, M., Sivamani, S., et al. (2019). Bioprocessing of Cassava Stem to Bioethanol Using Soaking in Aqueous Ammonia Pretreatment. Bioprocessing for Biomolecules Production, 429-441.
Banik, R. L. (2015). Morphology and growth. In Bamboo (pp. 43-89). Springer, Cham.
Banik, R. L. (2016). Bambusa. In Silviculture of South Asian Priority Bamboos (pp. 21-115). Springer, Singapore.
Bensah, E. C., Kemausuor, F., Miezah, K., et al. (2015). African perspective on cellulosic ethanol production. Renewable and Sustainable Energy Reviews, 49, 1-11.
Chandrasekaran, A. P., and Sivamani, S. (2018). Statistical modeling and optimization of pretreatment for fermentable sugars production from cotton gin waste. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 40(4), 400-405.
Chandrasekaran, A. P., Sivamani, S., and Ranjithkumar, V. (2017). Characterization of combined organic–inorganic acid-pretreated cassava stem. International Journal of Environmental Science and Technology, 14(6), 1291-1296.
Dai, N. H., Huynh, K. T. T., Nguyen, T. A. D., et al. (2021). Hydrothermal and Steam Explosion Pretreatment of Bambusa stenostachya Bamboo. Waste and Biomass Valorization, 12(7), 4103-4112.
Devi, A., Niazi, A., Ramteke, M., et al. (2021). Techno-economic analysis of ethanol production from lignocellulosic biomass–a comparison of fermentation, thermo catalytic, and chemocatalytic technologies. Bioprocess and Biosystems Engineering, 44(6), 1093-1107.
Gao, H., Wang, Y., Yang, Q., et al. (2021). Combined steam explosion and optimized green-liquor pretreatments are effective for complete saccharification to maximize bioethanol production by reducing lignocellulose recalcitrance in one-year-old bamboo. Renewable Energy, 175, 1069-1079.
Handler, R. M., Shonnard, D. R., Griffing, E. M., et al. (2016). Life cycle assessments of ethanol production via gas fermentation: anticipated greenhouse gas emissions for cellulosic and waste gas feedstocks. Industrial & Engineering Chemistry Research, 55(12), 3253-3261.
Hongbin, C., Gorgens, J., Macintosh, P., et al. (2014). Saccharification of bamboo by dilute acid pretreatment and enzymatic hydrolysis for cellulosic ethanol production. South African Journal of Chemical Engineering, 19(1), 46-52.
Huang, C., Zhan, Y., Du, X., et al. (2020). Modified alkaline peroxide pretreatment: an efficient path forward for bioethanol production from bamboo. Energy Conversion and Management 224, 113365.
Jeong, S. Y., and Lee, J. W. (2016). Optimization of pretreatment condition for ethanol production from oxalic acid pretreated biomass by response surface methodology. Industrial Crops and Products, 79, 1-6.
Jiang, Z., Fei, B., andLi, Z. (2016). Pretreatment of bamboo by ultra-high-pressure explosion with a high-pressure homogenizer for enzymatic hydrolysis and ethanol fermentation. Bioresource technology 214, 876-880.
Kootstra, A. M. J., Beeftink, H. H., Scott, E. L., et al. (2009). Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochemical Engineering Journal, 46(2), 126-131.
Li, Z., Fei, B., and Jiang, Z. (2014). Comparison of dilute organic and sulfuric acid pretreatment for enzymatic hydrolysis of bamboo. BioResources, 9(3), 5652-5661.
Li, Z., Fei, B., and Jiang, Z. (2015). Effect of steam explosion pretreatment on bamboo for enzymatic hydrolysis and ethanol fermentation. BioResources, 10(1), 1037-1047.
Li, Z., Jiang, Z., Yu, Y., et al. (2012). Effective of microwave-KOH pretreatment on enzymatic hydrolysis of bamboo. Journal of Sustainable Bioenergy Systems, Volume 2 2012; pp. 104-107. 2, 104-107.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry, 31(3), 426-428.
Pandian, C. A., Suganya, C., Sivamani, S., et al. (2016). Saccharification and single step fermentation of cassava peel by mixed culture of Saccharomycopsis fibuligera NCIM 3161 and Zymomonas mobilis MTCC 92. Am J Biomass Bioenergy, 5, 57-64.
Qiao, H., Cui, J., Ouyang, S., et al. (2019). Comparison of dilute organic acid pretreatment and a comprehensive exploration of citric acid pretreatment on corn cob. Journal of Renewable Materials, 7(11), 1197-1207.
Sathishkumar, G. K., Ibrahim, M., Akheel, M.M., et al. (2020). Synthesis and mechanical properties of natural fiber reinforced epoxy/polyester/polypropylene composites: A review. Journal of Natural Fibers, 1-24.
Shen, L., Yang, J., Zhang, R., et al. (2019). The benefits and barriers for promoting bamboo as a green building material in China—An integrative analysis. Sustainability, 11(9) 2493.
Sindhu, R., Binod, P., Satyanagalakshmi, K., et al. (2010). Formic acid as a potential pretreatment agent for the conversion of sugarcane bagasse to bioethanol. Applied biochemistry and biotechnology, 162(8) 2313-2323.
Sindhu, R., Kuttiraja, M., Binod, P., et al. (2014). Bioethanol production from dilute acid pretreated Indian bamboo variety (Dendrocalamus sp.) by separate hydrolysis and fermentation. Industrial Crops and Products, 52, 169-176.
Sivamani, S., and Baskar, R. (2018). Bioconversion of cassava stem to ethanol: oxalic acid pretreatment and co-culture fermentation. Biofuels, 9(5), 559-566.
Sivamani, S., Baskar, R., and Chandrasekaran, A. P. (2020). Response surface optimization of acid pretreatment of cassava stem for bioethanol production. Environmental Progress & Sustainable Energy, 39(2), e13335.
Sivamani, S., Baskar, R., and Lakshmi, B. (2021). Production of Fermentable Sugars from Cassava Stem using Hybrid Pretreatment Technology. Recent Progress in Materials, 3, doi:10.21926/rpm.2103037
Sivamani, S., Chandrasekaran, A. P., Balajii, M., et al. (2018). Evaluation of the potential of cassava-based residues for biofuels production. Reviews in Environmental Science and Bio/Technology, 17(3), 553-570.
Song, Y., Lee, Y. G., Cho, E. J., et al. (2020). Production of xylose, xylulose, xylitol, and bioethanol from waste bamboo using hydrogen peroxicde-acetic acid pretreatment. Fuel, 278, 118247.
Tang, H., Zheng, L., Li, Y., et al. (2021). Comparative genomic and secretomic characterisation of endophytic Bacillus velezensis LC1 producing bioethanol from bamboo lignocellulose. Archives of Microbiology, 1-11.
Tantayotai, P., Mutrakulchareon, P., Tawai, A., et al. (2019). Effect of organic acid pretreatment of water hyacinth on enzymatic hydrolysis and biogas and bioethanol production. In IOP Conference Series: Earth and Environmental Science (Vol. 346, No. 1, p. 012004). IOP Publishing.
Thatoi, H., Dash, P. K., Mohapatra, S., et al. (2016). Bioethanol production from tuber crops using fermentation technology: a review. International Journal of Sustainable Energy, 35(5), 443-468.
Tippayawong, N., and Chanhom, N. (2012). Conversion of bamboo to sugars by dilute acid and enzymatic hydrolysis. International Journal of Renewable Energy Research (IJRER), 1(4) 240-244.
Vanitha, S., Bharathi, S. V., and Sivamani, S. (2017). Statistical Modeling and Optimization of Bioethanol Production from Parthenium hysterophorus. In Bioremediation and Sustainable Technologies for Cleaner Environment (pp. 253-265). Springer, Cham.
Vena, P. F., Görgens, J. F., and Rypstra, T. (2010). Hemicelluloses extraction from giant bamboo prior to kraft and soda AQ pulping to produce paper pulps, value-added biopolymers and bioethanol. Cellulose Chemistry & Technology, 44(4), 153.
Wu, G., Wu, D., Li, Y., et al. (2020). Effect of acetone-n-butanol-ethanol (ABE) as an oxygenate on combustion, performance, and emission characteristics of a spark ignition engine. Journal of Chemistry 2020.
Yang, H., Shi, Z., Xu, G., et al. (2019). Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresource technology 274 261-266.
Yuan, Z., and Wen, Y. (2017). Evaluation of an integrated process to fully utilize bamboo biomass during the production of bioethanol. Bioresource technology 236 202-211.
Zahan, K. A., and Kano, M. (2018). Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies, 11(8) 2132.


