1Department of Biotechnology, Government College, Hisar, India.
2Advanced Centre for Plant Virology, Division of Plant Pathology, ICAR-Indian
Agricultural Research Institute, New Delhi, India.
Corresponding author email: sumit.jangra712@gmail.com
Article Publishing History
Received: 04/03/2021
Accepted After Revision: 13/06/2021
The non-renewable sources of energy are depleting all over the globe at an alarming rate. Bioethanol is considered as the fuel of modern era as it contributes around 10-14% of the total global energy supply. Researches are in continuous search of renewable resources for energy production. The use of biodegradable waste as a source of energy is gaining interest. In the present study, different renewable resources viz. sugarcane juice, molasses, and paddy straw were tested for their efficacy to produce bioethanol. Three different yeast strains designated as Y1, Y2, and Y3 were isolated from over-ripened fruits and were to ferment the different substrates used in the present study. Sugarcane and molasses were directly used for ethanol production while paddy straw was pre-treated biologically and chemically before using it as substrate for ethanol production.
For biological treatment, cellulolytic microorganisms were used to degrade paddy straw while acid and alkali treatments were given to paddy straw during chemical treatment. Thereafter, ethanol was produced using selected yeast strains. The study showed that the strain Y3 produced maximum ethanol with all three substrates viz. sugarcane juice (9.4%), molasses (9%) and paddy straw (4.21%). Paddy straw yielded very little ethanol as compared to sugarcane juice and molasses. The present study showed that sugarcane and molasses are very good substrates for the production of alcohol but lignocellulosic agricultural waste like rice straw can also be used for the production of alcohol but they need to be pre-treated. This research is expected to act as a milestone for future studies providing with credible baseline work.
Bioethanol, Molasses, Paddy Straw, Saccharomyces Cerevisiae, Sugarcane Juice
Nehra K.S, Jangra M.R, Sharma P, Aggarwa M, Mishra P, Bharti R, Sachdeva H, Poonia P, Jangra S. Production of Bioethanol from Sugarcane Juice, Molasses and Paddy Straw using Saccharomyces cerevisiae. Biosc.Biotech.Res.Comm. 2021;14(2).
Nehra K.S, Jangra M.R, Sharma P, Aggarwa M, Mishra P, Bharti R, Sachdeva H, Poonia P, Jangra S. Production of Bioethanol from Sugarcane Juice, Molasses and Paddy Straw using Saccharomyces cerevisiae. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/2QOkFvq“>https://bit.ly/2QOkFvq</a>
Copyright © Nehra et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Ethanol is the important fuel used in automotive industries and in other potable purposes. It is considered as a renewable alternative to fossil-based fuel. So, its production needs to be enhanced in the coming time by using modern techniques. Ethanol is basically produced by the fermentation of sugar or starch from agricultural crops by yeast or bacteria. A variety of substrates are used for the production of ethanol. These substrates include molasses, sugar beet pulp, and waste newspapers (Xin et al. 2010; López et al. 2012; Kasavi et al. 2012; Amid et al. 2021) etc. Substrate, strain, nutrient, and physiological factors like temperature and pH affect the fermentation process and ethanol production. The availability and cost of the substrate need to be assessed before beginning fermentation.
The use of cheap and readily available substrate is recommended to make the fermentation process cheaper. The production cost can be further reduced by using renewable sources like molasses, potato starch, sugarcane, and paddy straw (Oscar and Carlos 2008; Amid et al. 2021). Molasses are the most commonly used and easily available substrates and cheapest too but the increased demand has reduced the availability, thereby increasing the cost (Schweinitzer and Josenhans 2010; Amid et al. 2021).
So it is the need of hour to focus on alternative substrate for ethanol production. Recently, focus has shifted from molasses to paddy straw. Sugarcane is regarded as a good source of ethanol production because of its high sugar content and even infected plant juice can also be used for ethanol production. Moreover, the energy accumulated in the sugarcane biomass is directly from the products of photosynthesis, due to carbon dioxide fixation, which decreases the crop’s overall contribution to global warming. Alternatively, paddy straw is also a very good substrate for the completion of ethanol requirements because it contains 32-47% cellulose and 19-27% hemicellulose.
However, some problems are also associated with paddy straw if used for fuel production. One of the major problem is the presence of lignin, cell wall polysaccharides, and cellulose crystallinity. Lignin makes enzymatic degradation difficult as it covers cellulose and hemicellulose and hence protects polysaccharides from degradation. So removal of lignin is necessary so that cellulose becomes accessible to the enzymes and yeast action. Lignin can be hydrolyzed by using some chemical and biological methods (Krishna and Chowdary 2000; Malik et al. 2021).
The cellulose and hemicellulose become fermentable sugars by pretreatments either it is chemical or biological means, the later employing enzymes like cellulases and hemicellulases. Although, some advantages and limitations are there for both methods. Chemical hydrolysis though advantageous by being rapid but is limited by low sugar recovery efficiency, formation of furfural and other degradation products are poisonous to the fermentation microorganisms and raised environmental concerns due to disposal of acid.
The biological (enzymatic) methods, on the other hand, have the advantage of being highly specific, ecofriendly, and no degradation products of glucose are formed (Sukumaran et al. 2010; Hou et al. 2021). The present study, aims to utilize alternative renewable resources for ethanol production using both chemical and enyzymatic means of degradation. The use of paddy straw for ethanol production is a potential and a low-cost method that can be employed at commercial scale. Further, it will also help to solve the paddy straw burning problem in northern India.
MATERIAL AND METHODS
The present study was carried out in the Department of Biotechnology, Govt. College, Hisar (Haryana). All the material for yeast isolation and substrates for ethanol production was taken from the local market and field of Hisar. The chemicals and media ingredients were of AR or GR grade and procured from either M/s E. Merck or BDH or HI- MEDIA, India. The glassware used was of high-quality Borosil. For the yeast was isolated on YEPD medium from honey, cheese, tomato, curd, sugarcane, jaggery, and molasses procured from the local market of Hisar (Haryana) and then characterized. The isolated colonies of yeast were tested for their ethanol-producing abilities. These were tested on different substrates like sugarcane, paddy straw, and molasses (Kreger-van Rij 1984).
For the isolation of cellulolytic microorganisms, one gram of rice straw powder was taken and suspended in 9 ml of sterile distilled water. After serial dilution of this suspension (10-1 to 10-6 times), 100 μl from each dilution was spread on carboxymethyl cellulose (CMC) agar plates (1% CMC, 0.1% KH2PO4, 0.1% K2HPO4, 0.04% MgSO4, 0.005% NaCl, 0.000125% FeSO4, and 1.8% agar, pH 7.0) and incubated (NSW) at 37°C for 24-48 h which is suitable temperature for the growth of yeast. The isolated bacterial colonies forming clear-zones after application of 1% congo red dye solution were selected as cellulase producers. Bacterial isolates producing significant clear zone on CMC agar were identified based on cultural, morphological, and biochemical characteristics (Cowan and Steel 1974; Kasana et al. 2008).
For observations, plates were stained with 1% Congo red dye (15 min), followed by de-staining with 1MNaCl solution for 20 min. Cellulolytic strains were selected on the basis of the hydrolysis zone surrounding the colonies. The cultures were identified based on the cultural, morphological, and biochemical characteristics (Rifai 1969; Cowan and Steel 1974; Teather and Wood 1982). For the ethanol production from sugarcane, the samples of sugarcane juice were collected from local hand-operated cane crushers from different locations of Hisar. Different pretreatments like filtration, sterilization, and concentration were given to sugarcane juice. Then it was inoculated with a previously isolated yeast strain and incubated at 30oC for 24 h for fermentation.
For the ethanol production from paddy straw, the paddy straw saccharification was conducted by microorganisms. The isolated colonies of bacteria were then tested for their ability to grow and degrade rice straw. Rice straw was crushed and added into 100 ml of mineral salt medium in a 500 ml Erlenmeyer flask and autoclaved. The media was allowed to cool down and 3 ml of yeast suspension was added to it for fermentation. The cultures were incubated at 25°C for 14 days. After 14 days, culture aliquots were centrifuged (REMI) at 6000 rpm to remove solids. The supernatant was used as crude enzyme solution (Belal and El-Mahrouk 2010).
Now the supernatants were assayed for their enzymatic activity. Cellulase activity was determined by incubating 0.5 mL of the supernatant with 1% CMC and it was incubated at 60°C for 30 min. After incubation, the reaction was terminated by adding3 mL of 1% 3,5-dinitrosalicylic acid (DNS) reagent to1mL of the reaction mixture and heated for 10 min at 100oC. In these tests, reducing sugars were estimated calorimetrically using glucose as standard (Miller 1959; Belal and El-Mahrouk 2010).
For the alkali and acid treatment to the rice, paddy straw was procured from Ramray village of Jind district (Haryana). It was dried at 50oC, comminuted to small pieces using grinder fitted with sieves of different mesh sizes. For alkali treatment, about 50 g chopped (>2 cm length) dried rice straw was suspended in 1, 2, 3, 4, and 5% NaOH in a ratio of 1:10 (w/v). Thereafter, the samples were incubated in water bath at 85°C for 1 h. Finally, hydrolysate was passed through cheesecloth. For acid hydrolysis, about 50 g chopped dried rice straw was suspended in an 1:10 (w/v) sulfuric acid solution of 1, 3, 5, 7, and 9%. The mixtures were autoclaved at 121°C for 15 minutes and enzymatic treatment was given to slurry (1:10 of alkali-treated paddy straw in distilled water).
Commercial cellulose was added at a concentration of 7.5 FPU/g substrate and incubated at 35oC in an incubator. Reducing sugar was estimated by DNS method (Miller et al. 1959). For the ethanol production after treatment, the pretreated samples were inoculated with the isolated cultures of yeast for fermentation. After fermentation ethanol was estimated using dichromic method (Caputi et al. 1968). Absorbance was read at 600 nm against a suitable blank using spectrophotometer. The amount of ethanol was determined by referring to a standard curve plotted from different concentrations (1-7%) of absolute alcohol. For the statistical analysis, analysis of variance (ANOVA) was used to test the significant difference among the three strains viz. Y1, Y2, and Y3. Mean differences among the categories were separated by Tukey’s test at a confidence interval of 95%.
RESULTS AND DISCUSSION
In the present study, isolated cultures were screened for primary identification from different samples and fermented products as described above. A total of ten cultures were isolated from these samples out of which three were identified as yeast. Apple, orange, banana, and other fruits were locally available and thus served as readily available raw materials for the separation of ethanol-producing yeasts. Various strains of indigenous yeasts capable of producing ethanol were isolated from local fermented pineapple juice by Eghafona (1999). These isolated strains are used for the production of ethanol from different substrates (Belal et al. 2013).
A comparative study on ethanol production from molasses using Saccharomyces cerevisiae and Zymomonas mobilis was performed by Bansal and Singh (2003) and Hossain et al. (2014). Different concentration of glucose (50, 10, 30, 50, and 70g/l) was used as a sole source of sugar in the MGYP medium; the consequences showed that the maximum yeast biomass and maximum ethanol yield was obtained at high glucose concentration. The cultures were identified as yeasts based on colony characters, microscopic examination, and budding formation. Colonies formed by yeast isolates were circular, smooth, and cream (Aguilar 2011).
Colony size varies from small to large (Table 1). Individual cells were oval, elongate, ovoid to spherical when young and hexagonal when aged. Cells showed oval, globose, spherical and ellipsoidal budding. Based on differences in colony morphology, color, appearance, size, and margin these strains were designated as Y1 to Y3 (Table 1) (Moaris 1996; Aguilar 2011). The isolated yeast strains were analyzed microscopically under 40X resolution of compound microscope (Olympus) using wet mount. Viability of Saccharomyces sp. also studied by Moaris (1996) and Aguilar (2011). In 50% glucose, reported viability of 10-98.8% in different strains of yeast (Moaris 1996; Aguilar 2011).
Table 1. Colony characteristics of yeast isolates
| Yeast Isolate | Colony Color | Colony Nature | Appearance and Size | Elevation | Margin |
| Y1 | Cream | Smooth | Circular & small | Raised | Entire |
| Y2 | Cream | Smooth | Circular & Small | Raised | Entire |
| Y3 | Cream | Smooth | Yeast like & medium | Convex | Entire |
All the isolated strains were characterized on the basis of physiological parameters. All three selected yeast isolates were able to grow at 25°C-42°C. Therefore, the isolated yeast was considered to be thermo-tolerant. Ethanol tolerance of yeast was also checked and it was observed that yeast isolates can grow in liquid YEPD media containing up to 15% ethanol. Maximum growth was observed in 5% ethanol containing media. A very high or low concentration of ethanol is inhibitory for the growth of yeast. So, an optimum concentration is required for the growth of yeast. As far as we are concerned with sugar concentration, yeast was found to be resistant to sugar up to 10% (w/v) glucose concentration. The use of different concentrations of glucose (50, 10, 30, 50, and 70g/l) showed that the maximum yeast biomass and maximum ethanol yield was obtained at high glucose concentration (Nasreen et al. 2014).
Ethanol production from sugarcane bagasse was found to be 0.41g/L (Irfan et al. 2014). Rice straw (49 g/L) and wheat straw (34 g/L) also produced ethanol but their production was not good as compared to sugarcane bagasse. This difference in ethanol production was due to the availability of fermentable sugars from cellulose present in biomasses. Use of commercial enzyme for saccharification showed that treated rice straw gave better ethanol production (85 g/L) as compared to untreated (70 g/L) rice straw (Jalil et al. 2010). Pretreatment of sugarcane bagasse with 1 N NaOH resulted in 48% ethanol production by C. cladosporoides after 48 h of fermentation under static condition (Uma et al. 2010). A maximum ethanol production of 3.36 g/L was obtained from pretreated sugarcane bagasse under optimized process conditions in aerobic batch fermentation (Sasikumar and Viruthagiri 2010).
“Isolation of Cellulolytic microorganisms” Rotted rice straw residues were used as source for cellulolytic microorganisms in the present study. Only five microorganisms were isolated by using clear zone formation on MSA (mineral salt agar) containing carboxymethyl cellulose as a sole source of carbon. A preliminary classification based on cultural and morphological characteristics of the isolates revealed that the rice straw residues – degrading microorganisms belong to the group of fungi as well as to the group of bacteria, (Stella et al. 2015).
A total of three microorganisms were found to be gram-positive and rod-shaped. Results of identification showed that the rice straw degrading bacterial strain was identified as Bacillus. A microbial consortium consisting of 30 bacteria to study biodegradation of rice straw was developed by Stella et al. (2015). The microorganisms in the consortium were generally identified as Proteus mirabilis, Raoutella planticola, Serratia sp., Pseudomonas viridilivida, Klebsiella oxytoca, B. fusiformis, B. cereus, Klebsiella sp., B. licheniformis, Corynebacterium urealyticum, Cellulomicrobium cellulans, and B. subtilis using 16s rDNA molecular identification technique. “Ethanol production using molasses” Yeast isolate was examined for ethanol production using 25% (w/v) molasses, as substrate under the constant set of conditions. Ethanol production was estimated at 28°C and calculated after completion of fermentation. Yeast strains Y3 produced maximum ethanol (9%) followed by Y1 and Y2 (Figure 1) (Stella et al. 2015).
Figure 1: Percent ethanol produced from molasses after 24 h of fermentation
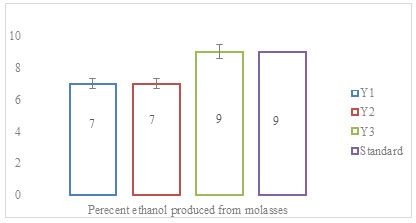
The data was statistically significant with a p-value of ≤0.02. Brooks (2008), isolated yeast strains from ripe banana peels for ethanol production and found, that isolates fermented 40% glucose at 30oC to yield 3.6 and 5.8% ethanol respectively. Molasses was used as a reference to check the production of ethanol in sugarcane and paddy straw. “Ethanol production using sugarcane juice” Sugarcane juice was explored for ethanol production. The process of ethanol production depends on the yeast strain employed (Stella et al. 2015).
The yeast strains differ considerably in the production of ethanol; therefore, it is essential to select suitable yeast strains for ethanol production from sugarcane. Y1, Y2, and Y3 yeast isolates of different morphology retrieved from different samples and sugarcane juice samples by dilution plating and enrichment culture technique were tested for ethanol production and their efficiency was compared against a standard culture of Saccharomyces cerevisiae (Giri 2008). Yeast strain Y3 give maximum ethanol production (8.21) but it was not higher than the standard culture of Saccharomyces cerevisiae. Y1 and Y2 produced almost similar i.e., 6.05% and 6.12% ethanol when sugarcane juice was used as substrate (Figure 2) (Giri 2008; Stella et al. 2015).
Figure 2: Percent ethanol produced from sugarcane juice after 24 h of fermentation
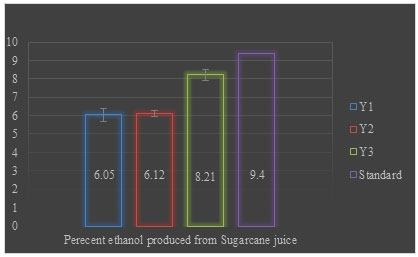
The data was statistically significant with a p-value of ≤0.003. Sugarcane (Saccharum officinarum) is a C4 plant having high capability to convert solar radiation into biomass (Black et al. 1969). It is the most important feedstock grown in tropical and subtropical countries that can be used as juice or molasses (by-product of sugar mills) for fuel ethanol production. Total fermentable sugar content in sugarcane juice is about 12–17% in which 90% of this sugar is sucrose and the remaining 10% is glucose and fructose (Wheals et al. 1999). Sugar content in juice varies based on variety, maturity, and harvest time (Dhaliwal et al. 2011).
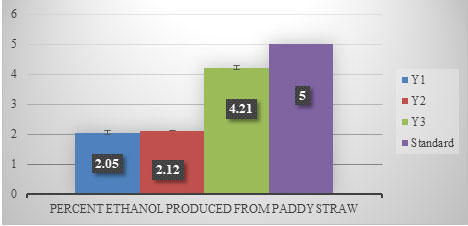
Sugarcane juice contains adequate amount of organic nutrients and minerals in addition to free sugars making it an ideal raw material for bioethanol production. “Ethanol production using paddy straw” An attempt was made to produce ethanol from paddy straw. In this experiment, paddy straw was de-lignified by using alkali and acid treatment. Thereafter, cellulosic enzymes were used for saccharification (Dhaliwal et al. 2011). After the treatment with cellulase, this solution was inoculated with three strains of yeast for fermentation. It was found that Y3 produced (4.21%) maximum ethanol followed by Y2 (2.12%) and Y1 (2.05%) (Figure 3). No statistical significance was observed in the data (p ≥2.99) (Dhaliwal et al. 2011).
Figure 3: Percent ethanol produced from paddy straw after 24 h of fermentation
Lignocellulosic residues including rice straw, sugarcane bagasse, wheat straw, corn stover, spruce and municipal solid waste have been researched by several workers for microbial and enzymatic bioconversion with commercial or in-house produced cellulase into glucose employing various pretreatment protocols including acid, alkali and steam (Li et al. 2007; Patel et al. 2007; Kovacs et al. 2009; Rabelo et al. 2009; Yoswathana and Phuriphipat 2010).
Following pretreatment, plant cell wall polysaccharides are more susceptible to enzymatic hydrolysis that breaks them into monomeric (single) sugars that can be fermented into ethanol. The multiple mean comparisons (Tukey’s test) of the average ethanol produced from molasses, sugarcane juice, and paddy straw using the three strains Y1, Y2, and Y3 showed that the average ethanol production from molasses was significantly different with paddy straw. A significant difference in ethanol production was also observed among sugar cane juice and paddy straw (Lynd et al. 1999; Yoswathana and Phuriphipat 2010).
CONCLUSION
Although the percentage of bioethanol produced during this study was not so high that can meet the increasing demand for fuel in modern time if parameters were optimized properly, this percentage can be improved. Clearly, the results exploring methodologies that allow the isolation of efficient ethanol-producing yeast strains from different source is limited and further research is needed. The opportunity exists to use such yeast strains to improve the efficiency of ethanol production from complex substrates like paddy straw. It should help reduce the current reliance on petroleum-based fuel. To take advantage of this opportunity, new approaches in the isolation of stable yeast strains capable of being used during high-temperature ethanol production will need to be developed.
ACKNOWLEDGEMENTS
The authors are thankful to Principal, Government College, Hisar for providing the necessary facilities to carry out the work. KSN, MRJ, and SJ conceived and designed the research. MRJ, PS, MA, PS, RB, HS, and PM conducted the experiments. SJ performed the statistical analysis. MRJ and SJ analyzed the data and wrote the manuscript.
Conflict of interests: All the authors have read and approved the final manuscript and declare no conflict of interest.
REFERENCES
Aguilar-Uscanga MG, Garcia-Alvarado Y, Gomez-Rodriguez J, Phister T, Delia ML and Strehaiano P (2011). Growth and ethanol production of Brettanomyces bruxellensis at different glucose concentrations. Lett Appl Microbiol 53(2): 141-149.
Amid S, Aghbashlo M, Tabatabaei M, Karimi K, Nizami AS, Rehan M, Hosseinzadeh-Bandbafha H, Soufiyan MM, Peng W and Lam SS (2021) Exergetic, exergoeconomic, and exergo environmental aspects of an industrial-scale molasses-based ethanol production plant. Energy Convers Manag 227: 113637.
Bansal R and Singh RS (2003). A comparative study on ethanol production from molasses using Saccharomyces cerevisiae and Zymomonas mobilis. Ind J Microbiol 43: 261-264.
Belal EB and El-Mahrouk ME (2010) Solid state fermentation of rice straw residues for its use as growing medium in ornamental nurseries. Acta Astronautica 67:1081–1089.
Black CC, Chen T and Brown R (1969) Biochemical basis for plant competition. Weed Science 338–344.
Brooks AA (2008) Ethanol production potential of local yeast strain isolated from ripe banana peels. Afri J Biotechnol 7(20): 3749-3752.
Cowan ST (1974) Cowan and steel’s manual for the identification of medical bacteria. 2nd ed. Ames: Cambridge University Press.
Eghafona NO, Aluyi HAS and Uduehi IS (1999) Alcohol yield from pineapple juice: Comparative study of Zymomonas mobilis and Saccharomyces uvarum. Niger Journal of Microbiology 13: 117-122.
Elsayed B and Belal (2013) Bioethanol production from rice straw residues. Brazilian Journal of Microbiology 44(1): 225-234.
Fernández-López CL, Torrestiana-Sánchez B, Salgado-Cervantes MA, Mendoza García PG and Aguilar-Uscanga MG (2012) Use of sugarcane molasses “B” as an alternative for ethanol production with wild-type yeast Saccharomyces cerevisiae ITV-01 at high sugar concentrations. Bioprocess Biosystem Engineering 35(4): 605–614.
Giri R (2008) Bioethanol production from sugarcane juice by yeast. M. Sc. Thesis submitted to CCS Haryana Agricultural University, Hisar.
Hosian Z, Golam F, Jaya NS, Mohmmad SA, Rosli H and Amru NB (2014) Bioethanol production from fermentable sugar juice. Scientific World J 1: 1-11.
Hou J, Zhang X, Zhang S, Wang K and Zhang Q (2021) Enhancement of bioethanol production by a waste biomass-based adsorbent from enzymatic hydrolysis. J Clean Prod 291: 125933.
Irfan M, Nadeem M and Syed Q (2014) Ethanol production from agricultural wastes using Sacchromyces cervisae. Braz J Microbiol 45(2):457-465.
Jalil R, Ibrahim WA, Ali MSM, Hashim S, Elham P, Tahar A and Zahidi NFA (2010). 7th Biomass Asia Workshop, November29-December 01, Jakarta, Indonesia.
Kasana RC, Salwan R, Dhar H, Dutt S and Gulati S (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol 57(5): 503 507.
Kasavi C, Finore I and Lama L (2012) Evaluation of industrial Saccharomyces cerevisiae strains for ethanol production from biomass. Biomass Bioenerg 45:230–238.
Kovacs K, Marcelli S, Szakacs G and Zacchi G (2009) Enzymatic hydrolysis of steam-pretreated lignocellulosic materials with Tricohderma aatroviride enzymes produced in-house. Biotechnol Biofuels 2(14) doi: 10.1186/1754-6834-2-14.
Kreger-van Rij NJW (1984) The Yeasts – A taxonomic study, 3rd edn. Amsterdam, Elsevier.
Krishna H and Chowdary GV (2000) Optimisation of simultaneous sacchrification and fermentation for the production of ethanol from lignocellulosic biomass. J Agric Food Chemi 48(5): 1971-1976.
Li A, Antizaar-Ladislaao B and Khraaisheh M (2007) Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst Eng 30:189–196.
Lynd LR, Wyman CE and Gerngross TU (1999) Biocommodity engineering. Biotechnol Prog 15:777–793.
Malik K, Salama ES, El-Dalatony MM, Jalalah M, Harraz FA, Al-Assiri MS, Zheng Y, Sharma P and Li X (2021) Co-fermentation of immobilized yeasts boosted bioethanol production from pretreated cotton stalk lignocellulosic biomass: Long-term investigation. Ind Crops Prod 159: 113122.
Miller GL (1959) Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428.
Moaris PB, Rosa CA, Linardi VR, Carazza F and Monato EA (1996) Production of fuel alcohol by Saccharomyces strains from tropical habitats. Biotechnol Lett 18: 1351- 56.
Nasreen Z, Shaista J, Muafia S, Shumaila U, Tehseen Y, Ammara Y and Saima N (2014) Production of alcohol by yeast isolated from apple, orange and banana. Int J Food Nutr Sci 1(2): 016-019.
Oscar JS and Carlos AC (2008) Trends in biotechnological production of fuel ethanol from different feed stocks. Bioresour Technol 99: 5270-5295.
Patel SJ, Onkarapp AR and Shobha KS (2007) Comparative study of ethanol production from microbial pretreated agricultural residues. Journal of Applied Science and Environmental Management 11:137–141.
Rabelo SC, Filhho RM and Costa AC (2009) Lime pretreatment of sugarcane bagasse for bioethanol production. Appl Biochem Biotechnol 53: 139–150.
Rifai MA (1969). A revision of the genus Trichoderma. Mycological Papers 116: 1-56.
Sasikumar E and Viruthagiri T (2010) Simultaneous saccharification and fermentation (SSF) of sugarcane bagasse – Kinetics and Modeling. International Journal of Chemical and Molecular Engineering 4(1): 93-100.
Schweinitzer T and Josenhans C (2010) Bacterial energy taxis: a global strategy? Arch Microbiol 192: 507-520.
Stella M and Emmyrafedziawati AK. (2015) Identification of rice straw degrading microbial consortium. J Trop Agric Fd Sc 43(2): 119-127.
Sukumaran RK, Surender VJ, Sindhu R, Binod P, Janu KU, Sajna KV, Rajasree KP and Pandey A (2010) Lignocellulosic ethanol in India: Prospects, challenges and feedstock availability. Bioresour Technol 101(13): 4826-4833.
Teather RM and Wood PJ (1982) Use of congo red-polysacharide interactions in enumeration and characterization of cellulolytic bacteria in the bovine rumen. Appl Environ Microbiol 43(4): 777–780.
Uma C, Muthulakshmi C, Gomathi D and Gopalakrishnan VK (2010) Fungal invertase as aid for production of ethanol from sugarcane bagasse. Res J Microbiol 5: 980-985.
Wheals AE, Basso LC, Alves DMG and Amorim HV (1999) Fuel ethanol after 25 years. Trends in Biotechnol 17(12): 482–487.
Xin F, Geng A, Chen ML and Gum MJM (2010) Enzymatic hydrolysis of sodium dodecyl sulphate (SDS) pretreated newspaper for cellulosic ethanol production by Saccharomyces cerevisiae and Pichia stipitis. Appl Biochem Biotechnol 162(4):1052–106.
Yoswathana N, Phuriphipat P, Teryawutthiwat P and Eshtaghi MN (2010) Bioethanol production from rice straw. Energy Research Journal 1: 26–31.


