Moscow State University of Food Production, Moscow, Russia.
Corresponding author email: ilmedv1@yandex.ru
Article Publishing History
Received: 14/09/2021
Accepted After Revision: 15/12/2021
To continue the fight against pig helminthiasis, a detailed analysis of the current prevalence of nematodes in these productive animals is required. The assessment of the material after the technological slaughter of pigs kept in the Moscow region revealed the presence of parasitization in their intestines of four nematodes (Ascaris suum, Metastrongylus spp., Trichocephalus spp., Strongyloides spp.) In the form of monoinvasion and any combination thereof. The total infestation of pigs with intestinal nematodes was 55.24%.
Trichocytic invasion (Trichocephalus spp.) Occurred in pigs most often (23.77%), ascarous invasion (Ascaris suum) reached 22.38%, strongyloid invasion (Strongyloides spp.) Was noted in 9.09% of cases, metastrongylous (Metastrongylus spp.) was present in 1.75% of cases. In conditions of an increase in the severity of monoinvasion, the size of helminth eggs decreased in Trichuris: size in length by 5.9%, size in width by 7.4%, in Ascaris: length by 4.9%, width by 4.6%, in strongulata eggs a decrease in size is also noted.
In the case of mixed Ascaris-Metastrongylous invasion, there was a clear antagonistic relationship, manifested in a decrease in the size of Ascaris eggs. The size of the eggs of Trichuris in the case of an invasion mixed with Ascaris decreased, which is apparently associated with the development of antagonism between them. Comparative analysis of information on the prevalence of nematodes in pigs in farms of the Moscow region and their size can help to increase the effectiveness of treatment and prophylactic measures against helminthiasis, and can be taken into account in the course of further examination of pigs in the Moscow.
Epizootology, Helminths, Monoinvasion, Mixed Invasion, Pigs.
Glamazdin I.G, Medvedev I.N, Sysoeva N.Y, Goryacheva M.M, Kryukovskaya G.M, Maryushina T.O. Prevalence of Swine Nematodes in Moscow, Russia. Biosc.Biotech.Res.Comm. 2021;14(4).
Glamazdin I. G, Medvedev I. N, Sysoeva N. Y, Goryacheva M. M, Kryukovskaya G. M, Maryushina T. O. Prevalence of Swine Nematodes in Moscow, Russia. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3EDzpRd“>https://bit.ly/3EDzpRd</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, sources the original author and sources are credited.
INTRODUCTION
The processes of growth and development in productive animals are of great economic importance (Bespalov et al. 2018; Zavalishina 2018a). The implementation of these processes depends on the species characteristics of animals, on the state of many biological processes occurring in different cells and tissues of their body, the level of nutrition, quality of nutrition, the presence or absence of pathological processes (Vorobyeva et al. 2018; Zavalishina 2018e). Pigs are one of the most reliable sources of quality and affordable protein products (Maksimov et al. 2018; Zavalishina 2018c).
Their development ensures the production of about 40% of all meat obtained in the world, the volume of which tends to grow. At the same time, the development of various diseases in animals during their entire ontogenesis, a significant proportion of which are various parasitic diseases, hinders the growth of the efficiency of pig breeding. Their presence contributes to the development of allergic processes in pigs, morphological disorders in tissues, weakening of various life processes, including a decrease in the absorption of nutrients (Laha et al. 2014; Fava et al. 2020; Lobanova 2021).
The development of these processes inhibits the growth of animals, reduces their growth, reduces the number of obtained offspring and increases the likelihood of death of pigs (Kumsa and Kifle 2014). At the same time, the presence of parasites in the body of pigs aggravates the course of any concomitant diseases and reduces the effectiveness of any vaccination. All these circumstances reduce the quality of meat with frequent culling of organs (Nur-E-Azam et al. 2015).
This dictates the need to control the level of prevalence of gastrointestinal helminthiasis in pigs at all stages of their maintenance (Allwin et al. 2015; Zavalishina 2018b). In pigs in the intestine, they are capable of parasitizing Ascaris, Trichuritsa, Strongylate, Metastrongyla individually and in various combinations (Yamov and Antropov 2008; Donnik and Sazhaev 2012; Ngowi et al. 2014; Allwin et al. 2015; Obebe et al. 2020).
A wide variation in the intensity of invasion by different types of nematodes is possible under conditions of monoinvasion and with mixed infection. The size of the parasites themselves and their eggs may also vary. The dynamics of these indicators can be influenced by the level of activity of the immune system of animals, housing conditions, peculiarities of their feeding, and it is possible that the processes of interspecific relationships. The purpose of the study is to assess the prevalence of nematodes in pigs kept in the Moscow region of Russia.
MATERIAL AND METHODS
The assessment of the prevalence of pig helminthiasis was carried out in animals kept on the territory of the Moscow region. Pigs were taken to the slaughterhouse. 286 samples of pig feces were taken from the upper sections of their large intestine. The Kotelnikov-Khrenov method was used using a saturated solution of ammonium nitrate, which acts as a flotation liquid. The extent of invasion was revealed as the ratio of the number of invaded animals to the total population, expressing the value as a percentage. The intensity of nematode invasion (infestation with parasites of one animal) was determined using a Goryaev counting chamber. The species of the detected eggs was identified by the available morphological characteristics (Anne and Gary 2012).
The process of morphometry of helminth eggs was carried out using the ImageJ software (Wayne Rasband of Research Services Branch of the National Institute of Mental Health). Statistical processing of the obtained data was carried out using the methodology for calculating the significance of differences in mean values according to the student’s t-test.
RESULTS AND DISCUSSION
As a result of the study of collected fecal samples from pigs kept in the Moscow region, a general infestation of them with helminthiases was revealed, the value of which was 55.24%. During the study, the following helminths were identified: Trichuris suis, Ascaris suum, Metastrongylus spp. and strongylata (Strongylata suborder), the severity of which is shown in Figure 1 and Table 1.
This observation is consistent with the work of Russian and foreign researchers who confirm the wide prevalence of helminths found in the work carried out in pigs. The performed study found that the most common in pigs is trichoid invasion, the frequency of which was 23.77%, which is consistent with earlier studies of domestic authors (Savelyev and Kulikova 2006; Laha et al. 2014; Petersen et al. 2020).
Figure 1: Extensiveness of invasion in pigs by varieties of helminths in the Moscow region of Russia
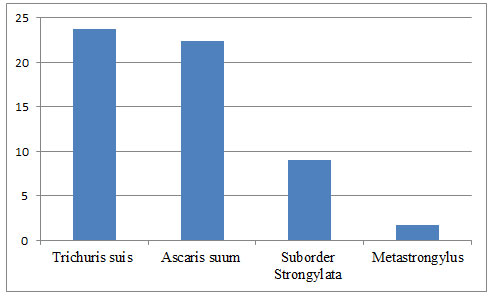
Table 1: Distribution of nematodes among the surveyed pig population
| Helminths | Number of positive samples, (n) | Extensiveness of invasion, % |
| Trichuris suis (monoinvasia) | 42 | 14.68 |
| Ascaris suum (monoinvasia) | 25 | 8.74 |
| Suborder Strongylata (monoinvasia) | 21 | 7.34 |
| Ascaris suum + Trichuris suis | 20 | 6.99 |
| Ascaris suum + Metastrongylus | 7 | 2.45 |
| Ascaris suum + suborder Strongylata | 8 | 2.79 |
| Ascaris suum + Trichuris suis+ suborder Strongylata | 2 | 0.69 |
| Trichuris suis (total) | 68 | 23.77 |
| Ascaris suum (total) | 64 | 22.38 |
| Suborder Strongylata (total) | 26 | 9.09 |
| Metastrongylus (total) | 5 | 1.75 |
| Total: | 158 | 55.24 |
The incidence of ascariasis in pigs was 22.38%. This frequency is confirmed in the works of other researchers (Safiullin 2009). It can be assumed that the prevalence of ascarous infestation among the pig population in the regions of Russia ranges from 10.0% to 30.0%. The level of invasion by intestinal strongylates in the work carried out reached 9.09%, and the incidence of invasion by metastrongylae was the lowest 1.75%.
It has been noticed that monoinvasions in pigs in the Moscow region of Russia occupy a leading place in the number of helminthiases (in more than 50% of cases). Di-invasions occurred in about 20% of cases, and invasions by three types of parasites were quite rare, which is confirmed in earlier studies (Ngowi et al. 2014). Among the mixed invasions, the following variants were noted: Ascaris + Metastrongyla, ascaris + Strongylata, Ascaris + Trichuris, ascaris + Trichuris + Strongylata.
Ascaris + Trichuris, which occurred in 6.99% of cases, were very frequent combinations in the study. The results of evaluating the size of the eggs of parasites are shown in tables 2 and 3. It was found that for certain types of helminths: ascaris, strongylata and metastrongyla, parasitism is characteristic when one animal is highly infested with parasites. It is rare in Trichuris suis nematodes, which is also confirmed by earlier studies (Allwin et al. 2015; Petersen et al. 2020).
Table 2: Sizes of helminth eggs in monoinvasion
|
Helminths |
Number of samples (n) | The size of eggs under conditions of different invasion intensity (M±m), μm | ||||||
| low | average | high | ||||||
| length | width | length | width | length | width | |||
| Trichuris suis | 15 | 78.2±1.48 | 34.6±0.73 | 73,8±1,52 | 32.9±1.24 | – | – | |
| V | 2.39 | 2.87 | 3,82 | 6.53 | – | – | ||
| Ascaris suum | 15 | 77.2±3.75 | 63.1±3.07 | – | – | 73.6±1.12 | 60,3±1,25 | |
| V | 9.88 | 10.63 | – | – | 6.12 | 7,03 | ||
| suborder
Strongylata |
12 |
130.7±10.65 | 70.7±6.52 | – | – | 86.3±2.25* | 51.3±0,62* | |
| 27.67 | 30.45 | – | – | 9.16 | 4.08 | |||
| V | ||||||||
* differences are significant in relation to parasite infestation of one animal (p <0.05) V – coefficient of variation,%
Table 3: Sizes of helminth eggs with mixed invasion
| Mixed invasion | Number of samples (n) | Size of eggs under conditions of different intensity of invasion (M ± m), μm | |||||
| low | average | high | |||||
| length | width | length | width | length | width | ||
| Ascaris suum
(Ascaris suum + Trichuris suis) |
8 | 77.2±2.63 | 60.3±2.26 | – | – | – | – |
| Ascaris suum
(Ascaris suum + metastrongylus) |
5 | – | – | – | – | 76.2±1.4* | 63.3*±0.82* |
| Trichuris suis
(Ascaris suum + Trichuris suis) |
8 | 73,8±0,36 | 33,8±0,27 | – | – | – | – |
| Metastrongylus
(Ascaris suum + etastrongylus) |
5 | – | – | – | – | 68.4±2.15 | 54.1±0.32 |
* differences are statistically significant compared with monoinvasion (p <0.05)
It was found that the size of the eggs of Ascaris in the case of monoinvasion reaches – 77.2 µm x 63.1 µm with a low infection with parasites of one animal and 73.6 µm x 60.3 µm in conditions of high infection with parasites of one animal. In previous studies, it was proved that the length of the eggs of Acan be from 50 µm to 100 µm (Anne and Gary 2012).
The value of the coefficient of variation in the size of Ascaris eggs with an increase in parasite infestation of one animal decreased for length from 9.88% to 6.11% (lengthwise), for width from 10.63 to 7.03%. In the case of an increase in the parasite infestation of one animal, the size of the Ascaris eggs decreased in length by 4.9%, in width by 4.6% (Petersen et al. 2020).
The size of Trichuris eggs was found to be 78.2 µm x 34.6 µm at a low invasion, and 73.8 µm x 32.2 µm in the case of an average invasion. The revealed sizes turned out to be somewhat larger than those observed in previous studies (Anne and Gary 2012). Apparently, this is due to the fact that in the work carried out, samples were taken from the upper sections of the colon and the eggs of Trichuris could be immature.
The value of the coefficient of variation of the size of the trichuroz eggs increased little with the growth of the parasite infestation of one animal, which indicates a high reliability of the obtained numerical values of their sizes. In the case of an increase in the severity of invasion, the size of Trichuris eggs decreased: length by 5.9%, width by 7.4% (Petersen et al. 2020; Romashov et al. 2021).
The size of strongylate eggs varied significantly: with low invasion from 130.7 μm x 70.7 μm, with high invasion up to 86.3 μm x 51.3 μm. The value of the coefficient of variation for the length of the eggs was 27.67% and for the width it corresponded to 30.45%, indicating a significant variability in their sizes.
Apparently, this depends on the place of sampling of feces from the intestine (there was an immaturity of the eggs of these helminths), when several species from the Strongylata order were parasitized together. At the same time, it is recognized that the eggs of helminths of this order belong to the genera Hyostrongylus, Trichostrongylus, Oesophagostomum and Globocephalus, it is difficult to distinguish visually (Anne and Gary 2012; Petersen et al. 2020; Romashov et al. 2021).
In the case of high parasite infestation of one animal with strongylates, the value of the variation coefficient decreased in length to 9.16% and in width up to 4.08%, which obviously needs to be regarded as a consequence of the presence of only one species of strongylate. Under conditions of increasing intensity of invasion by strongylates, the size of eggs decreased significantly: in length by 51.4%, in width by 37.8%. The size of the metastrongil eggs was 68.4 µm x 54.1 µm, which is higher than the values obtained by other researchers (Anne and Gary 2012).
The value of the coefficient of variation for these eggs was not clarified, since metastrongylous monoinvasion was not widespread, and the presence of ascaris eggs does not make it possible to draw final conclusions about the severity of variation in the eggs of these helminths. that their size in length and width under conditions of monoinvasion, as the severity of invasion in strongylates increases, statistically significantly decreases (p <0.05) (Petersen et al. 2020; Romashov et al. 2021).
From literary sources it is known that with increasing degree of invasion, helminths. Apparently, this regularity is also characteristic of the size of the eggs laid by helminthes (Zavalishina 2018d; Nasurdinova and Zhigileva 2007). The conducted studies revealed that the sizes of eggs of ascaris under conditions of monoinvasion and mixed invasions, a combination of Ascaris and Trichuris differed little under conditions of the same severity of ascaronous parasitism.
In the case of mixed ascaris-metastrongylous parasitism, there was a tendency to an increase in the length and an increase in the width of the ascaris eggs. It can be assumed that the size of A. Sum eggs is not affected by the concomitant trichurosis invasion, and metastrongylae apparently exhibit antagonistic effects on Ascaris, which is manifested in the size of their detected eggs. The sizes of eggs of Trichuris under the conditions of mixed with Ascaris invasion decreased in length and width compared to the conditions of monoinvasion, which indicates the depressive effect of A. suum on Trichuris suis (Romashov et al. 2021).
CONCLUSION
The finding of the present study suggests that the prevalence of infestation of pigs by intestinal nematodes in the Moscow region of Russia has reached 55.24%. Of the intestinal helminthiasis in pigs, the most common invasion is trichurosis – 23.77%, ascarous – 22.38%, strongylatous – 9.09% and metastrongylous – 1.75%. In the case of an increase in the intensity of the monoinvasion process, a decrease in the size of eggs was noted in all parasites. In the case of mixed invasion, a clear antagonistic relationship was noted, leading to a decrease in egg size. The information obtained on the interspecific relationships of helminths under conditions of mixed invasion can help in the development of anti parasitic measures in pigs.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
ACKNOWLEDGEMENTS
The study was conducted at the expense of the authors. The study was approved by the local ethics committee of the Moscow State University of Food Production (Protocol №11 of January 17, 2018). Authors thank the administration of the Moscow State University of Food Production for the opportunity to research its basis.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Allwin, B., Jayathangaraj, M.G., Palanivelrajan, M. et al. (2015). Prevalence of helminthic fauna in wild pigs in comparison with domestic pigs: A study in the adjoining areas of Mudumalai, Anamalai and Sathyamangalam tiger reserves, Tamil Nadu South India. J. Parasitol. Vector Biol., 7(3): 46-52.
Bespalov, D.V., Kharitonov, E.L., Zavalishina, S.Yu. et al. (2018). Physiological Basis For The Distribution Of Functions In The Cerebral Cortex. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 605-612.
Donnik, I.M., and Sazhaev, I.M. (2012). Distribution and species composition of pathogens of helminthiases and protozoses of pigs in livestock organizations. Agrarian Bulletin of the Urals, 9(101): 10-13.
Fava, N.M.N., Cury, M.C., Santos, H.A. et al. (2020). Phylogenetic relationships among Toxocara spp. and Toxascaris sp. from different regions of the world. Veterinary Parasitology this link is disabled, 282, 109133
Kumsa, В., and Kifle, E. (2014). Internal parasites and health management of pigs in Burau District, Oromia Regional State, Ethiopia. J. of the South African Vet. Assosiation, 85(1): 1-5.
Laha, R., Das, M., Goswami, A. et al. (2014). Prevalence of Gastrointestinal Parasitic Infections in Pigs of North Eastern Region of India. Indian J. of Hill Farming, 27(1): 110-117.
Lobanova, A.A. (2021). Pathogenic action of parasites on a macroorganism. In the collection: Actual problems of veterinary science and practice. Collection of materials of the All-Russian (national) scientific-practical conference. Omsk, 327-329.
Maksimov, V.I., Zavalishina, S.Yu., Parakhnevich, A.V. et al. (2018). Physiological Dynamics Of Microrheological Characteristics Of Erythrocytes In Piglets During The Phase Of Milk Nutrition. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 454-459.
Nasurdinova, N.M., and Zhigileva, O.N. (2007). Competition of helminths in parasitic communities of the common frog Rana arvalis. Bulletin of Tyumen State University, 6: 204-209.
Ngowi, H. A., Chenyambuga, S., Sambuta, A. et al. (2014). Coendemicity of cysticercosis and gastrointestinal parasites in rural pigs: a need for integrated control measures for porcine cysticercosis. Sci Parasitol., 15(1-4): 1-8.
Nur-E-Azam, Md., Sen, P., Tasneem, M. et al. (2015). Occurence of gastrointestinal parasitic infections in pigs of Dinajpur district, Bangladesh. Scientific J. of Veterinary Advances, 4(8): 57-66.
Obebe, O.O., Aluko, O.O., Falohun, O.O. et al. (2020). Parasitic contamination and public health risk of commonly consumed vegetables in Ibadan-Nigeria. Pan Afr Med J, 36: 126. doi: 10.11604/pamj.2020.36.126.19364.
Petersen, H.H., Takeuchi-Storm, N., Enemark, H.L. et al. (2020). Surveillance of important bacterial and parasitic infections in Danish wild boars (Sus scrofa). Acta Vet Scand, 62(1): 41. doi: 10.1186/s13028-020-00539-x.
Romashov, B.V., Odoevskaya, I.M., Romashova, N.B. et al. (2021). Ecology of trichinellosis transmission in the voronezh state nature reserve and adjacent areas, Russia. Nature Conservation Research, 6(2): 1-15.
Safiullin, R.T. (2009). Epizootic situation in pigs ascariasis by zones of the country and forecast. Theory and practice of animal parasitic diseases, 10: 344-348.
Savelyev, A.A., and Kulikova, O.L. (2006). Epizootology of pig intestinal nematodes in basic farms. Veterinary pathology, 1: 71-74.
Vorobyeva, N.V., Mal, G.S., Zavalishina, S.Yu. et al. (2018). Influence Of Physical Exercise On The Activity Of Brain Processes. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(6): 240-244.
Yamov, V.Z., and Antropov, V.A. (2008). Epizootology of pig helminthiasis in the Tyumen south. Agrarian Bulletin of the Urals, 5(47): 70-71.
Zajac, A.M., Conboy, G.A., Little, S.E. et al. (2021). Veterinary clinical parasitology. John Wiley & Sons.
Zavalishina, S.Yu. (2018a). Anti-Coagulant And Fibrinolytic Activity Of Blood Plasma In Healthy Calves Of Dairy-Vegetative Nutrition. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 753-758.
Zavalishina, S.Yu. (2018b). Functional Activity Of The Blood Clotting System In Calves During The Phase Of Milk And Vegetable Nutrition. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 720-725.
Zavalishina, S.Yu. (2018c). Physiological Dynamics Of The Blood Coagulation System Activity In Calves During The Phase Of Dairy Nutrition. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 680-685.
Zavalishina, S.Yu. (2018d). Physiological Mechanisms Of Hemostasis In Living Organisms. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 629-634.
Zavalishina, S.Yu. (2018e). Functional Properties Of Anticoagulant And Fibrinolytic Activity Of Blood Plasma In Calves In The Phase Of Milk Nutrition. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9(5): 659-664.


