Department of Speech Language Pathology and Audiology, National Guard Health Affairs, Ministry of National Guard, Riyadh Saudi Arabia
Corresponding author Email: rania2222@hotmail.com
Article Publishing History
Received: 27/12/2018
Accepted After Revision: 21/02/2019
The current review is aimed to provide an overview of etio-pathogenesis of otosclerosis. Otosclerosis affects bony labyrinth resulting a progressive conductive hearing loss, a sensorineural hearing loss, or a mixed-type hearing loss. It is a hereditary localized disease of the otic capsule typifi ed by new bone formation that is composed of different fi brillar and cellular pattern. The abnormal new bone produces ankylosis of the stapes footplate resulting in conductive deafness. The onset of the hearing loss due to otosclerosis is usually between 15 and 40 years of age. Otosclerosis is a disease of adult onset affecting more women than men in the world. The previous study also has identifi ed the link of otosclerosis with the family history. Additionally, women at an earlier age reported greater severity of otosclerosis symptoms compared to men. Despite the numerous theories suggested, the etiology of otosclerosis has remained obscure. Since histopathological inner ear changes due to otosclerosis is important, this review will provide an updated overview of prevalence and etio-pathological changes in otosclerosis.
Otosclerosis, Hearing Loss, Otospongiosis, Cochlear Lesions
Sharaf R. A. Prevalence and Pathogenesis of Otosclerosis: A Review. Biosc.Biotech.Res.Comm. 2019;12(1).
Sharaf R. A. Prevalence and Pathogenesis of Otosclerosis: A Review. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2YSOA46
Copyright © Sharaf , This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Otosclerosis is a disease of the bony labyrinth, which is characterized by fi xation of the footplate of the stapes secondary to abnormal bone formation (Quesnel et al., 2018; Guild, 1994; Schuknecht, 1993). The area of the labyrinthine capsule immediately in front of the footplate of the stapes called “fi ssula antefenestrum” is the site of predilection for otosclerosis. Other frequent sites include the border of the round window and even the footplate of the stapes (Mansour et al., 2018; Guild, 1994; Schuknecht, 1993).Virolainen (1972) reports that otosclerosis is usually bilateral. It is a hereditary localized disease of the otic capsule typified by new bone formation that is composed of different fibrillar and cellular pattern (Sho-man et al., 2014; Guild, 1994; Schuknecht, 1993). The abnormal new bone produces ankylosis of the stapes footplate resulting in conductive deafness (Henriques et al., 2016; Guild, 1994; Schuknecht, 1993). Other part of the labyrinth may be involved resulting in sensorineural deafness and vestibular abnormalities (Ciuman, 2013; Smyth, 1997).
“Otospongiosis” is the other term for this condition and is preferred by many European otologists, as the abnormal new bone is highly vascular (Bittermann, 2014; Causse and colleague, 1984). However, in UK, the term “otosclerosis” is commonly used, which refers to the fi nal inactive stage where there is sclerotic hard bone (Virolainen, 1972). According to Smyth (1997) neither of these terms is strictly correct.
Despite the numerous theories suggested, the etiology of otosclerosis has remained obscure. Delayed physiological development, inflammatory processes, cartilaginous deposits in the region of the fissula antefenestrum, disturbances in the blood supply, mechanical stimuli, etc. have all been described as possible cause of otosclerosis (Batson and Rizzolo, 2017; Cureoglu et al., 2006) (Figure 1). It is therefore possible that there are constitutional, local and activating factors, which could be responsible for the development of the otosclerotic focieither individually or jointly (Virolainen, 1972). This review aimed to provide an overview of prevalence and etio-pathogenesis of otosclerosis.
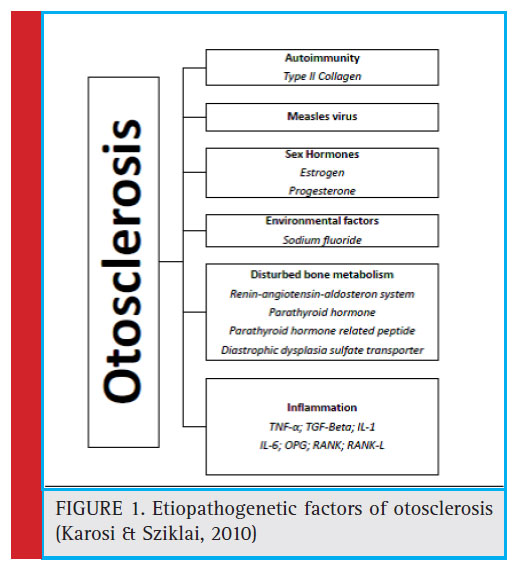 |
Figure 1: Etiopathogenetic factors of otosclerosis (Karosi & Sziklai, 2010) |
Review of Literature on Otosclerosis
In the past years the study of otosclerosis has principally been devoted to the histochemistry of otosclerotic foci (Abdel-Ghany et al., 2014). A signifi cant elevation in the activity of alkaline phosphatase and an equally significant fall in the activity of lactic acid dehydrogenase has been described (Moumoulidis et al., 2007). Some changes in the matrix of the otosclerotic bone have also been shown, for instance in the number and construction of the polysaccharides (Virolainen, 1972; Ziff, 2014).
Otosclerosis is a disease of adult onset affecting more women than men in the world (Crompton et al., 2019). The previous study also has identifi ed the link of otosclerosis with the family history (Crompton et al., 2019). Additionally, women at an earlier age reported greater severity of otosclerosis symptoms compared to men (Crompton et al., 2019). Clinical manifestation of otosceloris is more evident in White Europeans and people of Indian origin (Lin et al., 2007).
In additional to typical conductive hearing loss, patients suffering from otosclerosis also often have perceptive hearing loss (Cureoglu et al., 2010; Venail et al., 2007). Cochlear lesions are considered to be due to secretions from the active otosclerotic foci, which have found their way into the endolymph (Makarem and Linthicum, 2008). Vestibular disturbances even appear in patients suffering from otosclerosis (de Vilhena et al., 2016). These may be derived from otosclerotic vascular changes due to the disease or from biochemical changes in the inner ear fl uids (Ciuman, 2013).
Because hearing loss is the dominant feature of otosclerosis, these patients have not been examined for the other disorders associated with otosclerosis as carefully as for hearing (Declau et al., 2007). Unsteadiness and or dizziness have been frequently observed in these patients (Yetis˛er, 2018). Abnormal electronystagmographic findings have been reported in otosclerotics (Gupta and Mundra, 2015). Further studies of these changes would throw additional light on the general view about otosclerosis (Purohit, 2014).
Occurrence of Otosclerotic Foci
It’s important to distinguish between clinical, nonclinical or histological otosclerosis; the latter is about 10 times more common than the former (Smyth, 1997; McElveen and Kutz, 2018). In 1940, fi rst study revealed histological otosclerosis on the temporal bones in 12% of 200 autopsies (Virolainen, 1972). In 1944, Guild demonstrated histological otosclerosis in only 8.3% of 482 African races but much less in the wider population around 1%. A recent histological study reported lack of evidence for nonotosclerotic stapes fi xation in people undergoing stapedectomy (Quesnel et al., 2016).
Otosclerosis is usually bilateral, however the frequency of histological unilateral otosclerosis ranges between 13% and 30% (Foster and Backous, 2018; Quesnel et al., 2018). The area of the labyrinthine capsule immediately in front of the footplate of the stapes called ‘fi ssula antefenestrum’
is the site of predilection for otosclerosis; other frequent sites may include border of the round window and even the footplate of the stapes around the cochlea, the internal auditory canal or less often around the semicircular canal (Michaels and Soucek, 2011).
The focus nearly always originates in the endochondral layer of the labyrinthine capsule, extending in many cases into the periosteal layer as far as the mucosa of the tympanic cavity, and into the endosteal layer of the labyrinth (Naumann et al., 2005). Where the focus reaches the mucoperiosteum of the tympanum and the endosteum of the labyrinth, these show varying degrees of fi brous thickening and increased vascularity (Makarem et al., 2010).
Virolainen (1972) quotes Seligman and Shambaugh (1951) that assessment of 2086 post-operative surgery of ear revealed that cochlear nerve involvement is present twice as often when otosclerotic foci are situated in the horizontal semicircular canal, compared with the frequency of the involvement when the canal is not affected. Histological otosclerosis is at any rate quite frequently asymptomatic (Declau et al., 2007). In a previous review of Ménière’s disease, the lateral semicircular canal was found at autopsy to be full of otosclerotic bone (Oberman et al., 2017).
Pathological Changes in Otosclerosis
Otosclerosis is a disease of the bony labyrinthine capsule, frequently developing in areas where embryonic cartilage persists (Bloch, 2012; Babcock et al., 2018). The pathological process is characterized by resorption of the normal bone, often around the blood vessels, and by the replacement of normal bone with cellular fibrous connective tissue (Rudic et al., 2015). Areas undergoing active resorption are extensively vascularised (Rudic et al., 2015). Focus may contain areas at different stages of activity that can be described as active intermediate and inactive final stage (Karosi & Sziklai, 2010). Karosi et al., (2011) reported that the activity of alkaline phosphatase increases in the focus.
The term “otospongiosis” refers to the active phase of the disease, which is characterized by the presence of vascular space containing highly cellular fi broses tissue, mononuclear histiocytes together with osteocytes and osteoclasts (Parahy and Linthicum, 1984; Uppal et al., 2010). These cells contain many enzymes, which are released in to the surrounding tissues resulting in its absorption (Ziff, 2014). The term “otosclerosis” refers to the final stage of highly mineralized bone with a mosaic appearance (Quesnel et al., 2013). Osteoclasts have disappeared but osteocytes and/or osteoblasts may still be seen in the peripheral areas (Quesnel et al., 2013).
Previous study had suggested that some enzyme serve as the initiating factor of decalcification (Purohit & Hermans, 2014). Another study suggested that the focus shows alkaline phosphates during the first phase and the whole of secondary phase until the bone is completely calcified, alkaline phosphatase will then disappear (Gronowicz et al., 2014). Some studies observed the presence of abnormal accumulation of mucopolysaccharides in to the otosclerosis focus (Ricci, 1962; Ribári et al., 1991). A recent study reported higher level of total oxidant status and oxidative stress index in patients with otosclerosis (Baysal et al., 2017). Additionally, reactive oxygen species could be developed in otosclerotic foci (Rudic et al., 2015).
Perilymph Changes in Otosclerosis
The perilymph has an extra-cellular character with low potassium, glucose, amino acid, and high sodium concentration (Palmer et al., 2016). The blood-labyrinthine barrier permeability has been slightly increased in ears of patients with Ménière’s disease (Tagaya et al., 2011). Additionally, the blood-labyrinthine barrier permeability is also increased in other diseases, for instance sudden deafness (Sugiura et al., 2006; Yoshida et al., 2008), labyrinthine invasion of cholesteatoma (Sone et al., 2007; Sone et al., 2012), vestibular schwannoma (Yamazaki et al., 2009), and relapsing polychondritis (Kato et al., 2014). Endolymphatic hydrops (EHs) is often seen in patients with otosclerosis, and preoperative EH was one of the risk factor for inner ear disturbances following stapes surgery (Mukaida et al., 2015). Furthermore, increased permeability of the blood-labyrinthine barrier and endolymphatic hydrops could also causes sensorineural hearing loss in otosclerosis (Mukaida et al., 2015). A recent study assessed and compared cochlear lymph signal intensity in patients with otosclerosis, Ménière’s disease, and healthy individuals (Naganawa et al., 2016). The results of this study indicated an increased cochlear lymph signal intensity ratio in patients with otosclerosis than Ménière’s disease or healthy individuals (Naganawa et al., 2016). Many studies has been reported an association of EH with otosclerosis (Franklin et al., 1990; Yoon et al., 1990; Shea et al., 1994); however, there is a lack of understanding about the mechanism for the development of EH in patients with otosclerosis (Mukaida et al., 2015).
Conclusion
The histological and pathological changes of otosclerosis is still not fully explained. To enhance a better understanding of the pathogenesis of otosclerosis, it is vital to use modern biologic methods to examine pathological changes in human temporal bones with otosclerosis.
References
Abdel-Ghany, A. F., Osman, N. M., & Botros, S. M. (2014). Correlation between the size, CT density of otosclerotic foci, and audiological tests in cases of otosclerosis. The Journal of International Advanced Otology, 10(2), 156.
Babcock, T. A., & Liu, X. Z. (2018). Otosclerosis: From Genetics to Molecular Biology. Otolaryngologic Clinics of North America, 51(2), 305-318.
Batson, L., & Rizzolo, D. (2017). Otosclerosis: an update on diagnosis and treatment. Journal of the American Academy of PAs, 30(2), 17-22.
Baysal, E., Gulsen, S., Aytac, I., Celenk, F., Ensari, N., Taysi, S., … & Kanlikama, M. (2017). Oxidative stress in otosclerosis. Redox Report, 22(5), 235-239.
Bittermann, A. J. (2014). The treatment of patients with otosclerosis. From expert opinion to evidence-based practice (Vol. 67). Utrecht University.
Bloch, S. L. (2012). On the biology of the bony otic capsule and the pathogenesis of otosclerosis. Danish medical journal, 59(10), B4524-B4524.
Bretlau, P., & Jørgensen, M. B. (1968). Otosclerosis in the lateral semi-circular canal. The Journal of Laryngology & Otology, 82(1), 65-67.
Causse, J. R., & Causse, J. B. (1984). Otospongiosis as a genetic disease. Early detection, medical management, and prevention. The American journal of otology, 5(3), 211-223.
Causse, J. R., Uriel, J., Berges, J., Shambaugh, J. G., Bretlau, P., & Causse, J. B. (1982). The enzymatic mechanism of the otospongiotic disease and NaF action on the enzymatic balance. The American journal of otology, 3(4), 297-314.
Ciuman, R. R. (2013). Inner ear symptoms and disease: Pathophysiological understanding and therapeutic options. Medical science monitor: international medical journal of experimental and clinical research, 19, 1195.
Crompton, M., Cadge, B. A., Ziff, J. L., Mowat, A. J., Nash, R., Lavy, J. A., … & Dawson, S. J. (2019). The Epidemiology of Otosclerosis in a British Cohort. Otology & Neurotology, 40(1), 22-30.
Cureoglu, S., Baylan, M. Y., & Paparella, M. M. (2010). Cochlear otosclerosis. Current opinion in otolaryngology & head and neck surgery, 18(5), 357.
Cureoglu, S., Schachern, P. A., Ferlito, A., Rinaldo, A., Tsuprun, V., & Paparella, M. M. (2006). Otosclerosis: etiopathogenesis and histopathology. American journal of otolaryngology, 27(5), 334-340.
de Vilhena, D., Gambôa, I., Duarte, D., & Lopes, G. (2016). Vestibular disorders after stapedial surgery in patients with otosclerosis. International journal of otolaryngology, 2016.
Declau, F., Van Spaendonck, M., Timmermans, J. P., Michaels, L., Liang, J., Qiu, J. P., & Van De Heyning, P. (2007). Prevalence of histologic otosclerosis: an unbiased temporal bone study in Caucasians. In Otosclerosis and Stapes Surgery (Vol. 65, pp. 6-16). Karger Publishers.
Foster, M. F., & Backous, D. D. (2018). Clinical Evaluation of the Patient with Otosclerosis. Otolaryngologic Clinics of North America, 51(2), 319-326.
Franklin, D. J., Pollak, A., & Fisch, U. (1990). Ménière’s symptoms resulting from bilateral otosclerotic occlusion of the endolymphatic duct: an analysis of a causal relationship between otosclerosis and Ménière’s disease. The American journal of otology, 11(2), 135-140.
Gronowicz, G., Richardson, Y. L., Flynn, J., Kveton, J., Eisen, M., Leonard, G., … & Parham, K. (2014). Differences in otosclerotic and normal human stapedial osteoblast properties are normalized by alendronate in vitro. Otolaryngology–Head and Neck Surgery, 151(4), 657-666.
Guild, S. R. (1944). Histologic otosclerosis. Ann Otol Rhinol Laryngol, 53(1045), 71.
Gupta, S. K., & Mundra, R. K. (2015). Electronystagmography a very useful diagnostic tool in cases of vertigo. Indian Journal of Otolaryngology and Head & Neck Surgery, 67(4), 370-374.
Henriques, V., Teles, R., Sousa, A., Estevão, R., Rodrigues, J., Gomes, A., … & Fernandes, F. (2016). Abnormal Congenital Location of Stapes’ Superstructure: Clinical and Embryological Implications. Case reports in otolaryngology, 2016.
Karosi, T., & Sziklai, I. (2010). Etiopathogenesis of otosclerosis. European archives of oto-rhino-laryngology, 267(9), 1337-1349.
Karosi, T., Csomor, P., Szalmás, A., Kónya, J., Petkó, M., & Sziklai, I. (2011). Osteoprotegerin expression and sensitivity in otosclerosis with different histological activity. European archives of oto-rhino-laryngology, 268(3), 357-365.
Kato, M., Katayama, N., Naganawa, S., & Nakashima, T. (2014). Three-dimensional fl uid-attenuated inversion recovery magnetic resonance imaging fi ndings in a patient with relapsing polychondritis. The Journal of Laryngology & Otology, 128(2), 192-194.
Lin, C. Y., Yang, Y. C., Leon Guo, Y., Wu, C. H., Chang, C. J., & Wu, J. L. (2007). Prevalence of hearing impairment in an adult population in southern Taiwan. International journal of audiology, 46(12), 732-737.
Makarem, A. O., Hoang, T. A., Lo, W. W., Linthicum, F. H., & Fayad, J. N. (2010). Cavitating otosclerosis: clinical, radiological and histopathological correlations. Otology & neurotology: offi cial publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 31(3), 381.
Makarem, A., & Linthicum, F. H. (2008). Cochlear otosclerosis and endolymphatic hydrops. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 29(4), 571.
Mansour, S., Magnan, J., Nicolas, K., & Haidar, H. (2018). Otosclerosis. In Middle Ear Diseases (pp. 1-83). Springer, Cham.
McElveen, J. T., & Kutz, J. W. (2018). Controversies in the Evaluation and Management of Otosclerosis. Otolaryngologic Clinics of North America, 51(2), 487-499.
Michaels, L., & Soucek, S. (2011). Origin and growth of otosclerosis. Acta oto-laryngologica, 131(5), 460-468.
Moumoulidis, I., Axon, P., Baguley, D., & Reid, E. (2007). A review on the genetics of otosclerosis. Clinical otolaryngology, 32(4), 239-247.
Mukaida, T., Sone, M., Yoshida, T., Kato, K., Teranishi, M., Naganawa, S., & Nakashima, T. (2015). Magnetic resonance imaging evaluation of endolymphatic hydrops in cases with otosclerosis. Otology & Neurotology, 36(7), 1146-1150.
Naganawa, S., Kawai, H., Taoka, T., Suzuki, K., Iwano, S., Satake, H., … & Ikeda, M. (2016). Cochlear lymph fl uid signal increase in patients with otosclerosis after intravenous administration of gadodiamide. Magnetic Resonance in Medical Sciences, mp-2015.
Naumann, I. C., Porcellini, B., & Fisch, U. (2005). Otosclerosis: incidence of positive fi ndings on high-resolution computed tomography and their correlation to audiological test data. Annals of Otology, Rhinology & Laryngology, 114(9), 709- 716.
Oberman, B. S., Patel, V. A., Cureoglu, S., & Isildak, H. (2017). The aetiopathologies of Ménière’s disease: a contemporary review. Acta Otorhinolaryngologica Italica, 37(4), 250.
Palmer, J. C., Lord, M. S., Pinyon, J. L., Wise, A. K., Lovell, N. H., Carter, P. M., … & Green, R. A. (2016, August). Understanding the cochlear implant environment by mapping perilymph proteomes from different species. In 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 5237-5240). IEEE.
Parahy, C., & Linthicum Jr, F. H. (1984). Otosclerosis and otospongiosis: clinical and histological comparisons. The Laryngoscope, 94(4), 508 512.
Purohit, B., & Hermans, R. (2014). Imaging in otosclerosis: A pictorial review. Insights into imaging, 5(2), 245-252.
Quesnel, A. M., Ishai, R., & McKenna, M. J. (2018). Otosclerosis: temporal bone pathology. Otolaryngologic Clinics of North America, 51(2), 291-303.
Quesnel, A. M., Ishai, R., Cureoglu, S., Linthicum, F., Lopez, I. A., Nadol Jr, J. B., & McKenna, M. J. (2016). Lack of evidence for nonotosclerotic stapes fi xation in human temporal bone histopathology. Otology & neurotology: offi cial publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 37(4), 316.
Quesnel, A. M., Moonis, G., Appel, J., O’malley, J. T., McKenna, M. J., Curtin, H. D., & Merchant, S. N. (2013). Correlation of computed tomography with histopathology in otosclerosis. Otology & neurotology, 34(1), 22.
Ribári, O., Pereplica, M., & Sziklai, I. (1991). Oversulfated mucopolysaccharides in the otosclerotic bone. Acta oto-laryngologica, 111(2), 362-365.
Ricci, V. (1962). Histochemical research on the otosclerotic focus. Considerations on the pathogenesis of otosclerosis and its classifi cation among the” mesenchmopathies”. Minerva otorinolaringologica, 12, 575.
Rudic, M., Keogh, I., Wagner, R., Wilkinson, E., Kiros, N., Ferrary, E., … & Zarkovic, N. (2015). The pathophysiology of otosclerosis: review of current research. Hearing research, 330, 51-56.
Sakihara, Y., Parving, A. (1999). Clinical otosclerosis, prevalence estimates and spontaneous progress. Acta oto-laryngologica, 119(4), 468-472.
Schuknecht, H.F. (1993). Disorders of bone. In: Schuknecht HF, editor. Pathology of the ear. 2nd ed. Philadelphia7 Lea & Febiger; p. 365- 414.
Seligman, E., & Shambaugh Jr, G. E. (1951). XXXVI Otosclerosis of the Osseous Horizontal Semicircular Canal. Annals of Otology, Rhinology & Laryngology, 60(2), 375-381.
Shea, J. J., Ge, X., & Orchik, D. J. (1994). Endolymphatic hydrops associated with otosclerosis. The American journal of otology, 15(3), 348-357.
Shoman, N. M., Samy, R. N., & Choo, D. I. (2014). Congenital Malformations of the Ear. In Congenital Malformations of the Head and Neck (pp. 23-66). Springer, New York, NY. Smyth, G. D. L. (1997). Otosclerosis. Scott-Brown’s Otolaryngology- Otology, London, 1-14.
Sone, M., Mizuno, T., Sugiura, M., Naganawa, S., & Nakashima, T. (2007). Three-dimensional fl uid-attenuated inversion recovery magnetic resonance imaging investigation of inner ear disturbances in cases of middle ear cholesteatoma with labyrinthine fi stula. Otology & Neurotology, 28(8), 1029-1033.
Sone, M., Yoshida, T., Naganawa, S., Otake, H., Kato, K., Sano, R., … & Nakashima, T. (2012). Comparison of computed tomography and magnetic resonance imaging for evaluation of cholesteatoma with labyrinthine fi stulae. The Laryngoscope, 122(5), 1121-1125.
Sugiura, M., Naganawa, S., Teranishi, M., & Nakashima, T. (2006). Three‐dimensional fl uid‐attenuated inversion recovery magnetic resonance imaging fi ndings in patients with sudden sensorineural hearing loss. The Laryngoscope, 116(8), 1451-1454.
Tagaya, M., Yamazaki, M., Teranishi, M., Naganawa, S., Yoshida, T., Otake, H., … & Nakashima, T. (2011). Endolymphatic hydrops and blood–labyrinth barrier in Meniere’s disease. Acta oto-laryngologica, 131(5), 474-479.
Uppal, S., Bajaj, Y., & Coatesworth, A. P. (2010). Otosclerosis 2: the medical management of otosclerosis. International journal of clinical practice, 64(2), 256-265.
Venail, F., Lavieille, J. P., Meller, R., Deveze, A., Tardivet, L., & Magnan, J. (2007). New perspectives for middle ear implants: first results in otosclerosis with mixed hearing loss. The Laryngoscope, 117(3), 552-555.
Virolainen, E. (1972). Vestibular disturbances in clinical otosclerosis. Acta Oto-Laryngologica, 74(sup306), 5-34.
Yamazaki, M., Naganawa, S., Kawai, H., Nihashi, T., Fukatsu, H., & Nakashima, T. (2009). Increased signal intensity of the cochlea on pre-and post-contrast enhanced 3D-FLAIR in patients with vestibular schwannoma. Neuroradiology, 51(12),855.
Yetis˛er, S. (2018). Bilateral vestibulopathy due to severe cochlear otosclerosis: a well-known condition without any favorable solution. Turkish archives of otorhinolaryngology, 56(3),174.
Yoon, T. H., Paparella, M. M., & Schachern, P. A. (1990). Otosclerosis involving the vestibular aqueduct and Meniere’s disease. Otolaryngology—Head and Neck Surgery, 103(1), 107- 112.
Yoshida, T., Sugiura, M., Naganawa, S., Teranishi, M., Nakata, S., & Nakashima, T. (2008). Three‐dimensional fl uid‐attenuated inversion recovery magnetic resonance imaging fi ndings and prognosis in sudden sensorineural hearing loss. The Laryngoscope, 118(8), 1433-1437.
Ziff, J. L. (2014). A molecular and genetic analysis of otosclerosis [Doctoral dissertation, University College London (University of London)].


