1Department of Medical Microbiology and Parasitology, Faculty of Basic Clinical Sciences, College
of Health Sciences, Usmanu Danfodiyo University, Sokoto 840001, Sokoto State, Nigeria.
*2Faculty of Science, Northern Border University, Arar, Saudi Arabia.
Corresponding author email: alshrari@live.com
Article Publishing History
Received: 15/07/2020
Accepted After Revision: 28/09/2020
The development of novel severe acute respiratory syndrome-2 (SARS-CoV-2), the causative agent of the continuous coronavirus diseases-19 (COVID-19) pandemic, has become a global concern. The current COVID-19 pandemic should be extensively comprehended to deal with it and to forestall a future pandemic. Like other countries, the African countries have also taken measures to stop the spread of COVID-19 infection like full lockdown and enforcing travel limitations. This review aimed to highlight the factors associated with the emergence, surveillance, preparedness, containment of COVID-19, along with the biosafety research facilities in African countries. A literature search with the combined keywords “Africa and COVID-19” was performed using different search engines like Pubmed, Google Scholars, and Medline Plus. The data was collected and analyzed.
It has been observed that most infection spread is attributed to improper hygiene/protective measures, for example, hand washing and social distancing. Accordingly, the large scale advertisement and conduction of the COVID-19 educational programs are highly recommended. The African countries lack appropriate numbers of biosafety level 3 and 4 research facilities, trained personnel/emergency units, and funding resources to combat COVID-19 and similar pandemic. It is advisable to build up more biosafety research facilities, trained emergency response units, isolation units, and substantial funding agencies in every African country with clear rules to combat outbreaks like COVID-19. The African countries may also ask support from other countries with successful experience against COVID-19. The implementation of the suggested strategies will be helpful to African countries against COVID-19.
Africa, Containment of Biohazards, Coronavirus, Epidemiology, Pandemic
Hudu S. A, Alshrari A.S. Preparedness in containment of Coronavirus Disease-19 in the African Continent. Biosc.Biotech.Res.Comm. 2020;13(3).
Hudu S. A, Alshrari A.S. Preparedness in containment of Coronavirus Disease-19 in the African Continent. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2EWP5FZ
Copyright © Hudu and Alshrari This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Viral infections appearing within a given population with an increasing incidence or hovers to increase sooner rather than later are referred to as an emerging infection. This emerging infection can either be caused by an unknown or previously undetected infectious agent that has spread to new geographic areas or new populaces and whose role in the disease pathogenesis has gone unrecognized previously. Similarly, diseases that were once significant medical issues globally or in a specific country, and afterward declined drastically, yet are again turning out to be medical issues to a critical extent of the populace are referred to as re-emerging infections. The emergence and re-emergence of novel human viruses are of great concern, most notably with the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which was confirmed to be the causative agent of the current coronavirus disease-19 (COVID-19) pandemic that is ravaging the world (Weber et al, 2019; Weber et al, 2019; Luo and Gao, 2020; WHO, 2020a).
It is relevant to realize that before 2003, many coronaviruses were known to cause severe infections in animals, while human coronaviruses were ordinarily connected with moderate respiratory diseases. With the constant exposure of humans to animals viruses via the food that we eat, domestic animals we rear, the animals we keep as pets, and our connections with the natural surroundings, we often get infected by several animal viruses majority of which enters and pass through our gastrointestinal tract harmlessly or get neutralized and destroyed by our competent immune system (Guan et al., 2003; Vijaykrishna et al., 2007; Jiang et al., 2017; Davis et al., 2018; Vojdani et al., 2020; WHO, 2020a).
Be that as it may, on uncommon events, an animal virus bump into a human host and starts to duplicate itself, executing its whole lifecycle inside human cells and growing one virion into a populace of many. Replication of an animal virus in the body of this first human subject is the crucial moment in the zoonotic procedure because the infection transforms and develops under the specific limitations of the human body adjusting and developing itself for replication in this new host. High viral titers generated by viral replication encourage its spread to a subsequent human host, starting choice for variations with expanded ability to spread in the human populace. As a result, several animal viruses were reported to infect humans with severe consequences such as the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003, Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 (Centers for Disease Control and Prevention, 2013), swine acute diarrhoea syndrome coronavirus (SADS-CoV) in 2016 and now, the 2019 novel human coronavirus (SARS-CoV-2), that has brought about the pandemic.
Be that as it may, these rising infections are understudied in Africa yet should be extensively comprehended to deal with the present pandemic and forestall a future pandemic. This narrative aims to review the factors associated with the emergence of COVID-19, surveillance preparedness and response to pandemics, surveillance and containment at the borders, containment of COVID-19, total lockdown as a measure of containment, and biosafety laboratories in the African continent (Zhong et al., 2003; Plowright et al., 2017; Gallagher et al., 2018; Zhou et al., 2018; Warren and Sawyer, 2019; Lai et al., 2020; WHO, 2020a).
Factors Associated With the Emergence of COVID-19: There are numerous factors associated with the development of new viral diseases or the reappearance of viral infections. A portion of the factors results from standard procedures, for example, the advancements of pathogens after some time. Yet, many are an after effect of human conduct and practices, taking into account how the interaction between the human populace and our environment has changed, particularly in the last century as a result of population development, relocation from rural regions to urban communities, global air travel, destitution, and ecological destruction for economic development and land use. To establish an emerging infection, the infectious agent must enter into a vulnerable host strived and spread readily to another host, causing disease in the new host and sustain its transmission within the population. This is in line with the germ theory of disease transmission that was established by Louis Pasteur.
The expansion of human activities to new geographical areas destroying vegetation and the ecosystem, increases the chances of human contact with animals the potential zoonotic transmission of viruses. However, the genetic make-up of the host and the virus is significant in determining which animal virus will strive and replicate the first human host. This is because animal viruses required minimal mutations in other to jump species. About 70% of emerging human infections are of zoonotic origin, and two-thirds of them are acquired from wildlife. This is because unplanned urbanization leads to the destruction of the animal habitat, which exposes human contact with arthropod vectors of viral infection and animal reservoirs of viral infection. Human and animal interactions are hindering wild animals.
Most notably, in Africa, many animal species population has reduced drastically due to human activities, such as hunting, pastoralization, habitat modification, and bush burning. The significance of every one of these elements rely upon the species, its area and natural surroundings, and population density. This interaction increases human susceptibility to novel viral infection in the absence of immunity against the invading novel viruses leading a pandemic. A more extensive comprehension of how infections advance is currently being discovered by considering host hereditary components accountable for repelling infection intrusion (Pasteur et al., 1879; Broecker and Moelling, 2019; Spyrou et al., 2019; Esser et al., 2019; Tam et al., 2019; McLennan et al., 2019; Otieno et al., 2019; Ingala et al., 2019; Beena and Saikumar, 2019; Warren and Sawyer, 2019; Ramalho-Ortigao and Gubler, 2020; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Surveillance Preparedness and Response to Pandemics: Thoughtfulness over emerging and re-emerging infections have brought about some national, territorial, and global actions to re-establish and improve surveillance and control of transmittable infections. To strengthen surveillance, WHO member states passed a resolution urging all member states to reinforce their surveillances and capacity to detect emerging viral infection and the ability to identify novel viruses causing infectious diseases. The accomplishment of these resolutions relies upon the capacity to acquire data on viral diseases and the readiness to impart this data broadly and globally. This resolution has been made by WHO into the establishment of the Division of Emerging and other Communicable Diseases Surveillance and Control, whose duty is to reinforce national and worldwide limits in the prevention and control of infectious diseases for a useful and timely response.
Africa, as a region, has been characterized by a higher burden of infectious diseases and has the weakest public health structure for surveillance globally, which often results in lots of paperwork, administrative bottleneck, too many instructions, conflicting priorities, and terminologies. There is a need for the use of standard case definitions, streamlined communication, strengthened surveillance, and feedback systems and training and research opportunities to improve the situation. This is the right time for Africa to move towards integrated surveillance of diseases and pandemic preparedness and response (WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Similarly, “One Health” surveillance and emergency response should be integrated into addressing the persistent menace of emerging pandemic threats across Africa. This will offer first-hand prospects in understanding the interface of animal-human, environment, and increasing public health awareness of zoonotic diseases as well as resilience and preparedness strategies. To achieve optimal resource allocation and technical assistance, it is essential to explore emergency outbreak schemes initiative and integrated community health capacity development at all levels to alleviate the menace of future emerging outbreaks in Africa. Pandemic readiness requires exceptional degrees of political and economic related commitment. It is taxing, however realistic. The well-being of Africa, and the world, rely upon us all keeping our responsibilities (WHO, 1947; WHO, 1998; WHO, 2014; Errecaborde et al., 2019; Rivers et al., 2019; Mboussou et al., 2019; WHO, 2020 a).
Surveillance and Containment at the Borders: In several African countries, the quest for emerging viruses keeps many international researchers and their local collaborators busy. In European countries, it has motivated the enactment of preparation strategies like sorting passengers at the airport and drafted guidelines that will test health care response to the pandemic. It is in this light that some African countries recommend a technical solution to fight against emerging viruses by installing a non-contact thermometer that measures temperature remotely, without contact for screening people at the borders. The data obtained are transmitted to the situation room at the terminal to identify febrile individuals that should be quarantined at the airport to protect the public and to the health authorities for epidemiological action. This will enhance the ability to respond to the spread of infective viruses under surveillance. Nonetheless, Africa’s permeable land outskirts stay a reason for worry among health authorities and policy makers, because unchecked movement and transport between countries could spread infections rapidly, as observed with the current COVID-19 hitting hard on African countries with major international airports (Bowen and Laroe 2006; Gold et al., 2019; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020; WHO 2020a).
Containment of COVID-19: In the context of the 2019 coronavirus disease (COVID-19) containment, individuals with risk are highly recommended to be quarantine. Quarantine is defined as separation or restraining of the activities of persons who are exposed to an infectious agent to monitor symptoms and for early detection of cases. Initiating early quarantine measures in an outbreak will help in delaying the introduction of diseases to a country and delay the peak of the epidemic in an environment where local transmission is ongoing. Though quarantine may generate a further source of infection if not properly implemented with regards to the current COVID-19 episode, the worldwide control system incorporates the rapid identification of lab-confirmed cases and their management or isolation at home or in a health care facility (Anderson et al., 2020; WHO, 2020a; WHO, 2020b; Coomes et al., 2020; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
A quarantine facility should be appropriately organized in such a way that it provides adequate ventilation, spacious single rooms, toilets, and hand hygiene facilities. If single rooms are not available, a large room that is adequately ventilated with beds placed two meters apart, with an appropriate level of conforming sound waste management system, is required. Sadly, these standard quarantine measures are hardly available in most of the African nations due to their low socioeconomic status. At the same time, in some countries, there are available resources, which are not adequately channelled because of displacement of priority by the government and lack of political will. At the point when a home isolate is picked, the individual ought to involve an all-around ventilated single room.
If a separate room is beyond the realm of imagination, keep up a separation of two meters from other family individuals, limiting the utilization of shared spaces and cutlery and guaranteeing that common spaces, for example, kitchen and washroom are very much ventilated. In most African countries, home or self-isolate is practically unimaginable, most notably among poor people living in the ghettos and vagrants with no professional stability. This gathering of the individual needs to go out day by day to search for what they will eat and take care of their families on a daily bases; for them, home isolation implies starvation if there is no help from the government as palliative (Sorooshian, 2020; WHO, 2020a; WHO, 2020b; WHO, 2020c).
Total Lockdown as a Measure of Containment: African countries effortlessly duplicated the format of “stay at home” or lockdown orders, as in most Western nations, however, didn’t duplicate the exact circulation of monies to residents. Most African nations don’t have realistic demographic data to recognize and focus on the most vulnerable points, unlike in the western world. In Africa, money is given to individuals who will end up misappropriating it and post photographs to legitimize their spending. The COVID-19 cases continue expanding each day, and if the lockdown is an approach to lessen or end the spread of the virus, at that point from the outcomes, it’s not working and counterproductive indeed as cases proceed to double. People continue to stay at home without a wellspring of income and the necessities of life. So also, for those with little reserve funds, it will soon be depleted, and individuals will be compelled to come out to hustle for sustenance (Alfani et al., 2019; Hamelin et al., 2020; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Africans have had a high degree of poverty and earning, even without COVID-19. Presently, this equitable tosses Africa into more hunger and will soon lead to individuals having more medical problems results from malnutrition and hunger. In typical circumstances, thousands pass on consistently in Africa because of different sicknesses and transmittable infections, for example, cholera, jungle fever, Lassa fever, tuberculosis, Zika infection, measles, smallpox, and so forth. The lockdown intensified these ailments as most of the casualties now have almost no money to take care of themselves. The common man considers this as an elite problem because, to them, the “hunger Virus” is more terrifying than SARS-CoV-2.
The more significant part of the proprietor of micro, small and medium enterprises are probably going to devour their business capital during the lockdowns, with no reasonable helpline. This is because the palliative measure taking by some African countries won’t almost certainly arrive at the targeted population because of dishonesty and fraud. Horticulture in Africa depends on downpour and season. The lockdowns during the planting season could undermine food security. This will have adverse effects on the farmers and the consumers, along with the increased inflation (Alfani et al., 2019; Hamelin et al., 2020; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Biosafety Laboratories in African:At the point when we discuss containment laboratory, two things ring a bell; biosafety and biosecurity. Biosafety is a containment guideline, expertise, and practice that is executed to forestall unexpected exposure to biological agents and poisons or their negligent discharge. At the same time, biosecurity is the protection, control of organic agents and poisons inside the research lab, to forestall their misfortune, abuse, re-routing, unapproved access, or purposeful unapproved discharge. All tasks performed in bio-labs are classified by their biosafety levels. Biosafety levels (BSL) are utilized to recognize the protective measures required in a lab setting to ensure the protection of employees, the surrounding environment, and the general public. There are four biosafety levels. These are BSL-1, BSL-2, BSL-3, and BSL-4, with BSL-4 being the maximum containment level of all the BSL (Gronvall and Bouri, 2008; Gómez-Tatay and Hernández-Andreu, 2019; Iwen et al., 2020; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
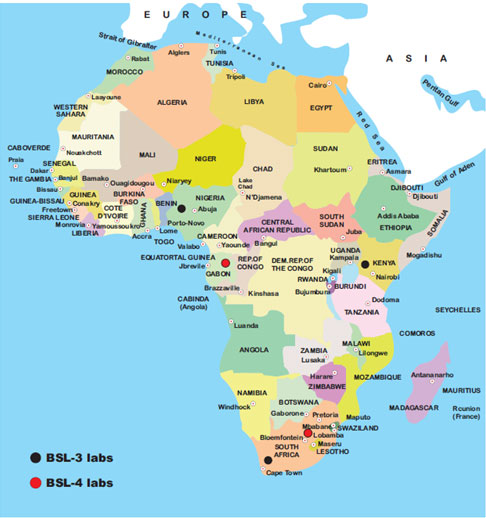
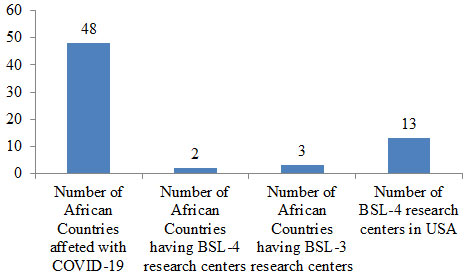
Level 4 BSL is used for studying high-risk infectious agents that are capable of aerosol transmission and life-threatening infection with no available vaccine-like SARS-CoV-2 causing the current COVID-19 pandemic. In Africa, there are just two BSL-4 research centers situated in Gabon and South Africa, and only three African nations have standard BSL-3 labs situated in Nigeria, Kenya, and South Africa (Figure 1). Contrary to this, the United States has 13 operational BSL-4 labs (Bressler and Hawley, 2006; Ahmad et al., 2015; Carpenter and Bhadelia, 2019; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Figure 1: Standard biosafety, level 3, and level 4 laboratories in the African continent
Figure 2: Number of BSL centers in Africa and the USA
It is because of the inadequacies of BSL 3 and 4 in Africa that stimulate WHO to come of African Emerging and Dangerous Pathogen lab Network (AFR-EDPLN) to provide diagnostic services for an array of emerging pathogens. The objective of the AFR-EDPLN is to improve readiness and response to Emerging and Dangerous Pathogens (EDP) by upgrading diagnostic abilities and giving better access to a scope of tests for EDP, encouraging increasingly quick reaction and improved outbreak control process. The EDP system, as of now, involved 14 national consisting of Uganda, South Africa, Sierra Leone, Senegal, Nigeria, Madagascar, Kenya, Ghana, Gabon, Democratic Republic of Congo, Côte d’Ivoire, Central African Republic, Cameroon, and Algeria. In any case, there is a requirement for a powerful, dependable and versatile system of research centers with the ability to distinguish emerging and re-emerging infections so that Africa is more ready to identify and react to future vulnerabilities to regional health security and reinforce the African ability to contain emerging and re-emerging infections (Boeras et al., 2016; WHO, 2017; Balajee et al., 2016; WHO, 2020a; Martinez-Alvarez et al., 2020; Chinazzi et al., 2020).
Most infections spread is attributable to improper hygiene and protective measures, for example, hand-washing, cleaning, and safe entombment practices. It is, therefore, critical to keeping up essential cleanliness and protective conduct, for example, social distancing. With fewer Biosafety Level 3 and 4 research facilities in African countries, it is essential to build up more labs in Africa or increases the number of regional labs. It will likewise be astute to set up an emergency response team or unit in every African country, having all the vital training and transparent rules for handling emergencies, which can act following an outbreak in collaboration with regional BSL-4 lab. African scientists and clinicians should be provided with training opportunities on biosafety and biosecurity as well as the standard containment principle in managing pandemic.
There is also a need to establish isolation units in specialized hospitals, along with the necessary infrastructure and protocols to monitor infected patients. There should be substantial funding allocated by governmental and non-governmental organizations to improve primary health care infrastructure in African countries to ensure that emergency medical situations are appropriately tackled. The large scale advertisement and education are also highly recommended. Finally, the African countries may also ask support from other countries with successful experience against COVID-19. The implementation of the suggested strategies will be helpful to African countries against COVID-19.
CONCLUSION
In conclusion, the large scale advertisement and conduction of the COVID-19 educational programs are highly recommended in African countries. They lack appropriate numbers of biosafety level 3 and 4 research facilities, trained personnel/emergency units, and funding resources to combat COVID-19 and similar pandemic. It is advisable to build up more biosafety research facilities, trained emergency response units, isolation units, and substantial funding agencies in every African country with clear rules to combat outbreaks like COVID-19.
Conflict of Interest: The authors declare that there is no conflict of interest.
Funding:None.
Authors’ Contribution:All listed author(s) have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Ethics Statement: This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
Ahmad, A., Ashraf, S., & Komai, S. (2015). Are developing countries prepared to face Ebola-like outbreaks?. Virologica Sinica, 30(3), 234-237. https://doi.org/10.1007/s12250-015-3564-9.
Alfani, F., Dabalen, A., & Fisker, P. et al. (2019). Vulnerability to stunting in the West African Sahel. Food Policy, 83, 39-47. https://doi.org/10.1016/j.foodpol.2018.11.002.
Anderson, R.M., Heesterbeek, H., & Klinkenberg, D. et al. (2020). How will country-based mitigation measures influence the course of the COVID-19 epidemic?. Lancet (London, England), 395(10228), 931-934. https://doi.org/10.1016/S0140-6736(20)30567-5.
arpenter, S., & Bhadelia, N. (2019). Maximum Containment Infectious Disease Laboratories as an Integral Part of Emergency Preparedness and Emergency Response. Defense Against Biological Attacks: Springer; 125-44.
Balajee, S.A., Arthur, R., & Mounts, A.W. (2016). Global Health Security: Building Capacities for Early Event Detection, Epidemiologic Workforce, and Laboratory Response. Health Security, 14(6), 424-432. https://doi.org/10.1089/hs.2015.0062.
Beena, V., & Saikumar, G. (2019). Emerging horizon for bat borne viral zoonoses. Virus Disease, 30(3), 321-328. https://doi.org/10.1007/s13337-019-00548-z.
Boeras, D.I., Peeling, R.W., & Onyebujoh, P. et al. (2016). The WHO AFRO external quality assessment programme (EQAP): Linking laboratory networks through EQA programmes. African Journal of Laboratory Medicine, 5(2), 560. https://doi.org/10.4102/ajlm.v5i2.560.
Bowen, J.T., & Laroe, C. (2006). Airline networks and the international diffusion of severe acute respiratory syndrome (SARS). The Geographical Journal, 172(2), 130–144. https://doi.org/10.1111/j.1475-4959.2006.00196.x.
Bressler, D.S., & Hawley, R.J. (2006). Safety Considerations in the Biosafety Level 4 Maximum-Containment Laboratory. Biological Safety . American Society of Microbiology; pp. 487-508.
Broecker, F., & Moelling, K. (2019). Evolution of Immune Systems From Viruses and Transposable Elements. Frontiers in Microbiology, 10, 51. https://doi.org/10.3389/fmicb.2019.00051.
Centers for Disease Control and Prevention (CDC) (2013). Updated information on the epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection and guidance for the public, clinicians, and public health authorities, 2012-2013. MMWR. Morbidity and Mortality Weekly Report, 62(38), 793–796.
Chinazzi, M., Davis, J.T., & Ajelli, M. et al. (2020). The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science (New York, N.Y.), 368(6489), 395–400. https://doi.org/10.1126/science.aba9757.
Coomes, E.A., Leis, J.A., & Gold, W.L. (2020). Quarantine. CMAJ : Canadian Medical Association Journal, 192(13), E338. https://doi.org/10.1503/cmaj.200393.
Davis, B.M., Foxman, B., & Monto, A.S. et al. (2018). Human coronaviruses and other respiratory infections in young adults on a university campus: Prevalence, symptoms, and shedding. Influenza and Other Respiratory Viruses, 12(5), 582–590. https://doi.org/10.1111/irv.12563.
Errecaborde, K.M., Macy, K.W., & Pekol, A. et al. (2019). Factors that enable effective One Health collaborations – A scoping review of the literature. PloS One, 14(12), e0224660. https://doi.org/10.1371/journal.pone.0224660.
Esser, H.J., Mögling, R., & Cleton, N.B. et al. (2019). Risk factors associated with sustained circulation of six zoonotic arboviruses: a systematic review for selection of surveillance sites in non-endemic areas. Parasites & Vectors, 12(1), 265. https://doi.org/10.1186/s13071-019-3515-7.
Gallagher, M.E., Brooke, C.B., & Ke, R. et al. (2018). Causes and Consequences of Spatial Within-Host Viral Spread. Viruses, 10(11), 627. https://doi.org/10.3390/v10110627.
Gold, L., Balal, E., & Horak, T. et al. (2019). Health screening strategies for international air travelers during an epidemic or pandemic. Journal of Air Transport Management, 75, 27–38. https://doi.org/10.1016/j.jairtraman.2018.11.006.
Gómez-Tatay, L., & Hernández-Andreu, J.M. (2019). Biosafety and biosecurity in Synthetic Biology: A review. Critical Reviews in Environmental Science and Technology, 49(17), 1587-1621. https://doi.org/10.1080/10643389.2019.1579628.
Gronvall, G.K., & Bouri, N. (2008). Biosafety laboratories. Biosecurity and Bioterrorism : Biodefense Strategy, Practice, and Science, 6(4), 299–307. https://doi.org/10.1089/bsp.2008.1126.
Guan, Y., Zheng, B.J., & He, Y.Q. et al. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science (New York, N.Y.), 302(5643), 276–278. https://doi.org/10.1126/science.1087139.
Hamelin, N., Nwankwo, S., & Gbadamosi, A. (2020). Social marketing and the corruption conundrum in Morocco: An exploratory analysis. World Development, 133, 104993. https://doi.org/10.1016/j.worlddev.2020.104993.
Ingala, M.R., Becker, D.J., & Bak Holm, J. et al. (2019). Habitat fragmentation is associated with dietary shifts and microbiota variability in common vampire bats. Ecology and Evolution, 9(11), 6508–6523. https://doi.org/10.1002/ece3.5228.
Iwen, P.C., Stiles, K.L., & Pentella, M.A. (2020). Safety Considerations in the Laboratory Testing of Specimens Suspected or Known to Contain the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). American Journal of Clinical Pathology, 153(5), 567–570. https://doi.org/10.1093/ajcp/aqaa047.
Jiang, L., Lee, V.J., & Cui, L. et al. (2017). Detection of viral respiratory pathogens in mild and severe acute respiratory infections in Singapore. Scientific Reports, 7, 42963. https://doi.org/10.1038/srep42963.
Lai, C.C., Shih, T.P., & Ko, W.C. et al. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents, 55(3), 105924. https://doi.org/10.1016/j.ijantimicag.2020.105924.
Luo, G.G., & Gao, S.J. (2020). Global health concerns stirred by emerging viral infections. Journal of Medical Virology, 92(4), 399–400. https://doi.org/10.1002/jmv.25683.
Martinez-Alvarez, M., Jarde, A., & Usuf, E. et al. (2020). COVID-19 pandemic in west Africa. The Lancet. Global Health, 8(5), e631–e632. https://doi.org/10.1016/S2214-109X(20)30123-6.
Mboussou, F., Ndumbi, P., & Ngom, R. et al. (2019). Infectious disease outbreaks in the African region: overview of events reported to the World Health Organization in 2018. Epidemiology and Infection, 147, e299. https://doi.org/10.1017/S0950268819001912.
McLennan, M.R., Howell, C.P., & Bardi, M. et al. (2019). Are human-dominated landscapes stressful for wild chimpanzees (Pan troglodytes)? Biological Conservation, 233, 73-82. https://doi.org/10.1016/j.biocon.2019.02.028.
Otieno, T.O., Goheen, J.R., & Webala, P.W. et al. (2019). Human-and risk-mediated browsing pressure by sympatric antelope in an African savanna. Biological Conservation, 232, 59-65. https://doi.org/10.1016/j.biocon.2019.01.028.
Pasteur, Joubert, and Chamberland on the Germ Theory. (1879). Edinburgh Medical Journal, 25(3), 265–268.
Plowright, R.K., Parrish, C.R., & McCallum, H. (2017). Pathways to zoonotic spillover. Nature Reviews. Microbiology, 15(8), 502–510. https://doi.org/10.1038/nrmicro.2017.45.
Ramalho-Ortigao, M., & Gubler, D.J. (2020). Human Diseases Associated With Vectors (Arthropods in Disease Transmission). Hunter’s Tropical Medicine and Emerging Infectious Diseases: Elsevier; pp. 1063-9.
Rivers, C., Chretien, J.P., & Riley, S. et al. (2019). Using “outbreak science” to strengthen the use of models during epidemics. Nature Communications, 10(1), 3102. https://doi.org/10.1038/s41467-019-11067-2.
Sorooshian, S. (2020). Quarantine Decision due to Coronavirus Pandemic. Electronic Journal of General Medicine, 17(4), em206. https://doi.org/10.29333/ejgm/7862.
Spyrou, M.A., Bos, K.I., & Herbig, A. et al. (2019). Ancient pathogen genomics as an emerging tool for infectious disease research. Nature Reviews. Genetics, 20(6), 323–340. https://doi.org/10.1038/s41576-019-0119-1.
Tam, V., Turcotte, M., & Meyre, D. (2019). Established and emerging strategies to crack the genetic code of obesity. Obesity Reviews : An Official Journal of the International Association for the Study of Obesity, 20(2), 212–240. https://doi.org/10.1111/obr.12770.
Vijaykrishna, D., Smith, G.J., & Zhang, J.X. et al. (2007). Evolutionary insights into the ecology of coronaviruses. Journal of Virology, 81(8), 4012–4020. https://doi.org/10.1128/JVI.02605-06.
Vojdani, A., Vojdani, E., & Vojdani, C. (2020). The Immune System: Our Body’s Homeland Security Against Disease. Integrative and Functional Medical Nutrition Therapy: Springer; pp. 285-302.
Warren, C.J., & Sawyer, S.L. (2019). How host genetics dictates successful viral zoonosis. PLoS Biology, 17(4), e3000217. https://doi.org/10.1371/journal.pbio.3000217.
Weber, D.J., Sickbert-Bennett, E.E., & Kanamori, H. et al. (2019). New and emerging infectious diseases (Ebola, Middle Eastern respiratory syndrome coronavirus, carbapenem-resistant Enterobacteriaceae, Candida auris): Focus on environmental survival and germicide susceptibility. American Journal of Infection Control, 47S, A29–A38. https://doi.org/10.1016/j.ajic.2019.03.004.
WHO. (1947). CONSTITUTION of the World Health Organization. Chronicle of the World Health Organization, 1(1-2), 29–43.
WHO. (1998). Disease surveillance–WHO’s role. Releve Epidemiologique Hebdomadaire, 73(43), 333–334.
WHO. (2014). Service availability and readiness assessment (SARA): an annual monitoring system for service delivery: reference manual. World Health Organization, https://www.who.int/healthinfo/systems/sara_introduction/en/[accessed 02 April 2020]
WHO. (2017). Health Emergencies Programme in the African Region: Annual Report 2016. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/. [accessed 14 May 2020].
WHO. (2020a). https://www.afro.who.int/health-topics/coronavirus-covid-19 [Last accessed on August 22,].
WHO. (2020b). Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID-19), Interim guidance, 29 February 2020. https://www.who.int/publications-detail/considerations-for-quarantine-of-individuals-in-the-context-of-containment-for-coronavirus-disease-(covid-19) [accessed 10 April 2020]
WHO. (2020c). Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID-19). Website https://www. who. int/publications-detail/considerations-forquarantine-of-individuals-in-the-context-of-containment-forcoronavirus-disease-(covid-19) [accessed 12 April 2020].
Zhong, N.S., Zheng, B.J., & Li, Y.M. et al. (2003). Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet (London, England), 362(9393), 1353–1358. https://doi.org/10.1016/s0140-6736(03)14630-2.
Zhou, P., Fan, H., & Lan, T. et al. (2018). Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature, 556(7700), 255–258. https://doi.org/10.1038/s41586-018-0010-9.