1Department of Chemistry, Sri Aurobindo College, University of Delhi, New Delhi-110017, India.
2School of Life Sciences, Jawaharlal Nehru University, New Delhi-110067,India
Corresponding author email: rangnathravi@gmail.com
Article Publishing History
Received:
Accepted After Revision:
Multimetallic nanoparticles (NPs) have extraordinary properties and therefore, drew the attention regarding their synthesis and applications in the form of bi and tri metallic nanoparticles. Bimetallic (BNPs) and trimetallic nanoparticles (TNPs) are gaining enormous attention than that of monometallic nanoparticles. Both NPs can be synthesized by different methods such as microwave, selective catalytic reduction, micro-emulsion, co-precipitation and hydrothermal etc. Using physical and chemical methods have more disadvantages such as production of toxic byproduct, use of excess energy and additional use of stabilizer. In addition, nanocomposites of bimetallic and trimetallic can be synthesized with inorganic and organic compounds such as: carbon, graphene, gelatin, cellulose, starch, chitosan, alginate, etc. The combination of two or more phases in these nanoscale materials provide them high surface area to volume ratio and possess higher degree of porosity that help in enhancing their adsorption and reusability found more helpful in removing the toxic pollutants from the environment.
Further these nanomaterials can also be fabricated in such a way that reduces the electron hole recombination, which induces synergetic effect between the constituent moieties that help in the degradation of pollutants. For instance the synthesis of trimetallic nanostructures with defined design along with the required morphology as well as mesoporous and magnetic characteristics have shown their versatile properties find applications in many industries such as conducting magnetic inks, memory devices, catalysis, bio-medical and especially in water treatment. Although, to obtain the nanoparticles with desired morphology and size is relatively difficult, which involves expensive non-eco-friendly reagents. In this review, we discussed in detail about the synthesis and role of Bimetallic and Trimetallic NPs as an adsorbent.
Nanoparticles, Bimetallic, Trimetallic, Waste Water treatment and Adsorption
Ravi R, Mishra A. Preparation of Bimetallic and Trimetallic Nanomaterials and their Role in Waste Water Treatment: A Review. Biosc.Biotech.Res.Comm. 2020;13(3).
Ravi R, Mishra A. Preparation of Bimetallic and Trimetallic Nanomaterials and their Role in Waste Water Treatment: A Review. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2HehSGB
Copyright © Raviand Mishra This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The significance of nanotechnology and nanoscience has been mainly associated with fabrication, characterization and applications of nanoscale materials in the form of nanorods, nanotubes, nanoparticles, nanosheets and nanoporous structures. They have been developed through the association of atomic and molecular clusters (Pitkethly 2004). Nanoparticles (NPs) may be defined as they possess at least one of the dimensions of nanosize. They are lying in the nanoregime i.e. in between 1nm to 100nm range (Jain et al., 2006). They act as the bridge between the bulk and their atomic structures (Luo et al.,2006). Bulk materials exhibit regular physiochemical properties regardless of their size. However, when the same materials acquire the nano-size, they start to show highly enhanced useful physico-chemical, electronic, electrical, magnetic, optical, catalytic properties etc. as compared to their conventional counterparts (Jiang et al., 2008).This can be attributed to the high surface area to volume ratio (Schrand et al., 2010). Because of these characteristic properties NPs have shown tremendous potential for their applications in the field of engineering, environmental, biological sciences as well as in biotechnology (Mishra et al.,2015). NPs also play a pivotal role in many catalytic and biochemical processes (Guildford et al. 2009, Wang et al. 2019).
The concept of nanoparticle and their application in biological systems also has advantages over other materials because their size is very much close to the size of cellular components (Yang et al. 2019). For instance, the size of a DNA molecule is about 2.5 nm, thickness of biological membrane is 6 nm and a protein is approximately 50 nm wide (Liu et al. 2018). Besides this, NPs plays significant role to analyze toxic dye removal from industrial wastewater (Leite et al. 2018, Sonkusare et al. 2020).
The NPs classification, synthetic procedures and their applications in dye removal and cancer treatment are discussed in the proceeding units.
Classification of NPs: Based on structure, morphology and size, the NPs of various shapes are presented in fig 1 a-b and further discussed as follows.
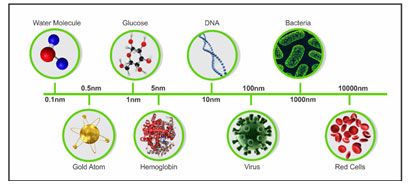
Figure 1a: Comparison of size in nanoparticles, (Brar et al., 2010)
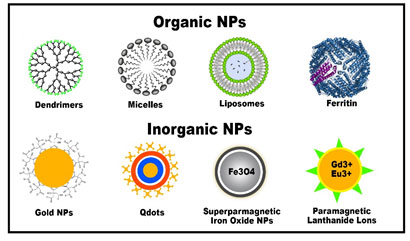
Figure 1b: Examples of nanoparticles based on constituents and structures, (Silva et al., 2019)
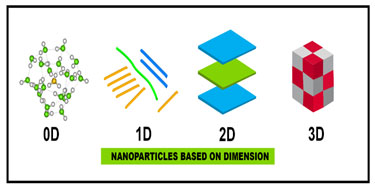
Based on dimensions:Based on their dimension and aspect ratio, NPs can be classified into four classes. The general characteristics of these nanoparticles are discussed in Table1.1, covering their definitions and area of applications, while their two and three dimensional structures are given in Fig. 2.
Table 1.1. Types of NPs, their definition along with examples and their uses.
| Types of NPs | Definition | Examples | Uses | Ref |
| Zero Dimension (0D) | NPs having each of three dimensions limited in the nanoscale. | Fullerenes
Composite NPs Core Shell NPs |
In various biomedical applications | (Barnakov et al. 2019) |
| One Dimension (1D) | NPs have two dimensions in the nanoscale. | Nanotubes
Nanorods |
Energy harvesting, Storage efficiency | (Linfeng et al. 2019) |
| Two dimensional (2D) | NPs having one dimension in nanoscale | Nano films, Nanosheets | Biochemical sensors, Catalysis | (Yola et al. 2018) |
| Three dimensional (3D) | NPs which no dimension are confined to the nanoscale | Bulk powders,
Nanowire bundles |
Biomedical | (Yang et al. 2019) |
Figure 2: Nanoparticles based on dimensions (Pal et al., 2011)
Based on uniformity:NPs can be classified in the forms of their states such as distributed aerosols, nanoclusters, colloidal solution and suspension (Kumar et al., 2018). These behaviors are based on the notion of dispersed phase and nature of dispersion medium along with their chemical and electromagnetic properties. Effectively, slightly loaded particles and magnetic particles display aggregation unless some stabilizing agent, like polymers are added and covers their surface. Agglomerated NPs act as macromolecules and loses the specific characteristics of NPs. NPs like nanocubes and spherical NPs are isometric due to their equal sizes (Adamiano et al., 2018, Saleh et al., 2020). There are some anisometric NPs like nano-stars, nanorods and nano-plates etc (Bansal et al., 2020).
Metal Oxides NPs : For the last several decades, metal oxides have gained a considerable interest for the maximum numbers of nanomaterials due to their different promising constituents and versatile nature. Metal oxides are most easily available, fast and effective materials for their application in water treatment without the involvement of undesirable by-products. It can also be applied for many other applications including biomedical sciences because of their nontoxic and anti-microbial behavior. They can provide the oxygenated sites for the surface complexation with foreign elements thus can be most suitable for water remediation technology. Moreover, metal oxides due to their high surface area are expected to be more suitable for water treatment under the water quality constraints. For this, the separation of adsorbent and post adsorption is necessary; therefore, the use of magnetic metal oxide NPs in the field of water treatment technology has more pronouncedly emerged, with these characteristics the metal oxide NPs are further classified as follows:
Monometallic NPs: As the name suggests monometallic NPs (MNPs) consist of single metal atoms, which alone determines the properties of these NPs. They can be prepared by many methods, out of which chemical method is the most common. From the past few decades, MNPs have attained greater interest owing to their enhanced physical and chemical properties (Pantidos et al., 2014). The most important examples of monometallic oxides are: Iron oxide (FeO, Fe2O3 and Fe3O4), titanium oxide (TiO2), aluminium oxide (Al2O3), zirconium oxide (ZrO2), manganese dioxide (MnO, MnO2 and Mn2O3), copper oxide (CuO and Cu2O) and zinc oxide (ZnO). These NPs have been widely used for several applications such as in electronic, dye adsorption (Zhang et al., 2016), catalysis ( Gawande et al., 2016), optical (Maruthupandy et al., 2017), and as antimicrobial agents against a few microorganisms such as Escherichia coli (Ribeiro et al., 2018) and Streptococcus mutans (Ramar et al., 2015, Lima et al., 2020).
Bimetallic NPs and Trimetallic NPs: Bimetallic nanoparticle (BNPs) are the mixture of two different metals. BNPs can be formulated by using two inorganic materials in order to enhance the desired properties, which cannot be achieved by single metal atom. Furthermore, by the virtue of tiny size and greater volume to surface area ratio, these are significantly used in adsorption of various dyes for water purification, anticancer properties, catalyst etc. (Nasrabadi et al., 2016, Sharma et al., 2017).
In addition to magnetic adsorptive property, adsorbent used in removal of pollutants like arsenite As(III), preoxidation of As(III) to As(V) is also necessary, which can be achieved by the doping of oxidants like chlorine. However, they can also increase the risk of the formation of un-healthy by-products by reacts with natural organic matter present in water. Trimetallic NPs (TNPs) are the blend of three different metals and have advantage over MNPs. The volume to surface area ratio of TNPs is reasonably unstable, which can be stabilized by using different stabilizers such as organic ligands and surfactants leads (Martinez et al., 2018). TNPs as well as BNPs have acquired more interest than the MNPs in terms of scientific and technological point of view (Ravi et al., 2019). The properties of BNPs and TNPs can be same or differ from the pure elemental particles and may acquire unique size, and extra optical, electronic, thermal and catalytic properties (Sharma et al., 2017, Ali et al., 2020).
In past few years, extensive studies in the field of BNPs and TNPs have recorded.
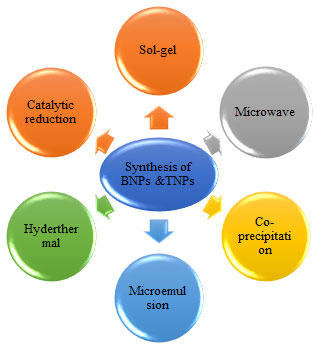
Preparation of Bimetallic and Trimetallic NPs: BNPs and TNPs can be synthesized by various important methods such as sol-gel, microwave radiation, co-precipitation, catalytic reduction and hydrothermal etc. These methods are important to prepare nanoparticles of different size, shape and composition. Some of the methods used for the synthesis of the nanoparticles are discussed below (Table 1.2 and Fig.3).
Figure 3: Different approaches of synthesis for Bimetallic and Trimetallic NPs.
Table 1.2. Fabrication, mode of synthesis and significant applications of some important inorganic and organic nanoparticles.
| Sr. No. | Inorganic/organic NPs | Mode of synthesis | Significant applications | Ref. |
| 1 | Titanium dioxide | Sol gel, Hydrothermal, sonochemical, solvothermal, reverse micelles |
Photo-catalysis, antimicrobial applications, gas and humid sensor, sunscreen products, wastewater treatment etc. | (Morshed et al., 2018, Baranowska-Wójcik et al., 2020) |
| 2 | Zinc oxide | Homogeneous precipitation, microwave method, thermal evaporation, Sol–gel, and chemical synthesis |
Gas and humid sensor, photocatalytic degradation of toxic pollutants from wastewater, skin care products, biomedical applications such as anticancer, antifungal and antibacterial etc. | (Rajabi et al., 2017) |
| 3 | Aluminium oxide | Sol–gel, flame spray pyrolysis, reverse micro emulsion | Removal of heavy metal ions from waste water treatment, antimicrobial applications, separation chamber, catalysis, biomedical applications etc. |
(Su et al. 2018) |
| 4 | Silica | Flame synthesis, sol-gel, micro emulsion | Gene and drug delivery, biosensor, enzyme immobilization etc. | (Sodipo et al.2016) |
| 5 | Magnetic | Sol–gel, Co-precipitation, solvothermal, and sonochemical method |
Bio-separation, dye and arsenic removal from wastewater, MRI, Immobilization of enzymes | (Srikar et al.2016) |
| 6 | Silver | Chemical reduction, photochemical method, Microwave and gamma irradiation, Biological synthesis by plant extract, enzymes, carbohydrate etc. | Antimicrobial, anticancer, catalysis, biosensor, water purification | (Natsuki et al.2015) |
| 7 | Gold | Reduction by chemicals, photochemical reaction, microwave irradiation | Biosensors, catalysis, drug delivery, anticancer etc. | (De souza et al. 2019, Priyadarshini et al. 2017) |
| 8 | Starch | Acid Hydrolysis, Ultrasonication, Gamma Irradiation | Drug carrier, wastewater treatment, tire making, fat replacers and emulsion stabilizers | (Kim et al. 2016) |
| 9 | Chitosan | Ionic gelation method, Reverse micelles method | Delivery system for vaccines, prevent infection in wounds and wound-healing process by enhancing the growth of skin cells, antibacterial agent | (Chandra et al. 2016) |
Applications of Bimetallic and Trimetallic nanoparticles: NPs because of their versatile properties and many folds enhanced chemical, catalytic, structural, magnetic and electrical characteristics, they find wide applications in the field of electronic, optical, energy, biological, medicinal and environmental industries (Fig.4). Among these applications, our review discusses the use of these BNPs and TNPs in the field of environmental pollution (water treatment) and nanomedicine for the treatment of lethal diseases, (Chen et al. 2016).
Figure 4: Various applications of metallic NPs.
Application of BNPs and TNPs in Water treatment: Industrial wastewater is the major source of water pollution. As per the WHO report (WHO), thousands of pollutants are present in environment in the form of air and water pollutants, some of these pollutants have severe thread for living organisms specially for aquatic systems, besides these pollutants are also responsible for various types of deadly diseases like cancer. Globally 9.6 million deaths per annum due to cancer in 2018 (WHO 2018). The alarming death rate due to cancer has become a worldwide challenge to the bio scientists and physicians. Literature reports that the toxic dyes used in textile industries is one of the main sources of pollutants causing cancer and danger for aquatic life (Mishra et al. 2015). The effects of textile dyes on the wealth of society and aquatic systems have been shown in Fig. 5 (Sha et al. 2016). Thus, the water pollution acts as the biggest challenge to the mankind (Carolin et al. 2017).
Adsorption technique is found to be more suitable, simple and cost effective, which has been efficiently used for the removal of wide range of water pollutants (Lata et al. 2016). These adsorbents maybe of organic (Cellulose, organic fibers, agricultural wastes and their fibers etc.) and inorganic (sands, metallic ferrites, metal sulphides, oxides etc.) nature (Soltani et al. 2015). Among these various types of magnetic metal ferrites have been successfully used for the removal of toxic dyes and other pollutants due to their easy reusability high absorbance capacity and more simple mode of application and rechargeability (Chang et al.,2020). The use of various types of metal NPs as adsorbents for the removal of different types of dyes are highlighted in Table 1.3.
Figure 5: Pictorial representation of the effect of textile dyes in health and aquatic system.
Moreover, the literature on the application of various types of NPs in removal of dyes is highlighted in Table 1.3.
Table 1.3. List of adsorbents used against the removal of various dyes
| S.N. | Adsorbent | Dyes | Ref. |
| 1 | Fe–Ni bimetallic NPs | Reactive Blue 21 | (Kale et al. 2019) |
| 2 | ZnO NPs | Methyl orange | (Zafar et al. 2019) |
| Amaranth | |||
| 3 | Partially oxidized graphite nanoparticles (POG-NPs) | Congo red | (Mahmoud et al. 2019) |
| Malachite green | |||
| 4 | Fe3O4@SiO2@PIL nanocomposite | Acid orange II | (Yang et al. 2019) |
| Thionin acetate | |||
| 5 | ZnO | Malachite Green | (Zhang et al. 2019) |
| Congo Red | |||
| 6 | FexCo3-xO4 NPs | Congo Red | (Liu et al. 2019) |
| 7 | Ni-Ag bimetallic NPs | Sunset Yellow | (Mirzajani et al. 2019) |
| Tartrazine | |||
| 8 | Agar@Fe/Pd Bimetallic NPs | Methylene blue | (Patra et al. 2019) |
| Rhodamine B | |||
| 9 | ZnO NPs stabilized on MWCNTs | Reactive blue 203 | (Bagheri et al. 2019) |
| 10 | Ag-NPs using Albizia procera leaf extract | Methylene blue | (Rafique et al. 2019) |
| 11 | Fe3O4@Tb/AMP core-shell NPs | Alizarin Red | (Huang et al. 2018) |
| Congo Red | |||
| 12 | CeO2 NPs | Reactive Green 19 | (Sane et al. 2018) |
| Reactive Orange 84 | |||
| Reactive violet 1 | |||
| Reactive Yellow 81 | |||
| 13 | WOx NPs | Rhodamine B | (Ying et al. 2018) |
| 14 | Alginate/γ-Fe2O3 | Methylene Blue | (Talbot et al. 2018) |
| 15 | ZnO NPs using alginate | Methylene Blue | (Tamer et al. 2018) |
| 16 | Iron oxide NPs | Reactive Black 5 | (Chang et al. 2018) |
| 17 | Nickel ferrite | Methyl orange | (Moghaddam et al. 2018) |
| Congo red | |||
| 18 | carbon dots/ZnFe2O4 (CDs/ZFO) | Methyl orange | (Shi et al. 2018) |
| 19 | Zr-based magnetic Metal-Organic Frameworks composites | Methylene Blue | (Huang et al. 2018) |
| 20 | SrFe2O4 | Erichrome black T | (Zafar et al. 2018) |
| Methylene blue | |||
| 21 | ZnO | Congo red | (Kataria et al. 2017) |
| Brilliant green | |||
| 22 | ZnO loaded activated carbon | Orange G | (Nasrollahzadeh et al. 2018) |
| Rhodamine B | |||
| 23 | NiFe2O4@AlMCM-41-Cu2O | Methylene blue | (Sohrabnezhad et al. 2017) |
| 24 | MnFe2O4/diatomite nanocomposite | Methylene blue | (Sun et al. 2017) |
| 25 | Ag NPs | Methylene blue | (Saha et al. 2017) |
Future Applications: Bimetallic and trimetallic are very promising nanomaterials for waste water treatment as compared to other types of NPs. To get the best result further experiments and research should be carried out. Further, these NPs could be used for other applications including biomedical application and catalysis.
CONCLUSION
Bimetallic and Trimetallic nanoparticles are more important than that of the monometallic nanoaprticles. The bimetallic and trimetallic nanoparticles are synthesized by various method such as sol-gel, microemulsion, sputtering, co-precipitation etc. Different shape and size of the BNPs and TNPs can be obtained by the various methods. Bimetallic and Trimetallic NPs are used as an excellent adsorbent due to their high adsorbing capacity and shown outstanding results for reusability purpose.
ACKNOWLEDGMENTS
Authors are thankful to Department of Chemistry, Sri Aurobindo College, University of Delhi and Jawaharlal Nehru University, New Delhi India for providing the facilities.
REFERENCES
Adamiano, A., I. Michele, M. Sandri, M. Basini, P. Arosio, T. Canu, G. Sitia et al. (2018) On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 73, 458-469.
Ali, S., Sharma, A. S., Ahmad, W., Zareef, M., Hassan, M. M., Viswadevarayalu, A., Chen, Q. (2020). Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Critical Reviews in Analytical Chemistry, 1-28.
Bagheri, M., N.R. Najafabadi, and E. Borna (2019) Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J. King Saud Uni., Sci.
Bansal, S. A., Kumar, V., Karimi, J., Singh, A. P., & Kumar, S. (2020). Role of gold nanoparticles in advanced biomedical applications. Nanoscale Advances.
Barnakov, Y. A., U. I. Ighodalo, A.B. Sergey, A.T. Trevor, and D.R. Evans (2019) Uncovering the mystery of ferroelectricity in zero dimensional nanoparticles. Nanoscale Adv., 1(2) 664-670.
Baranowska-Wójcik, E., Szwajgier, D., Oleszczuk, P., Winiarska-Mieczan, A. (2020). Effects of titanium dioxide nanoparticles exposure on human health—A review. Biological Trace Element Research, 193(1), 118-129.
Brar, S. K., Verma, M., Tyagi, R. D., & Surampalli, R. Y. (2010). Engineered nanoparticles in wastewater and wastewater sludge–Evidence and impacts. Waste management, 30(3), 504-520.
Carolin, C. F., P. S. Kumar, A. Saravanan, G. J. Joshiba, and Mu Naushad (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng., 3, 2782-2799.
Chandra, H., Krushna, S. Prabha, R. Chandra, B. Ahmed, and S. Nimesh (2016) Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artificial cells, Nanomed., Biotechnol., 44, 305-314.
Chang, M., and Y.H. Shih (2018) Synthesis and application of magnetic iron oxide nanoparticles on the removal of Reactive Black 5: Reaction mechanism, temperature and pH effects. J. Environ. Manag., 224, 235-242.
Chang, S., Zhang, Q., Lu, Y., Wu, S., Wang, W. (2020). High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Separation and Purification Technology, 238, 116400.
Chen, G., I. Roy, C. Yang, and P. N. Prasad (2016) Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev., 116, 2826-2885.
Cruz-Martínez, H M.M. Tellez-Cruz, H. Rojas-Chávez, C.A. Ramírez-Herrera, et al., (2018) NiPdPt trimetallic nanoparticles as efficient electrocatalysts towards the oxygen reduction reaction. Int. J. Hydro. Ener.
De Souza, C. D., B. Ribeiro Nogueira, and M. E. CM Rostelato. (2019) Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd.
Gawande, M. B., A. Goswami, Francois-Xavier Felpin, Tewodros Asefa, Xiaoxi Huang, Rafael Silva, Xiaoxin Zou, Radek Zboril, and Rajender S. Varma. (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev., 116 (6) 3722-3811.
Goshen, M., Katrin, and S. Magdassi. (2012) Organic nanoparticles from microemulsions: Formation and applications. Curr. Opin. Colloid Interface Sci., 17 (5) 290-296.
Guildford, A. L., T. Poletti, L. H. Osbourne, A. Di Cerbo, A. M. Gatti, and Matteo Santin (2009) Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interface., 41, 1213-1221.
https://www.who.int/cancer/PRGlobocanFinal.pdf
https://www.who.int/news-room/fact-sheets/detail/drinking-water
Huang, L., M. He, B. Chen, B. Hu (2018) Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere.,199, 435-444.
Huang, W., J. Xu, D. Lu, J. Deng, G. Shi, T. Zhoua (2018) Rational design of magnetic infinite coordination polymer core-shell nanoparticles as recyclable adsorbents for selective removal of anionic dyes from colored wastewater. Appl. Surf. Sci., 462, 453-465.
Jain, P. K., Lee, K. S., El-Sayed, I. H., & El-Sayed, M. A. (2006). Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. The journal of physical chemistry B, 110(14), 7238-7248.
Jiang, J., Oberdörster, G., Elder, A., Gelein, R., Mercer, P., Biswas, P. (2008). Does nanoparticle activity depend upon size and crystal phase?. Nanotoxicology, 2(1), 33-42.
Kale, R.D. and P.B. Kane (2019) Colour removal of phthalocyanine based reactive dye by nanoparticles. Groundwater Sustain. Dev., 8, 309-318.
Kataria, N. and V.K. Garg (2017) Removal of Congo red and Brilliant green dyes from aqueous solution using flower shaped ZnO nanoparticles. J. Environ. Chem. Eng., 5(6) 5420-5428.
Khan, J. A., M. Qasim, B. R. Singh, S. Singh, M. Shoeb, W. Khan, D. Das, A. H Naqvi (2013) Synthesis and characterization of structural, optical, thermal and dielectric properties of polyaniline/CoFe2O4 nanocomposites with special reference to photocatalytic activity. Spectrochim. Acta A. Mol. Biomol. Spectroscop.,109 ,313-321.
Kim, H.Y., S. S. Park, and S.T. Lim (2015) Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B: Biointerfaces., 126, 607-620.
Kumar, N., and S. Sinha Ray (2018) Synthesis and functionalization of nanomaterials.” In Processing of Polymer-based Nanocomposites, pp. 15-55. Springer, Cham.
Lata, S., and S. R. Samadder (2016) Removal of arsenic from water using nano adsorbents and challenges: a review. J. Environ. Manag., 166, 387-406.
Leite, M. L., N. B. da Cunha, and F. C. Fabricio (2018) Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol. Ther., 183, 160-176.
Lima, R. A., de Souza, S. L. X., Lima, L. A., Batista, A. L. X., de Araújo, J. T. C., Sousa, F. F. O., Bandeira, T. D. J. P. G. (2020). Antimicrobial effect of anacardic acid–loaded zein nanoparticles loaded on Streptococcus mutans biofilms. Brazilian Journal of Microbiology, 1-8.
Linfeng, C., S. Bin, and L. Jiang (2019) Recent advances in one-dimensional assembly of nanoparticles. Chem. Soc. Rev., 48 (1) 8-21.
Liu, J., N. Wang, H. Zhang, J. Baeyensa, Adsorption of Congo red dye on FexCo3-xO4 nanoparticles., J. Environ. Manag., 238 (2019) 473-483.
Liu, X., F Zhang, J. Xinxin, P. Muchen, P. Liu, L. Wei, Bowen Zhu et al (2018) Complex silica composite nanomaterials templated with DNA origami. Nature., 559 593.
Luo, X., Morrin, A., Killard, A. J., & Smyth, M. R. (2006). Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 18(4), 319-326.
Mahmoud, M.E., M. F. Amira, S. M. Seleim, M. E. Abouelanwar (2019) In situ microwave-assisted oxidation of graphite into partially oxidized graphite nanoparticles for microwave-sorptive removal of anionic and cationic dyes. J. Mol. Liq., 288, 110979.
Maruthupandy, M., Yong Zuo, Jing-Shuai Chen, Ji-Ming Song, He-Lin Niu, Chang-Jie Mao, Sheng-Yi Zhang, and Yu-Hua Shen. (2017) Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl. Surf. Sci., 397, 167-174.
Mishra, A., M. Sardar, (2015) Cellulase assisted synthesis of nano silver and gold: Application as immobilization matrix for biocatalysis. International Journal of Biological Macromolecules. 77 105-113.
Mishra, A., R. Ahmad, M. Sardar, (2015) Biosynthesized iron oxide nanoparticles mimicking peroxidase activity: application for biocatalysis and biosensing. Journal of Nanoengineering and Nanomanufacturing, 5(1) 37-42.
Mirzajani, R. and S. Karimi, Ultrasonic assisted synthesis of magnetic Ni-Ag bimetallic nanoparticles supported on reduced graphene oxide for sonochemical simultaneous removal of sunset yellow and tartrazine dyes by response surface optimization: Application of derivative spectrophotometry., Ultrason. Sonochem.,50 (2019) 239-250.
Moghaddam, A. Z., E. Ghiamati, A. Pourashuri, A. Allahresani (2018) Modified nickel ferrite nanocomposite/functionalized chitosan as a novel adsorbent for the removal of acidic dyes. Int. J. Biol. Macromol., 120, 1714-1725.
Mohammed, L., H. G. Gomaa, D. Ragab, and J. Zhu (2017) Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology, 30, 1-14.
Morshed, Md N., X. Shen, H. Deb, S. Al Azad, X. Zhang, and R. Li. (2018) Sonochemical fabrication of nanocryatalline titanium dioxide (TiO2) in cotton fiber for durable ultraviolet resistance. J. Nat. Fib., 1-14.
Nasrabadi, H. T., E. Abbasi, S. Davaran, Md Kouhi, and A. Akbarzadeh. (2016) Bimetallic nanoparticles: preparation, properties, and biomedical applications. Artificial cells, Nanomed., Biotechnol., 44, 376-380.
Nasrollahzadeh, M.S., M. Hadavifar, S. S. Ghasemi, M. A. Chamjangali. (2018) Synthesis of ZnO nanostructure using activated carbon for photocatalytic degradation of methyl orange from aqueous solutions. Appl. Water Sci., 8(4) 104.
Natsuki, J., T. Natsuki, and Y. Hashimoto (2015) A review of silver nanoparticles: synthesis methods, properties and applications. Int. J. Mater. Sci. Appl, 4,325-332.
Patra, S., E. Roy, R. Madhuri, P. K. Sharma (2016) Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. J. Indus. Eng. Chem.,33, 226-238.
Pal, S. L., Jana, U., Manna, P. K., Mohanta, G. P., & Manavalan, R. (2011). Nanoparticle: An overview of preparation and characterization. Journal of applied pharmaceutical science, 1(6), 228-234.
Pantidos, N., & Horsfall, L. E. (2014). Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. Journal of Nanomedicine & Nanotechnology, 5(5), 1.
Pitkethly, M. J. (2004) Nanomaterials–the driving force. Mater. Today 7,12, 20-29.
Priyadarshini, E., and N. Pradhan (2017) Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sens. Actuat. B: Chem., 238 ,888-902.
Rafique, M., Iqra Sadaf, M. Bilal Tahir, M. S. Rafique, G. Nabi, T. Iqbal, K. Sughra (2019) Novel and facile synthesis of silver nanoparticles using Albizia procera leaf extract for dye degradation and antibacterial applications. Mater. Sci. Eng: C., 99,1313-1324.
Rajabi, H. R., R. Naghiha, M. Kheirizadeh, H. Sadatfaraji, A. Mirzaei, and Z. M. Alvand. (2017) Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: synthesis, characterization, and biological properties. Mater. Sci. Eng: C., 78 ,1109-1118.
Ramar, M., B. Manikandan, P. N. Marimuthu, T. Raman, A. Mahalingam, P. Subramanian, S. Karthick and A. Munusamy. (2015) Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta A: Mol. Biomol. Spectroscopy, 140 ,223-228.
Ravi, R., S. Iqbal, A. Ghosal, and S. Ahmad (2019) Novel mesoporous trimetallic strontium magnesium ferrite (Sr0.3Mg0.7Fe2O4) nanocubes: A selective and recoverable magnetic nanoadsorbent for Congo red. J. Alloys Compd., 791, 336-347.
Ribeiro, M.S., Luciana SA de Melo, S. Farooq, A. Baptista, I. T. Kato, S. C. Núñez, and R.E. de Araujo (2018) Photodynamic inactivation assisted by localized surface plasmon resonance of silver nanoparticles: In vitro evaluation on Escherichia coli and Streptococcus mutans. Photodiagnos. Photodyn. Ther., 22 ,191-196.
- N. Tambat, S. Sontakke, P. Nemade (2018) Visible light removal of reactive dyes using CeO2 synthesized by precipitation. J. Environ. Chem. Eng., 6(4) 4476-4489.
Saha, J., A. Begum, A. Mukherjee, S. Kumar (2017) A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain. Environ. Res., 27(5) 245-250.
Saleh, T. A. (2020). Nanomaterials: Classification, properties, and environmental toxicities. Environmental Technology & Innovation, 101067.
Schrand, A. M., Rahman, M. F., Hussain, S. M., Schlager, J. J., Smith, D. A., & Syed, A. F. (2010). Metal‐based nanoparticles and their toxicity assessment. Wiley interdisciplinary reviews: Nanomedicine and Nanobiotechnology, 2(5), 544-568.
Sha, Y., I. Mathew, Q. Cui, M. Clay, F. Gao, X. J. Zhang, and Z. Gu (2016) Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere, 144, 1530-1535.
Sharma, G., A. Kumar, S. Sharma, Mu Naushad, R. P. Dwivedi, Z. A. ALOthman, and G. T. Mola. (2017) Novel development of nanoparticles to bimetallic nanoparticles and their composites: a review. J. King Saud Uni. Sci.
Sharma, G., D. Kumar, A. Kumar, H. Ala’a, D. Pathania, Mu Naushad, and G. T. Mola. (2017) Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: a review. Mater. Sci. Eng: C., 71 ,1216-1230.
Shi, W., F. Guo, H. Wang, C. Liu, Y. Fu, S. Yuan, H. Huang, Y. Liu, Z. Kang (2018) Carbon dots decorated magnetic ZnFe2O4 nanoparticles with enhanced adsorption capacity for the removal of dye from aqueous solution. Appl. Surf. Sci., 433,790-797.
Silva, S., Almeida, A. J., & Vale, N. (2019). Combination of cell-penetrating peptides with nanoparticles for therapeutic application: a review. Biomolecules, 9(1), 22.
Sodipo, B. K. and A. A. Aziz (2016) Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magnet. Magnet. Mater., 416, 275-291.
Sohrabnezhad, S., and M. Rezaeimanesh (2017) Synthesis and characterization of novel magnetically separable NiFe2O4@AlMCM-41-Cu2O core-shell and its performance in removal of dye. Adv. Powder Technol., 28(11) 3039-3048.
Soltani, N., A. Bahrami, M. I. Pech-Canul, and L. A. González (2015) Review on the physicochemical treatments of rice husk for production of advanced materials. CHEM ENG J., 264 ,899-935.
Sonkusare, V. N., Chaudhary, R. G., Bhusari, G. S., Mondal, A., Potbhare, A. K., Mishra, R. K., Abdala, A. A. (2020). Mesoporous Octahedron-Shaped Tricobalt Tetroxide Nanoparticles for Photocatalytic Degradation of Toxic Dyes. ACS omega, 5(14), 7823-7835.
Sun, Z., G. Yao, M. Liu, S. Zheng (2017) In situ synthesis of magnetic MnFe2O4/diatomite nanocomposite adsorbent and its efficient removal of cationic dyes, J. Taiwan Inst Chem E, 71, 501-509.
Srikar, S. K., D. D. Giri, D. B. Pal, P. K. Mishra, and S. N. Upadhyay (2016) Green synthesis of silver nanoparticles: a review. Green Sustain. Chem., 6, 34.
Su, Z., M. Yao, M. Li, W. Gao, Q. Li, Q. Feng, and Xi Yao. (2018) A novel and simple aluminium/sol–gel-derived amorphous aluminium oxide multilayer film with high energy density. J. Mater. Chem. C., 6 (21) ,5616-5623.
Talbot, D., S. Abramson, N. Griffete, A. Bee (2018) pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng., 25,301-308.
Tamer, T.M., W.M. Abou-Taleb, G.D. Roston, M.S. Mohyeldin, A.M Omer, R.E. Khalifa, A.M. Hafez (2018) Formation of zinc oxide nanoparticles using alginate as a template for purification of wastewater. Environ. Nanotech. Monit. Manag., 10,112-121.
Wang, C., and A. Didier (2018) Recent developments of metallic nanoparticle-graphene nanocatalysts. Prog. Mater. Sci., 94 ,306-383.
Wang, C., E. Guan, L. Wang, X. Chu, Z. Wu, J. Zhang, Z. Yang, Y. Jiang, L. Zhang, X. Meng and B.C. Gates (2019) Product Selectivity Controlled by Nanoporous Environments in Zeolite Crystals Enveloping Rhodium Nanoparticle Catalysts for CO2 Hydrogenation. J. Am. Chem. Soc.
Yang, Y. Chen, and S. Jianlin. (2019) Mesoporous silica/organosilica nanoparticles: Synthesis, biological effect and biomedical application. Mater. Sci. Eng. R., 137 66-105.
Yola, M. L., and N. Atar. (2018) Gold nanoparticles/two-dimensional (2D) hexagonal boron nitride nanosheets including diethylstilbestrol imprinted polymer: electrochemical detection in urine samples and validation. J. Electrochem. Soc., 165 (14) 897-902.
Yang, H., J. Zhang, Y. Liu, L. Wang, L. Bai, L. Yang, D. Wei, W. Wang, Y. Niu, H. Chen (2019) Rapid removal of anionic dye from water by poly(ionic liquid)-modified magnetic nanoparticles. J. Mol. Liq., 284, 383-392.
Yang, Yi, W Yulan, J. Seon-Mi, Xu Jiangping, H. Zaiyan, R. Jingli, (2019) 3D confined assembly of polymer-tethered gold nanoparticles into size-segregated structures. Mater. Chem. Front., 3(2) 209-215.
Ying, Y. L., S. Y. Pung, M. T. Ong, Y. F. Pung (2018) Rhodomine B dye removal and inhibitory effect on B. subtilis and S. aureus by WOx nanoparticles. J. Indus. Eng. Chem., 67,437-447.
Zafar, M.N., M. Amjad, M. Tabassum, I. Ahmad, M. Zubair (2018) SrFe2O4 nanoferrites and SrFe2O4/ground eggshell nanocomposites: Fast and efficient adsorbents for dyes removal. J. Clean. Prod., 199,983-994.
Zafar, M.N., Q. Dar, F. Nawaz, M. N. Zafar, M. Iqbal, M. F. Nazar (2019) Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol., 8(1) 713-725.
Zhang, F., X. Chen, F. Wu, and Yuefei Ji. (2016) High adsorption capability and selectivity of ZnO nanoparticles for dye removal. Colloids Surf. A: Physicochem. Eng. Asp., 509, 474- 483.