Department of Zoology , H M M College for Women Kolkata W.B India
Corresponding author email: zoologist.rehan@gmail.com
Article Publishing History
Received: 12/10/2020
Accepted After Revision: 14/12/2020
COVID-19 has spread in over 210 nations and territories shortly after China. On 29 February, 2020, World Health Organization listed it in a category of high risk and on 11 March 2020 it was designated a pandemic after the declaration being Public Health International Emergency on 30 January, 2020. The countries around the globe are working and making efforts to control and contain this virus. The outbreak yet again proves the capabilities of the zoonotic contaminations. Although the substantial evidence advises possibility of preliminary emergence of zoonotic evolution, it is early to make confirmations on the role hosts that are intermediate such as turtles, pangolins, snakes and the other wild animals in origin of the SARS-CoV-2. Moreover, in addition to bats, the natural host of numerous coronaviruses like MERS-CoV and SARS- CoV. The facts gained from the previous episodes of SARS-CoV and MERS-CoV are being subjugated to retort the virus. Recognizing the probable emergence of zoonotic link and exact mechanism that is responsible for the initial spread will aid to design the control and preventive strategies against the spread of SARS-CoV-2. The present review explains about the SARS-CoV/ COVID-19 with particular focus on role of the animals, zoonotic and the veterinary links with the control and prevention strategies based on the approach of One Health.
COVID -19, SARS –CoV-2, Animals, Transmission, Veterinary.
Ahmad S. R. Possible Zoonotic links for Corona Virus Disease-19: An Updated Review. Biosc.Biotech.Res.Comm. 2020;13(4).
Ahmad S. R. Possible Zoonotic links for Corona Virus Disease-19: An Updated Review. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/3a4oy6M”>https://bit.ly/3a4oy6M</a>
Copyright © Ahmad This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Earlier in the days of December where the people were planning to welcome The New Year along with the Chinese New Year on January 2020 the media houses reported the suffering of many individuals with an intermittent and a clustered situation of pneumonia of which the origin was still unknown to the world in Wuhan, China. Subsequently, after recording the first case in the month of 12 December 2019, the causal agent was easily identified as one of those from the family of Coronaviridae moreover on 12 January 2020, World Health organization declared it to be a fast scattering virus as the 2019 novel corona virus and the Novel Coronaviral pneumonia and CoV- related diseases were addressed as “Covid 19” by the WHO on 11 February 2020 (Du et al. 2020; Gralinski and Menachery 2020).
Later this year the emerging virus was also addressed as the “SARS CoV-2” by the CSG or the “Coronavirus Study Group” of the International Committee as on Taxonomy of the Viruses. On 12 March 2020 the world health organization finally declared the condition as pandemic that is threatening to the mankind to a very far extent (Chatterjee et al. 2020; Zheng 2020; Phadke and Saunik 2020; Rundle et al. 2020). As today, “SARS CoV-2” is believed as seventh coronavirus which is infecting humans. While other coronaviruses include the following OC43, HKU1, 229E, NL63, SARS-CoV and the MERS-CoV. Among all those the MERS-COV and SARS-CoV are zoonotic yet have concluded in massive outbreak due to the very high mortality rate in past two decades whereas the others usually are associated with the illness of minor upper respiratory tract (Wei et al. 2020). Sometimes this leads to the complicated disease when stirring in the immunocompromised individual. (Villamil-Gomez et al. 2020).
To be specific the culinary habits of the Chinese people includes the consumption of various wild animals. The general motivation which is responsible for such consumption by humans in China is because of the belief that consumption of few wild animals and their driven products hold certain medicinal values an also promotes human health in many aspects (Harypursat and Chen 2020). The circumstantial proof which links the first ever case of novel coronavirus to Huanan a south seafood market that offers numerous exotic live animals moreover our former knowledge that the coronaviruses are derived from animals helped us to conclude that the possible zoonotic communication in the SARS-CoV-2. However, it is early to conclude anything as our knowledge of the main source of such infection is restricted (Jalava 2020).
To discover the origins of SARS-CoV-2 would aid us to loosen the actual mechanism that is responsible for the preliminary transmission. After the attainment of a remarkable progress in a developing field concerned with as well as a highly precise lab based diagnostics , much consideration that has been surfaced on emerging operative vaccine and therapeutics for obstructing person to person transmission, old age contagions and the healthcare care employees infection (Chen et al. 2020), which is serious for developing suitable preventive and the regulate strategies against fast dispersal of SARS-CoV-2 contamination. Looking at advantageous propositions of the hydroxychloroquine the multicentric randomised learning that is ongoing to assess its operative as prophylactic measure in limiting the secondary SARS- CoV-2 contaminations along with related clinical indications evolution plummeting general spread of virus (Mitja and Clotet 2020).
Initiating from central of china, SARS-CoV-2 pandemic did not only spread in the 369 cities of China and only crossed the international margins within a very short period from December to March 2020. On 2 May, 2020, COVID-2019 has triggered persons in almost 210 nations and major territories in Asia, Africa, North America, Europe and the Latin America (Rodriguez-Morales et al. 2020a; WHO 2020). Thus, because of a very high rate of transmissibility all over the world it was hence declared as an emergency of public health of international interest by the World Health Organization on 30 January, 2020 and soon after as a pandemic (DuToit 2020; Habibzadeh and Stoneman 2020; Liu et al. 2020a, 2020b; wood 2020; WHO 2020).
In the beginning of the 21st century the other coronaviruses like MERS-CoV and SARSCoV, in the year 2012 and 2002 respectively have been the reason for (SARD) severe acute respiratory distress outbreak however, the present Covid 19 affected the population in larger numbers leading to a number of around 4.71 million confirmed affected as well as death toll by 17 May, 2020 of 0.31 million (WHO). These Statistics are relatively higher than the cases of MERS-CoV A and SARS-CoV however with lower fatality rate. The current situation of the pandemic has significantly affected the economies around the globe moreover, intensively affecting the developing nations. This pandemic not only adversely affected the operations of the world like disappointed multinational businesses, interrupted global trading, transportation, trade, tourism but has resulted in reduction in the income derived from the market, (Ayittey et al. 2020).
China is known as a home for many farms which rears several species of animals like deer, porcupines, snakes, civets, turtles, foxes, minks, bamboo rats, bears and birds. These kinds of farms can be helpful in understanding and knowing the origins of the SARS-CoV-2 (Zhai et al. 2020). Before addressing dogs, snakes and pangolins as the host of the SARS-CoV-2, a bunch of principles known as Koch’s assumptions have to be fulfilled. Thus, it is absolutely unethical to discard these animals without proper conclusive evidence of the SARS-CoV spread from animals to humans (Brownlie 2020). The current reports of the SARS-CoV-2 in the animals such as cats, dogs and one tiger have concluded in needless fear among general public along with pet owners moreover have affected the welfare of the animals, (Parry 2020). The current compilation in brief highlights bound SARS-CoV-2, causing increasing cases of Coronavirus (COVID-19) in humans related to the role of that of animals, zoonotic aspects, veterinary importance and noticeable prevention and strategies of control that focuses on the one-health tactics to refrain and fight the situation of virus.
The virus of SARS-COV-2: The coronaviruses are RNA viruses with positive sense. The newly developed SARS-CoV-2 is a member of order Nidovirales, the family Coronaviridae, the sub family of Orthocoronavirinae under this belong four genera known as Alphacoronavirus, Betacoronavirus, the Gammacoronavirus and lastly the Deltacoronavirus are characterised. SARS-CoV-2 have its place to genus Betacoronavirus and the subgenus of Sarbecovirus. The SARS-CoV-2 and the MERS-CoV also belongs to the genus of Betacoronavirus. However, the SARS-CoV-2 is quite different that the mentioned two on genetic levels. SARS-CoV-2 is said to be 88-89% identical to the two-bat origin of SARS coronaviruses. (bat-SL-CoVZC45) and bat-SL- CoVZXC21, also known as ZC45 and ZXC21), while it is said to be 82% identical to the humans SARS-CoV Tor2 in addition to the human SARS-CoV BJ01 2003 at nucleotide level as (Drexler et al. 2014; Hu et al. 2017; Hu et al. 2018; Chan et al. 2020; Malik et al. 2020a). Only around 50 to 51.8% identities were observed between the MERS-CoV-2 and SARS-CoV and 79% between the SARS-CoV and SARS-CoV-2. Furthermore, the molecular level of the phylogenetic analyses showcases that SARS-Cov-2 is closer to bat origin of SARS-CoV (Mohd et al. 2016; Ramadan and Shaib 2019; Ren et al. 2020; Malik et al 2020a). The highly advanced and in-depth study of the genome identifies the presence of 380 substitutions of amino acids between the arrangements of the SARS-CoV-2 the (HB01) in contrast to equivalent consensus arrangements of SARS-CoV and the SARS-CoV-2 viruses. The amino acid substitution may have made contributions to the functional along with the pathogenic deviation.
The range of Host: The coronaviruses do not only infect humans but also animals of both wild and domestic kind the infections most of the times remain sub-clinical (Ji et al. 2020a; Li et al. 2020a; Salata et al. 2020). However, this clinical form differs from colitis in horses, cattle and swine, the upper breathing tract disease mainly in cattle, felines, dogs and poultry in addition to common cold to a highly fatal infections of respiration in man (Dhama et al. 2020a, 2020b). Midst the main four genera in coroaviridae family, the alphacoronavirus and the betacoronavirus commonly contaminate mammals and possess probable origins of bat meanwhile the Gammacoronavirus and the Deltacoronavirus contaminate fishes, birds and mammals and the assumption is that they are from pig origin (Woo et al. 2012; Hu et al. 2017; Cui et al. 2019). The Betacoronavirus genes have probable zoonotic pathogens like the SARS- CoV and the MERS-CoV that possess bats as the primary host moreover secondary hosts such as civet cat for the former and dromedary camels for latter (Wang and Eaton 2007; ArGouilh et al. 2018; Ramadan and Shaib 2019).
Many CoVs that has been improved or recovered from the birds like Bulbul coronavirus HKU11, Wigeon coronavirus HKU20, Night- Heron coronavirus HKU19, Munia coronavirus HKU13 and the general moorhen coronavirus HKU21. The general swine contaminating infections from coronavirus includes Transmissible Gastroenteritis Virus, Porcine Coronavirus HKU15, Porcine Epidemic Diarrhoea virus and the Porcine Hemagglutinating Encephalomyelitis virus that are being testified across globe (Ma et al., 2008). Furthermore, another list of species of animal are also states harbouring CoVs like horses, swine, cattle, cats, dogs, rabbits, rodents, camels, ferrets, birds, mink, snake (like krait and Chinese cobra), bats, frogs, hedgehogs (the Erinaceus europaeus), marmots, Javan or Malayan or Sunda pangolin (the manisjavanica), while many othe significant wild animals and the role they play as reservoir must be given even more attention by the world ( WHO 2203; Dhama et al. 2014a, 2014b 2020a; Monchatre- Leroy et al. 2017; Ji et al. 2020a; Malik et al., 2020b; Xu 2020).
SARS-CoV-2/ Covid19: zoonotic links, veterinary, animals and transmission
Animals being affected by Coronavirus: As it is known that the coronaviruses possess a wide animal hosts, few species of animals also harbour the pathogens and a very few of such animals get affected by serious contamination (Cui et al. 2019; Andersen et al. 2020). The coronaviruses such as rat hepatitis virus, guinea pig virus, rat sialodacryoadenitis coronavirus and the rabbit coronavirus are few important viruses that are responsible for the enteritis, hepatitis and the breathing infections in the lab animals. Amongst a large number of animals, the bovine coronaviruses (BoCoVs) possess zoonotic potential known as being different from the asymptomatic children and found affecting few domestic animals and the wild ruminants where bloody diarrhoea among adult cattle, calf diarrhoea in the neonates and the respiratory form of fever in all groups irrespective of age in cattle are global implication (Zhang et al. 1994; Suzuku et al. 2020).
The Feline coronavirus affects respiratory tract, the major nervous system, gastrointestinal tract, abdominal cavity in order to produce infectious peritonitis and enteritis (Tekes and Thiel 2016). The Canine enteric CoVs of the Alphacoronavirus and the Betacoronavirus genera bother enteric and the respiratory region respectively, (Erles and Brownlie 2008; Licitra et all. 2014).In poultry industry the virus of infectious bronchitis, the member of the Gammacoronavirus genes results in extensive loss in economic operations by developing respiratory issues, infection in the urinary tract and the reproductive issues (Dhama et al. 2014a, 2014b). The swine acute diarrhoea syndrome CoVs a member of the Alphacoronavirus genes that produces very serious enteritis in the suckling piglets that causes significant mortality. Upon such genomic study, the virus was found in 95-96% of identical to the horseshoe origin of bat (Rhinolophus sp.) CoVs and the known HKU2 coronavirus (Wanh and Jin 2020). Furthermore, it has been suggested that there is a possibility of the host skipping from bats to the pigs by crossing species obstacle either by recombination of genes or by developing changes at level of (RBD) receptor binding domain (Zhous et al. 2018; Yang et al. 2019).Among all the species of animals another new coronavirus named as the SW1 is recognised by making use of the technology of panviral microarray in liver tissues of captive beluga whale (Mihindukulasuriya et al. 2008).
Zoonotic links and Animals of SARS-CoV-2: Coronaviruses in he past has crossed the barrier of species during MERS and SARS outbreaks, and therefore SARS-CoV-2 appears to be an outcome of the barrier of the species jumping for third time. Among the CoVs, the current zoonotic ones like MERS-CoV, SARS-CoV and the SARS-CoV-2 has gained higher significance due to the seriousness of the disease in the humans moreover their global blowout (Rothan and Byrareddy 2020). The development of the new CoVs and the extensive host range it possesses is may be because of the instability of replicase enzyme, the RNA is dependent on the RNA polymerase, the polybasic furin cleavage site and the O-linked glycans, deficiency mechanism of proofreading, the advanced mutations rate in RBD of the genetic recombination and the spike gene (Su et al. 2016; Chen 2020; Patel and Jernigan 2020). Multiple Research show that the SARS-CoV and the SARS-CoV-2 both make use of the ACE2 as a common cell access receptor (Zhou et al. 2020a). Because of the mutation in the region of the RBD of gene S of CoVs, the range of host gets prolonged in order to infect the other host species whether humans or animals, the transmissibility and pathogenicity of the virus may get increased on altered in the future becoming a situation of global concern. (Chen 2020; Patel and Jernigan 2020).
While conducting a search of the origination of the SARS-CoV-2, the observation was made that initially the infected person has a general exposure spot. The Wet seafood market of wholesale in south china in the city of Wuhan is known for restaurants that offers large and small domestic and wild animals moreover live animals that includes rabbits, bats, poultry, snakes, turtles, pangolins,hedgehogs, marmots and badgers for consumption by humans. (Hu et al. 2015; Hui et al. 2020; Ji et al. 2020b; Liu et al. 2020a, 2020b; Lu et al. 2020b; Wang et al. 2020; Wu et al. 2020b). The inferences in the beginning from Wuhan’s seafood marketplace hypothesised the source of animals’ attachment and the wild animals for spill over of the SARS-CoV-2. The results of the Research indicated the chances of zoonotic basis because the CoVs circulate between certain species of animals, vertebrate and the humans as there are wide range of hosts. There was an assumption that the SARS-CoV-2 was initially transmitted from the animals to humans, and maintaining a human to human spread (Hui et al. 2020; Ji et al. 2020a; Nishiura et al. 2020).
In case of the MERS-CoV, there are evidences which states biological RNA is released by the nasal secretions and the faeces also from the milk, stating the danger of food borne spread of the MERS-CoV (Reusken et al. 2014). Moreover, a number of camels that are offered for slaughtering in few studies showcased evidences of shedding of nasal MERS-CoV (Farag et al. 2015). Furthermore, the chances of SARS-CoV-2 as being a food borne infection of CoVthat is spread by route of respiration (Jalava 2020). The literature documents showed that there are a few of origin of bat SARS-CoV and were likely enough to infect human beings. As noted formerly, bats were overserved to be transmitting the SARS-CoV and the MERS-CoV, hence the researchers predicted the role played by bats in the transmission and origin for the present pandemic of the SARS-CoV-2 (Fan et al. 2020a; Malik et al. 2020a; Wong et al. 2019; Zhou et al. 2020a).
Presently it is understandable that SARS-CoV-2 is intimately related to coronavirus of the bat which is believed to be a preservation of the hosts of the former CoVs related to SARS. Therefore, SARS-CoV-2 may have been developing from the recombination of sequential combinations that occurs between the viruses related to SARS and precursors. On the basis of the usage of the codon bias snake was projected as the reservoir of the SARS-CoV-2 (Ji et al. 2020a). Nevertheless, this projection was contradicted by few researchers. This was the cause for questioning the presence of any intermediate host (animal) which can be responsible for the spill over of zoonotic transmission among humans (Weiss and Leibowitz 2011; Murdoch and French 2020).
In a similar way not just only from bats the coronavirus is associated with SARS that was transmitted from the humans to swine (Chen et al. 2005). It is relevant to state that the swine had been one of the predominant species for evolution in the past of various strains of Influenza A when existing in an intimate association with the avian and the species pf human and as the CoVs of bats that infect pigs, possibility of development of any novel virus involving corona and influence can’t be left out including the present situation of the increasing SARS-CoV-2 cases, explorative studies and researches are required for such hypothesis (Brown 2001; Dhama et al. 2012; Malik et al. 2020a).
The given conditions at any time, swine that serve as the vessel of mixing of viruses carrying influenza (Ma et al. 2009) needs to be considered with safety as it remains in the proximity with human and the cycles of domestic sylvatic that involves contact with various wild animals and the circumstances might get worse (Ma et al. 2008). Nevertheless, for the current time, researches of Shi et al. (2020) have not disclosed any important vulnerability of swine to SARS-CoV-2. Civets, camels and bats have been in the list of current animal carriers of the human infections of CoV (Cui et al. 2019). In the latest, the bats (Wu e al. 2020b) and the pangolins (Zhang et al. 2020a) are believed to be the possible origin source of the SARS-CoV-2 (Andersen et al. 2020).
Till now the real intermediate nature and host that led to emergence are yet to be discovered and explored. The two scenarios of the development of SARS-CoV-2 are taken into projections. In the first the natural selection of the viruses which might have been occurring in any animal host formerly before the spread in the humans and secondly it is the natural selection of the viruses that has occurred in the humans after zoonotic spread (Anderson et al. 2020). The advanced researches that involves the cuture of cell or the animal models may help in answering these hypothesis (Ge et al. 2013; Anderson et al. 2020).
The Bats: Bats are considered to be the superlative reservoir hosts for the CoVs, as the virus are continuously present in bats and are asymptomatic. They tend to travel across the jungles in quest of and spread virus to different kinds of hosts as they get in contact with (Fan et al. 2019). In china bats are of significant purposes as they not only are sold for consumption purposes but also for medicinal purposes and the wild bats are often used to attaincompounds derived from bats. Even though bats possessmedicinal value, a severe risk is posed by them for acquiring novel zoonotic infection (Riccucci 2012; Wassenaar and Zou 2020). In present situation novel coronavirus pandemic, findings of the laboratory confirmed that the SARS-CoV-2 is constituted 96% alike to bats Cov at genomic therefore bats might be the principal source of the zoonotic spill over (Tang et l. 2006; Rodreiguez Morales et al. 2020b; Zhou et al 2020a).
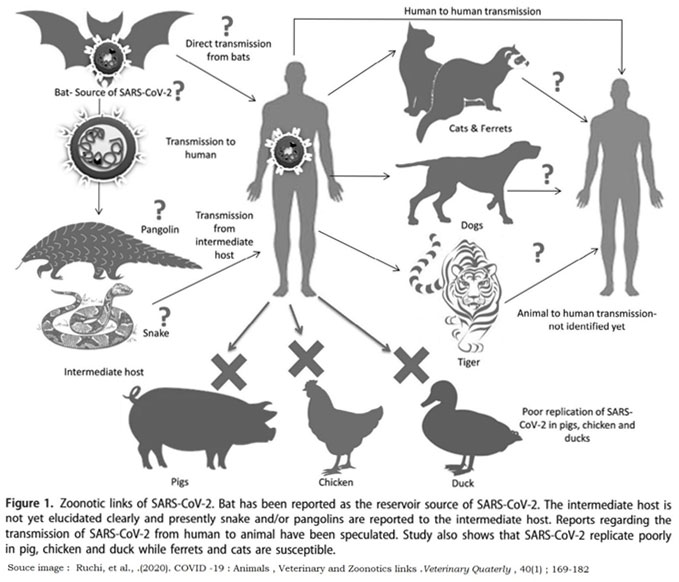
The Pangolins: The bats are not the only reservoir of coronavirus but Malayan pangolins are also isolated for coronavirus and RBD is the protein S of the SARS-CoV-2 was very similar to the of the Pangolin-V thus, the pangolins may be the intermediate host of the novel coronavirus (Xiao et al. 2020). The conclusions of Zhang et al. (2020b) also support the stated research. Most interestingly, coronavirus secluded from the pangolins (SRR101068377 and SRR10168378) didn’t possess RRAR motif. SARS-CoV-2 virus secluded from the individuals that are infected showcased a huge alikeness to Beta COV/Yunnan/ CoV/RaTG13/2013 virus that is compared to those that were secluded from pangolins (Li et al. 2020b). Such researches suggested that pangolins had little probability of acting as one of the intermediate hosts of the SARS-CoV-2. Further the studies required to recognise intermediate host and in order to confirm their part in the development and origin of the virus in man.
Felines and Canines: Until now the SARS-CoV-2 infection is identified in two dogs, where both are reported from the city of Hong Kong (Almendros 2020a). The primary case was identified and reported in a Pomeranian dog which was 17-year-old which gave a positive RTPCR outcomes in both the cases of nasal and oral samples (Almendros 2020a; American Veterinary Medical Association 2020). Although the serological test done initially gave a negative result, the blood tests that were carried out later in the future gave a weak positive outcome. The reason for this might be because of the fact that the development in the antibody may take 14 days or more (Almendros and Gascoigne 2020b). A research report of the seroconversion in dogs showcased that animal has formed antibodies against the SARS-CoV-2. This suggests that the virus imposed weak infection in dog and such result was due to the immune system. Therefore, the studies suggest an unbiased and true contamination in the case of dogs which was transferred from humans to animals (Almendros and Gascoigne 2020b). In a similar way one such case of SARS-CoV-2 contamination was identified in German Shepherd in Hong Kong. Interestingly, that both mentioned cases of the canine SARS-CoV-2 contaminations were identified with positive owners of SARS-CoV-2 (American Veterinary Medical Association 2020). Presently, there are no substantial proof that the dogs get infected from SARS-CoV-2 or are able to spread the virus to the humans (Almendros 2020a).
In another case where animals were again involved was of two cats, one from Hong Kong and the other from Belgium was tested positive for the virus of COVID 19 (American Veterinary Medical Association 2020). Scientist from the Harbin Veterinary Research Institute has stated that the cats can be infected with the SARS-CoV-2 under the conditions of experiment and are able to transmit to the other cats that are susceptible and are pet together in the same house,(Mallapaty 2020; Shi et al. 2020). The research was on the basis on the experimental inoculations and might not stand accurate in the natural conditions. None of those cats who were infected displayed any symptoms or signs or any sickness, that indicates their low capability for spreading the infections (Mallapaty 2020).
The serological study that was carried out among the cats from the city of Wuhan noticed the attendance of the neutralizing antibodies of SARS-CoV-2. This study specifies that the cats are not immune to the infection of SARS-CoV-2 under the natural conditions that results in a response that is antibody (Zhang et al. b). Nevertheless, among cats that were tested positive, an advanced titre of the neutralizing antibodies was seen in cats who were staying in a close contact with the owners who were SARS-CoV-2 positive (Zhang et al. 2020b). In a recent case, Malayan tiger from the Bronx Zoo in the New York city was also identified as a SARS-CoV-2 positive. This big cat is also assumed to be contaminated with the virus because of the positive tested asymptomatic keeper of the same zoo. The carnivores were being tested for the virus when they began to display of minor respiratory sickness (United States Department of Agriculture 2020).
Animal and human interactions are a risk factor: Many researchers stated the traditional way of cooking in China as one of the reasons responsible in a way for the situation of infection of the novel coronavirus in human beings because as stated by the customs of Chinese food that the live slaughtering animals are believed to be even more nutritious however the same time, many get in contact with the any or all types of pathogens that are possibly present (SARS-CoV, Rotavirus, Nipah virus, Highly Pathogenic influenza virus) that could be present in the food items that are offered (FAO/WHO 2008). Human and animals’ interactions on a regular basis either in market place or in animal industry without taking better ecological biosecurity were believed as an important risk factor for the increasing number of zoonotic diseases, predominantly in the communities that lives in the rural china in the southern part (Daszak 2020).
After such reports, china has temporarily banned the sale of wild animals and trading of bats after the breakout of novel coronavirus. In addition, Wuhan’s food market has been closed to refrain the spread virus and development of novel variant of any virus can be barred. Furthermore, it has been advised to ignore any such contact with wildlife and farm without use of protective personal gear (Benvenuto et al. 2020). In addition to this, the need that arises is to waft strategies for surveillance and guidelines for prevention to get an in-depth analysis of the betacoronavirus. Specifically, in the bat family of Rhinolophus as in previous cases of MERS, SARS and currently SARS-CoV-2 pandemic that has created panic (Daszak et al. 2020).
Therefore, the bat appears as the source od origin or natural reservoir of the SARS-CoV-2 (Li et al. 2020) which leads to the zoonotic infection in people through a middle host that is yet to be decoded with current investigations on the ferrets, pangolins and probably snakes. Nevertheless, the future developments might disclose the original intermediate hosts of the virus SARS-CoV-2 that is responsible for the zoonotic spread (Almenndros 2020a; Dhama et al. 2020d; She et al. 2020; Zhang et al. 2020a, 2020b).
Animal Models: Even though the current studies on animal models lack for the SARS-CoV-2, a current study explored the utility of the rhesus macaque, non-human primate as a model that carries the SARS-CoV-2 studies. Earlier these non- human primates helped in calculating the antivirals and the vaccines against the MERS-CoV (de Wit et. Al 2020). The work process on the SARS-CoV-2, rhesus macaques showcased the settlement of the infection of SARS-CoV-2 which possessed high amount of virus in the rectal swabs and oral-naso. Apparently, the lesions of the disease in radiographs of the lungs and the clinical signals lastingup to a period of 16 days evidently proved the effectiveness of diseases and helping in establishing and testing the antivirals and vaccines (Munster et al. 2020).The isolation SARS-CoV-2 from the dogs is also noted (OIE. 2020). Currently, Shi et al. (2020) have stated the susceptibility of cats, dogs, ferrets and various domestic animals to SARS-CoV-2 by an experiment inoculation and stated that SARS-CoV-2 is poorly replicated in pigs, chickens, ducks and dogs however efficiently in cats and ferrets. Cats can transmit the infection via their droplets (Shi et al. 2020). Nevertheless, exact exploration needs specific models, specifically animals with the ACE2 receptors which are similar to those people (Andersen et al. 2020).
Establishing an accurate animal models will aid in understanding process of the disease and also developing therapeutics and prophylactics (TBRI 2020; Dhama et al. 2020c). The non-human primates are believed to be suitable models of the human diseases, where in case of exploration to-pathogenesis of disease and the immune response, other modes are preferable (TBRI 2020). The non-human mice, hamster and primates (Gretebeck and Subbarao 2015) were used as the animal models for the MERS and SARS, and few may have probability in SARS-CoV-2. The golden Syrian hamster has been investigated for studies on protection of the vaccine against the strains of SARS-CoV-2 (Roberts et al. 2008). Moreover, advised to be the capable animal model that reveals the pathogenesis and CoV pathology along with the efficiency of the vaccine that is to be tested. In case of transgenic animals like mice have better relativity of stimulating the SARS-CoV-2 as there are presence of structural differences in the ACE 2 receptors in some species of animals to which the receptors are binding the domain of protein of spike of SARS-CoV-2 (Liu et al. 2020b; Wang 2020).
On modelling the receptors of ACE2 between some animals like cats, orangutans, ferrets, pigs, monkeys, species of bats and human possess alike levels of affinity for the virus SARS-CoV-2 that is based on the similarity of structures of the receptors of ACE2 (Jarvis 2020). Therefore, these might have probable role that can be used as the animal’s models with furthermore investigative researches. The smalls animal’s model is preferable generally like rabbits and mice as it is cheaper and easier to manipulate and easily available (Dhama et al. 2020c). Primarily, mice were thought to be challenging because of the distinction in the ACE2 receptor usage pattern, however for SARS-CoV-2 the transgenic mice now are believed to be the applicable models (Li et al. 2020a, 2020b Wang 2020; Zhou et al. 2020b). Animals like Ace2 knokout mouse, inbred mouse, Tmprss2 knockout mouse, Transgenic HLA mouse and Stat1 knockout mouse are being speculated as he animal modesl for the COVID-19/ SARS-CoV-2 (Hoffmann et al. 2020; Taconic Biosciences 2020; Wang 2020).
Control and Prevention: In the past and the current situations of Ebola, Zika, viruses of Bird Flu, Nipah (Munjal et al. 2017; Dhama et al. 2012, 2018; Singh et al. 2019), and knowledge earned from past threats of the coronaviruses (MERS- and SARS-CoVs) along with the advance in field of science have paved the path to counter developing pathogens like SARS-CoV-2. For this situation, high efforts are made to control and contain the transmission of the virus that is haunting the lives of many around the globe and posing a situation of pandemic even now. Therefore, works for strict vigilance, rapid diagnosis, quarantine procedures and appropriate isolation are being done to refrain the further transmission. Moreover, enhanced surveillance and observation, intensive care unit, better medical facilities, rapid communication, networking programs, awareness programs for the public, efforts to establish effective anti-viral, drugs and vaccines are being speculated and discovered optimally.
Global collaborative readiness and efforts to handle the further emergency to a level of the situation of pandemic capability along with the proper and applicable heath approach to fight this virus are being used effectively (Bonilla- Aldana et al. 2020, Dhama et al. 2013a; 2013b; 2020a; 2020c; Malik et al. 2020a, 2020b; Rodriguez- Morales et al. 2020c). The vaccines look long lasting solution for the pandemic. Nevertheless, presently there are no such vaccines available to fight against it (Shang et al. 2020; Chen et al. 2020). Ideas are being motivated from the structures of virus, pathogenesis and the connected coronavirus (Ahmed et al. 2020; Shang et al. 2020).
There are various vaccines that are being evaluated by the different companies and institutes (Shang et al. 2020) with few of them under trials. Moderna, Ma, Cambridge, USA a biopharma company initiated the mRNA-1273 vaccine collaborating with the CanSino in Hong Kong the Special Administrative Region, situated in China (Flanagan 2020). Birmingham’ s University of Alabama (UAB), USA, AI, Birmingham in coordination with the Altimmune Inc., Md, USA, Gaithersburg, is working on development of an intranasal antiviral against the COVID-19 and termed it AdCOVID on pattern of the pandemic vaccine for influenza and inhalation anthrax (Hansen et al. 2020). Clover Biopharmaceuticals firm, China, Chengdu has come up with SARS-CoV-2 protein that is based on the subunit vaccine (Clover Biopharmaceuticals 2020).
As on 20th March, 2020, The WHO has calculated over 44 candidates for vaccine that targets SARS-CoV-2 among these only a few are under the clinical observation while others are under development by institutions and companies.Theyincluded live attenuated, formaldehyde inactivated, protein subunit, DNA, m-RNA, VLP, replicating, and non-replicating vector-based SARS-CoV-2 vaccines. Adenovirus type 5 vector vaccine by CanSino biological Inc., Hong Kong Special Administrative Region, China, and Beijing Institute of Biotechnology, Beijing, China, andLNP-encapsulated mRNA vaccine developed by Moderna/NlAlD, Bethesda, Md, USA, is under phase-I clinical evaluation.
Few in pre-clinical stage of clinical evaluation against COVID-19 include DNA plasmid vaccine by ZydusCadila, Ahmedabad, India, DNA plasmid vaccine through electroporation device by Inovio pharmaceuticals, Plymouth Meeting, Pa, USA,DNA vaccine by Takis/Applied DNA Sciences/Evvivax, Rome, Italy, formaldehyde inactivated alum vaccine by Sinovac, Beijing, China, live attenuated virus vaccine by Codagenix, Farmingdale, New York, USA/ Serum Institute of India, Pune, India, MVA encoded VLP by GeoVax/BravoVax, Smyrna, Ga, USA, Ad 26 by Janssen pharmaceutical companies, Beerse, Belgium, ChAdOx1 by university of Oxford, Oxford, UK, adenovirus-based NasoVAX expressing SARS-CoV-2 spike protein by Altimmune, Gaithersburg, Md, USA, Ad5 S (named as GREVAXTM) by Greffex, Aurora, Co, USA, oral vaccine by Vaxart, South San Francisco, Car USA.
Animal derived Drosophila S2 insect cell expression system using VLPs as protein subunit vaccine by ExpreS2ion, Horsholm, Denmark, S protein based by WRAIR/ USAMRIID, Fort Detrick, Md, USA, another S protein by AJ Vaccines, København, Denmark, S-trimer by Clover Biopharmaceuticals Inc., Chengdu, China/GSK, Brentford, UK, peptide based vaccine by Vaxil Bio, Toronto, Ontario, Canada, S protein through baculovirus production system by Sanofi Pasteur company, Swiftwater, Pa, USA, full length S trimers nanoparticle with Matrix M by Novavax, Rockville, Md, USA, gp-96 backbone based vaccine by Heat Biologics, Morrisville, NC, USA, or University of Miami, Miami, Fl, USA, Sl or RBD protein based by Baylor College of Medicine, Houston, TX, USA, adjuvanted microsphere peptide vaccine candidate by University of Saskatchewan, Saskatoon, Saskatchewan, Canada, LNP encapsulated mRNA encoding RBD by Fudan University/Shanghai JiaoTong University/RNACure Biopharma, Shanghai, China, sa-RNA (small activating ds-RNA) based COVID-19 vaccine by Imperial College London, London, UK, among others (https://www.who.int/blueprint/priority-diseases/key-action/novelcoronavirus-landscape-ncov.pdf?ua=l).
Though till April 1st, 2020 US Food and Drug Administration (FAO) had not announced any confirmed commercial therapeutic or prophylactic vaccine against SARSCoV-2, nevertheless they have enlisted few potential vaccine candidates which are currently under either preclinical or clinical trials such as mRNA-1273 by Moderna Inc., Bethesda, Md, USA, Inovio’s DNA vaccine INO-4800 against COVID-19 by Inovio Pharmaceuticals, Plymouth Meeting, Pa, USA, along with Ology Bioservices, Alachua, Fl, USA, BNT162 a mRNA vaccine by BioNTech, Mainz, Germany, plantbased COVID-19 vaccine by Medicagor Quebec, Quebec, Canada, oral recombinant COVID-19 vaccine by Vaxart, South San Francisco, Ca, USA, li-Key peptide COVID-19 vaccine by Generex Biotechnology, Toronto, Ontario, Canada, among others (PrecisionVaccination2020). (https://www.precisionvaccinations.com/vaccines/coronavirus-vaccines).
Till the time vaccines are set, alternate diseases control and prevention strategies require the focus. There is a requirement for strengthening he capacity and infrastructure that is build with trained employees, health workers and services to recognise the patients that are affected by SARS with isolation of the patients after they are suspected of the COVID-19. In order to apply any measures for prevention the primary step is check the case with speed and accuracy. While the confirmation of the deadly case, the guidelines of the Centre for Disease of and Prevention or CDCmust be implemented. As suspected cases are good source of any nosocomial blowout, the health employees should follow the precautionary practices when handling COVID-19 cases. It is notable that a facility with the adverse air pressure is advised for keeping the confirmed cases of SARS-CoV-2.
Applications of the telemedicine that have supervision and, monitoring, tele-visits, consultation and interpretation (Serper and Volk 2018) is proven to be effective in modifying the lingering diseases. Tele-model is being applied to the current situation of COVID-19 specifically in the isolated area that have limited medical reach that saves both the resources and manpower (Au 2020).Even though the infections of respiratory tract and transmission of virus from the naso-oral secretions is well explained, the primary isolation of the SARS-CoV in China of a positive patient displayed the importance of digestive tract in the conjunction to respiratory tract. Ever since, when planning the strategies for control, this alternative path of transmission of virus was kept in mind that includes a focus on asymptomatic patients and clinical sufferers or persona who have mild or no signs (Gu et al. 2020).
CONCLUSION
The markets for live animals, just like one in the South China, Huanan sea food market, will remain to be the ideal point which encourages the inter-species interaction between the domestic and wild animals. Therefore, the probability inter-species spread of the CoVcontaminations at hot spot is a centre of worry to the human being because of the adaptive recombination of genetic, that is present in these viruses. The perpetual ban on trade of wild animal should not be executed as it would only move the trade to black market. It is better to control and monitor the trading of wild animals rather than a complete ban. The spread of the newer zoonotic contaminations like the SARS-CoV-2 is unavoidable in the days to come. Thus, the international and local regulatory bodies must develop and enforce mechanisms for diseases’ control that is effective enough to decrease the probability of human acquaintance to the wild animals. The breakout of the SARS-CoV-2 is another example which proves the presence of any intimate but upfront interaction between the animals, people and environmental health which can potentially result in development of live threatening pandemic. The recent years have showcased the destructive capabilities of few zoonotic coronavirus contaminations like MERS, SARS, and presently the SARS-CoV-2 which needs call for the applications of the framework of One Health to safeguard the humankind from the evolving pathogens soon.
Conflict of Interests: The author declares that there exist no commercial or financial relationship that could, in any way, lead to potential conflict of interest.
Funding Declaration: Author declared that he hasn’t received any financial assistance from any organization for conducting above mentioned work.
REFERENCES
Ahmed SF, Quadeer AA, McKay MR. 2020. Preliminary identification of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 12(3):254. 0000
Almendros A. 2020a. Can companion animals become infected with Covid-19? Vet Rec. 186(12):388–389.
Almendros A, Gascoigne E. 2020b. Can companion animals become infected with Covid-19? Vet Rec. 186(13):419–420.
Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. 2020. The proximal origin of SARS-CoV-2. Nat Med. 26(4):450–452.
Au S. 2020. Revisiting the role of telemedicine under the 2019 novel coronavirus outbreak. EJGG. 2(1):26–27.
Ayittey FK, Ayittey MK, Chiwero NB, Kamasah JS, Dzuvor C. 2020. Economic impacts of wuhan 2019-nCoV on China and the world. J Med Virol. 92(5):473–475.
Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. 2020. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 92(4):455–459.
Bonilla-Aldana DK, Dhama K, Rodriguez-Morales AJ. 2020. Revisiting the one health approach in the context of COVID-19: a look into the ecology of this emerging disease. Adv Anim Vet Sci. 8(3):234–237.
Brown IH. 2001. The pig as an intermediate host for influenza a viruses between birds and humans. Int Congr Ser. 1219:173–178.
Brownlie J. 2020. Conclusive proof needed for animal virus reservoirs. Vet Rec. 186(11):354–354.
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. 2020. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 9(1):221–236.
Chen W, Yan M, Yang L, Ding B, He B, Wang Y, Liu X, Liu C, Zhu H, You B, et al. 2005. SARS-associated coronavirus transmitted from human to pig. Emerg Infect Dis. 11(3):446–8.
Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, Gupta N, Gangakhedkar RR. 2020. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res. 0(0):0.
Chen J. 2020. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 22(2):69–71.
Chen WH, Strych U, Hotez PJ, Bottazzi ME. 2020. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. :1–4. doi: 10.1007/s40475-020-00201-6
Clover Biopharmaceuticals. 2020. Clover initiates development of recombinant subunit-trimer vaccine for Wuhan coronavirus (2019-nCoV). Available at: https://pipelinereview.com/index.php/2020012873644/Vaccines/Clover-Initiates-Development-of-Recombinant-Subunit-Trimer-Vaccine-for-Wuhan-Coronavirus-2019-nCoV.html
Cui J, Li F, Shi ZL. 2019. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 17(3):181–192.
Daszak P. 2020. A qualitative study of zoonotic risk factors among rural communities in southern China. Int Health. 12(2):77–85.
Daszak P, Olival K, Li H. 2020. A strategy to prevent future epidemics similar to the 2019-nCoV outbreak. Biosaf Health. 2(1):6–8. http://dx.doi.org/10.1016/j.bsheal.2020.01.003.
deWit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci Usa. 117(12):6771–6776.
Dhama K, Verma AK, Rajagunalan S, Deb R, Karthik K, Kapoor S, Mahima Tiwari R, Panwar PK, Chakraborty S. 2012. Swine flu is back again: A review. Pak J Biol Sci. 15(21):1001–1009.
Dhama K, Chakraborty S, Kapoor S, Tiwari R, Kumar A, Deb R, Rajagunalan S, Singh R, Vora K, Natesan S. 2013a. One world, one health – veterinary perspectives. Adv Anim Vet Sci. 1(1):5–13.
Dhama K, Verma AK, Tiwari R, Chakraborty S, Vora K, Kapoor S, Deb R, Karthik K, Singh R, Munir M, et al. 2013b. A perspective on applications of geographical information system (GIS); an advanced tracking tool for disease surveillance and monitoring in veterinary epidemiology. Adv Anim Vet Sci. 1(1):14–24.
Dhama K, Pawaiya RVS, Chakrabort S, Tiwari R, Saminathan M, Verma AK. 2014a. Coronavirus infection in equines: a review. Asian J Anim Vet Adv. 9(3):164–176.
Dhama K, Singh SD, Barathidasan R, Desingu PA, Chakraborty S, Tiwari R, Kumar MA. 2014b. Emergence of avian infectious bronchitis virus and its variants need better diagnosis, prevention and control strategies: a global perspective. Pak J Biol Sci. 17(6):751–767.
Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, Kumar D, Ramakrishnan MA, Malik YS, Singh R, et al. 2018. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Front Immunol. 9:1803.
Dhama K, Sharun K, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, BonillaAldana DK, Rodriguez-Morales AJ. 2020a. Coronavirus disease 2019 – COVID-19. Preprints. doi: 10.20944/preprints202003.0001.v2
Dhama K, Patel SK, Pathak M, Yatoo MI, Tiwari R, Malik YS, Singh R, Sah R, Rabaan AA, Bonilla-Aldana DK, et al. 2020b. An update on SARS-COV-2/COVID-19 with particular reference on its clinical pathology, pathogenesis, immunopathology and mitigation strategies – a review. Preprints. doi: 10.20944/preprints202003.0348.v1.
Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, Chaicumpa W. 2020c. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 18:1–7.
Dhama K, Patel SK, Sharun K, Pathak M, Tiwari R, Yatoo MI. 2020d. SARS-CoV-2: Jumping the species barrier, lessons from SARS and MERS, its zoonotic spillover, transmission to humans, preventive and control measures and recent developments to counter this pandemic virus. Preprints. doi: 10.20944/preprints202004.0011.v1.
Drexler JF, Corman VM, Drosten C. 2014. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antiviral Res. 101:45–56.
Du B, Qiu HB, Zhan X, Wang YS, Kang HYJ, Li XY, Wang F, Sun B, Tong ZH. 2020. Pharmacotherapeutics for the new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 43(0):E012.
Du Toit A. 2020. Outbreak of a novel coronavirus. Nat Rev Microbiol. 18(3):123.
Erles K, Brownlie J. 2008. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet Clin North Am Small Anim Pract. 38(4):815–825, viii.
Fan Y, Zhao K, Shi ZL, Zhou P. 2019. Bat coronaviruses in China. Viruses. 11(3):210.
Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). 2008. Microbiological hazards in fresh leafy vegetables and herbs: meeting report. Microbiological Risk Assessment Series No. 14. Rome. 151.
Farag EABA, Reusken CBEM, Haagmans BL, Mohran KA, Stalin Raj V, Pas SD, Voermans J, Smits SL, Godeke G-J, Al-Hajri MM, et al. 2015. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect Ecol Epidemiol. 5:28305.
Flanagan C. 2020. First results from moderna covid-19 vaccine may take two more months. [Accessed 2020 April 2]. https://www.bloomberg.com/news/articles/2020-03-26/first-look-at-moderna-covid-19-vaccine-may-take-two-more-months. [Google Scholar]
Gao ZC. 2020. [Efficient management of novel coronavirus pneumonia by efficient prevention and control in scientific manner]]. Zhonghua Jie He He Hu Xi Za Zhi. 43(0):E001.
Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, et al. 2013. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 503(7477):535–538.
Gorbalenya AE, Baker SC, Baric RS, deGroot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, et al. 2020. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming It SARS-CoV-2.. Nat Microbiol. 5(4):536–544.
Gralinski LE, Menachery VD. 2020. Return of the coronavirus: 2019-nCoV. Viruses. 12(2):135.
Gretebeck LM, Subbarao K. 2015. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 13:123–129.
Gu J, Han B, Wang J. 2020. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 158(6):1518–1519. published online ahead of print, 2020 Mar 3].
Habibi R, Burci GL, deCampos TC, Chirwa D, Cinà M, Dagron S, EcclestonTurner M, Forman L, Gostin LO, Meier BM, et al. 2020. Do not violate the international health regulations during the COVID-19 outbreak. Lancet. 395(10225):664–666.
Habibzadeh P, Stoneman EK. 2020. The novel coronavirus: a bird’s eye view. Int J Occup Environ Med. 11(2):65–71.
Hansen J, Brown W, Robinson A. 2020. UAB will test a COVID-19 vaccine candidate created by Altimmune Inc. [Accessed 2020 April 1]. https://www.uab.edu/news/research/item/11203-uab-will-test-a-covid-19-vaccine-candidate-created-by-altimmune-inc.
Harypursat V, Chen YK. 2020. Six weeks into the 2019 coronavirus disease (COVID-19) outbreak- it is time to consider strategies to impede the emergence of new zoonotic infections. Chin Med J (Engl). 133(9):1118–1120.
Hellewell J, Abbott S, Gimma A, Bosse NI, Jarvis CI, Russell TW, Munday JD, Kucharski AJ, Edmunds WJ, Funk S, et al. 2020. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 8(4):e488–e496.,
Hoffmann M, KleineWeber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280.e8. 2020.
Knowles G. 2020. Will they ever learn? Chinese markets are still selling bats and slaughtering rabbits on blood-soaked floors as Beijing celebrates ‘victory’ over the coronavirus. Mail Online. https://www.dailymail.co.uk/news/article-8163761/Chinese-markets-selling-bats.html.
Hu B, Ge X, Wang LF, Shi Z. 2015. Bat origin of human coronaviruses. Virol J. 12:221.
Hu B, Zeng LP, Yang XL, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, et al. 2017. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 13(11):e1006698
Hu D, Zhu C, Ai L, He T, Wang Y, Ye F, Yang L, Ding C, Zhu X, Lv R, et al. 2018. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg Microbes Infect. 7(1):154
Hui DS, IAzhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, et al. 2020. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 91:264–266
Jalava K. 2020. First respiratory transmitted food borne outbreak? Int J Hyg Environ Health. 226:113490.
Jarvis C. 2020. Mar 16, 2020 which species transmit Covid-19 to humans? we’re still not sure. [Accessed 2020 April 1). https://www.the-scientist.com/news-opinion/which-species-transmit-covid-19-to-humans-were-still-not-sure-67272. [Google Scholar]
Ji W, Wang W, Zhao X, Zai J, Li X. 2020a. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 92(4):433–440.
Ji W, Wang W, Zhao X, Zai J, Li X. 2020b. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross-species transmission from snake to human. J Med Virol. 92 (4):433–440.
Li X, Song Y, Wong G, Cui J. 2020a. Bat origin of a new human coronavirus: there and back again. Sci China Life Sci. 63(3):461–462.
Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, Chaillon A. 2020b. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 92(6):602–611. 10.1002/jmv.25731.
Licitra BN, Duhamel GE, Whittaker GR. 2014. Canine enteric coronaviruses: emerging viral pathogens with distinct recombinant spike proteins. Viruses. 6(8):3363–3376.
Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, Trilling M, Lu M, Dittmer U, Yang D. 2020a. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 92(5):491–494.
Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L. 2020b. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 92(6):595–601. 10.1002/jmv.25726.
Lu H, Stratton CW, Tang YW. 2020a. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 92(4):401–402.
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. 2020b. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574.
Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, Richt JA. The role of swine in the generation of novel influenza viruses. Zoon Pub Health. 2009;56(6-7):326–337.
Ma W, Kahn R E, Richt J A. 2008. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. Journal of Molecular and Genetic Medicine : An International Journal of Biomedical Research. J Mol Genet Med. 3(1):158–166. 19565018
Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. 2020a. Emerging novel Coronavirus (2019-nCoV)-Current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 40(1):68–76.
Malik YS, Sircar S, Bhat S, Vinodhkumar OR, Tiwari R, Sah R, Rabaan AA, Rodriguez-Morales AJ, Dhama K. 2020b. Emerging coronavirus disease(COVID-19), a pandemic public health emergency with animal linkages: Current status update. Indian J Anim Sci. 90(3):158–173.
Mallapaty S. 2020. China set to clamp down permanently on wildlife trade in wake of coronavirus. Nature. doi: 10.1038/d41586-020-00499-2
Mallapaty S. 2020. Coronavirus can infect cats – dogs, not so much. Nature. doi: 10.1038/d41586-020-00984-8
Mihindukulasuriya KA, Wu G, St Leger J, Nordhausen RW, Wang D. 2008. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J Virol. 82(10):5084–5088.
Mitjà O, Clotet B. 2020. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 8(5):e639–e640.
Mohd HA, Al-Tawfiq JA, Memish ZA. 2016. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir. Virol J. 13:87.
Monchatre-Leroy E, Boue F, Boucher JM, Renault C, Moutou F, Ar Gouilh M, Umhang G. 2017. Identification of Alpha and Beta Coronavirus in wildlife species in France: bats, rodents, rabbits, and hedgehogs. Viruses. 9(12):364.
Munster VJ, Feldmann F, Williamson BN, van Doremalen N, Pérez-Pérez L, Schulz J, Meade-White K, Okumura A, Callison J, Brumbaugh B, et al. 2020. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2. Nature. doi: 10.1038/s41586-020-2324-7.
Munjal A, Khandia R, Dhama K, Sachan S, Karthik K, Tiwari R, Malik YS, Kumar D, Singh RK, Iqbal HMN, et al. 2017. Advances in developing therapies to combat Zika virus: current knowledge and future perspectives. Front Microbiol. 8:1469.
Murdoch DR, French NP. 2020. COVID-19: another infectious disease emerging at the animal-human interface. N Z Med J. 133(1510):12–15.
Nishiura H, Linton NM, Akhmetzhanov AR. 2020. Initial cluster of novel coronavirus (2019-nCoV) Infections in Wuhan, China is consistent with substantial human-to-human transmission. JCM. 9(2):488.
OIE. 2020. Animal and environmental investigation to identify the zoonotic source of the COVID-19 virus. [Accessed 2020 March 3]. COVID19_21Feb.pdf.
Parry NMA. 2020. COVID-19 and pets: when pandemic meets panic. Forensic Science International: Reports. 2:100090.
Patel A, Jernigan DB, 2019-nCoV CDC Response Team. 2020. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak – United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 69(5):140–146.
Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, Baghbanzadeh M, Aghamohammadi N, Zhang W, Haque U. 2020. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned?. Int J Epidemiol. doi: 10.1093/ije/dyaa033
Phadke M, Saunik S. 2020. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res. doi: 10.1002/ddr.21666.
Ramadan N, Shaib H. 2019. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 9(1):35–42.
Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, Jiang YZ, Xiong Y, Li YJ, Li H, et al. 2020. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl). 133(9):1015–1024.
Reusken CB, Farag EA, Jonges M, Godeke GJ, ElSayed AM, Pas SD, Koopmans MP. 2014. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 19(23):20829.
Riccucci M. 2012. Bats as materia medica: an ethnomedical reviewe and implications for conservation. Vespertilio. 16:249–270.
Roberts A, Lamirande EW, Vogel L, Jackson JP, Paddock CD, Guarner J, Zaki SR, Sheahan T, Baric R, Subbarao K. 2008. Animal models and vaccines for SARS-CoV infection. Virus Res. 133(1):20–32.
Rodríguez-Morales AJ, Gallego V, Escalera-Antezana JP, Mendez CA, Zambrano LI, Franco-Paredes C, Suárez JA, Rodriguez-Enciso HD, Balbin-Ramon GJ, Savio-Larriera E, et al. 2020a. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. doi: 10.1016/j.tmaid.2020.101613.
Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, Pagliano P, Esposito S. 2020b. History is repeating itself, a probable zoonotic spillover as a cause of an epidemic: the case of 2019 novel Coronavirus. Infez Med. 28(1):3–5.
RodriguezMorales AJ, BonillAldana DK, Tiwari R, Sah R, Rabaan AA, Dhama K. 2020c. COVID-19, an emerging coronavirus infection: current scenario and recent developments – an overview. J. Pure Appl. Microbiol. 14(1):6150.
Rothan HA, Byrareddy SN. 2020. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak [published online ahead of print, 2020 Feb 26]. J Autoimmun. 109:102433.
Rundle AG, Park Y, Herbstman JB, Kinsey EW, Wang YC. 2020. COVID-19 Related School Closings and Risk of Weight Gain Among Children. Obesity (Silver Spring). doi: 10.1002/oby.22813.
Salata C, Calistri A, Parolin C, Palu G. 2020. Coronaviruses: a paradigm of new emerging zoonotic diseases. Pathog Dis. 77(9):ftaa006. doi: 10.1093/femspd/ftaa006.
Serper M, Volk ML. 2018. Current and Future Applications of Telemedicine to Optimize the Delivery of Care in Chronic Liver Disease. Clin Gastroenterol Hepatol. 16(2):157–161.e8.
Shang W, Yang Y, Rao Y, Rao X. 2020. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 5:18.
Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, et al. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 8:eabb7015.
Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S, Khandia R, Munjal A, Vora KS, Latheef SK, Karthik K, Singh Malik Y, Singh R, et al. 2019. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies – a comprehensive review. Vet Q. 39(1):26–55.
Song P, Karako T. 2020. COVID-19: Real-time dissemination of scientific information to fight a public health emergency of international concern. Biosci Trends. 14(1):1–2.
Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. 2016. Epidemiology, genetic recombination, and pathogenesis of Coronaviruses. Trends Microbiol. 24(6):490–502.
Suzuki T, Otake Y, Uchimoto S, Hasebe A, Goto Y. 2020. Genomic characterization and phylogenetic classification of bovine Coronaviruses through whole genome sequence analysis. Viruses. 12(2):183.
Tang XC, Zhang JX, Zhang SY, Wang P, Fan XH, Li LF, Li G, Dong BQ, Liu W, Cheung CL, et al. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J Virol. 80(15):7481–7490.
Tekes G, Thiel HJ. 2016. Feline coronaviruses: pathogenesis of feline infectious peritonitis. Adv Virus Res. 96:193–218.
Villamil-Gómez WE, Sánchez A, Gelis L, Silvera LA, Barbosa J, Otero-Nader O, Bonilla-Salgado CD, Rodríguez-Morales AJ. 2020. Fatal Human Coronavirus 229E (HCoV 229E) and RSV–Related Pneumonia in an AIDS patient from Colombia. Travel Med Infect Dis. :101573. doi: 10.1016/j.tmaid.2020.101573.
Wang LF, Eaton BT. 2007. Bats, civets and the emergence of SARS. Curr Top Microbiol Immunol. 315:325–344.
Wang G, Jin X. 2020. The progress of 2019 Novel Coronavirus (2019-nCoV) event in China. J Med Virol. 92(5):468–472.
Wang W, Tang J, Wei F. 2020. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 92 (4):441–447.
Wassenaar TM, Zou Y. 2020. 2019_nCoV: rapid classification of betacoronaviruses and identification of traditional Chinese medicine as potential origin of zoonotic coronaviruses. Lett Appl Microbiol. 70(5):342–348.
Wei X, Li X, Cui J. 2020. Evolutionary perspectives on novel Coronaviruses identified in pneumonia cases in China. Natl Sci Rev. 7(2):239–242.
Weiss SR, Leibowitz JL. 2011. Coronavirus pathogenesis. Adv Virus Res. 81:85–164.
Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, et al. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 86(7):3995–4008.
Wood C. 2020. Infections without borders: a new coronavirus in Wuhan, China. Br J Nurs. 29 (3):166–167.
Wong ACP, Li X, Lau SKP, Woo P. 2019. Global epidemiology of bat coronaviruses. Viruses. 11(2):174.
WHO. 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 103. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200502-covid-19-sitrep-103.pdf?sfvrsn=d95e76d8_4.
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, et al. 2020a. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 27(3):325–328.
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, et al. 2020b. A new coronavirus associated with human respiratory disease in China. Nature. 579(7798):265–269.
Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou JJ, Li N, Guo Y, Li X, Shen X, et al. 2020. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan Pangolins. bioRxiv. doi: 10.1101/2020.02.17.951335
Xu Y. 2020. Genetic diversity and potential recombination between ferret coronaviruses from European and American lineages. J Infect. 80(3):350–371.
Yang Y-L, Qin P, Wang B, Liu Y, Xu G-H, Peng L, Zhou J, Zhu SJ, Huang Y-W. 2019. Broad cross-species infection of cultured cells by Bat-HKU2 –related swine acute diarrhea syndrome coronavirus and identification of its replication in murine dendritic cells in vivo highlight its potential for diverse interspecies transmission. J Virol. 93(24):e01448–19.
Zhang T, Wu Q, Zhang Z. 2020a. Pangolin homology associated with 2019-nCoV. bioRxiv. doi: 10.1101/2020.02.19.950253.
Zhai S-L, Wei W-K, Lv D-H, Xu Z-H, Chen Q-L, Sun M-F, Li F, Wang D. 2020. Where did SARS-CoV-2 come from? Vet Rec. 186(8):254–254.
Zhang T, Wu Q, Zhang Z. 2020. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Current Biology : CB. Curr Biol. 30(7):1346–1351.e2. doi:10.1016/j.cub.2020.03.022. 32197085
Zhang Q, Zhang H, Huang K, Yang Y, Hui X, Gao J, Jin M. 2020b. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. doi: 10.1101/2020.04.01.021196
Zhang XM, Herbst W, Kousoulas KG, Storz J. 1994. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J Med Virol. 44(2):152–161.
Zheng J. 2020. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 16(10):1678–1685.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. 2020a. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–273.
Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, Zhu Y, Zhang YW, Xie QM, Mani S, Zheng XS, et al. 2018. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature. 556(7700):255–258.
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. 2020b. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579(7798):270–273