Department of Botany and Research Centre, University
College, Thiruvananthapuram, India
Corresponding author email: gssandhia@gmail.com
Article Publishing History
Received: 16/07/2021
Accepted After Revision: 18/09/2021
Endophytes are microorganisms that can live within the inter or intracellular regions of various plant tissues without causing any harmful effects. The endophytes possess different properties which are beneficial to its host plant. They can directly or indirectly promote the plant growth. Hemidesmus indicus (L.) R.Br is a laticiferous twining shrub that belongs to the family Apocynaceae. It is an important medicinal plant that is widely used in various systems of medicine and the plant has pharmacological, phytochemical and ethnobotanical importance. The tuber of the plant is widely exploited in traditional medicine. The microbial population associated with this plant is less explored. In the current study, ten different endophytic bacteria were isolated from the tuber of the plant by following standard isolation procedure. These bacteria were analysed for plant growth promoting potentials.
Of all the isolates, the one designated as SVH1 showed maximum plant growth promoting properties like IAA production, Phosphate solubilization, ACC deaminase production and Siderophore production. SVH1was identified as Bacillus species following morphological, biochemical and molecular characterisation. IAA production from this endophyte was 23.48 µg/ml which is significantly higher than many of the previous reports. The production of IAA was also confirmed by RP- HPLC analysis. The phosphate solubilization index of SVH1 was noticed as 60 which is also a significantly higher value. The isolate SVH1 which is also positive for ACC deaminase and siderophore production can be expected to have growth promoting role in H. indicus. This strain can also be explored in future for the production of valuable bioactive compounds similar to that of H. indicus.
ACC Deaminase, Bacillus Sp, Hemidesmus Indicus, IAA, Phosphate Solubilization, Siderophore
Shyamili C V, Subramaniyan S, Sandhia G S. Plant Growth Promoting Potentials of SVH1 Bacillus Sp. from Hemidesmus indicus. Biosc.Biotech.Res.Comm. 2021;14(3).
Shyamili C V, Subramaniyan S, Sandhia G S. Plant Growth Promoting Potentials of SVH1 Bacillus Sp. from Hemidesmus indicus. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3xL5G4Z“>https://bit.ly/3xL5G4Z</a>
Copyright © Shyamili et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Microorganisms that are symbiotically associated with plants are called endophytes. Plants are the potential reservoir of these indigenous microbes. Endophytic bacteria provide reward to the host plant in many ways – by producing plant growth regulators, inhibiting the phytopathogens and also by helping in phytoremediation. They exist in the plant body asymptomatically. Bacterial endophytes promote plant growth either directly or indirectly.
The growth of a plant can be influenced by the endophytes and they also produce phytohormones. Indole 3 Acetic Acid (IAA) is one the major auxin produced by them (Elbeltagy et al. 2000; Lodewyckx et al. 2002; Schulz and Boyle 2006; Lin and Xu 2013). Endophytes also help the plants to procure nutrients like nitrogen, phosphorus and iron (Yaish et al. 2005; Nandy et al. 2020).
Ethylene is a natural product synthesised by the plant which controls the growth and developmental responses and the response of plants in biotic and abiotic stresses. The hike in ethylene production in a plant is disadvantageous. This may even lead to death of the plant. The endophytic bacteria within the plant helps them to overcome this situation to an extent by producing an enzyme,1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase).
This enzyme breaks the compound ACC into α-keto butyrate and ammonia which can be further utilized by the plants for other purposes (Santoyo et al. 2016). Plant growth is promoted by the endophytic bacteria indirectly by competing with the pathogenic microbes for nutrients and other factors thereby protecting the plants from them (Gray and Smith 2005; Miethke and Marahiel 2007; Nandy et al. 2020).
Moreover, some endophytic Plant Growth Promoting Bacteria (PGPB) also have the proficiency to solubilise unavailable phosphate to available form. Siderophores are iron-chelating compounds secreted by some microorganisms under iron-limiting conditions. This is the strongest soluble iron binding compound. Siderophore production by associated endophytes supports the plants to survive difficult environments and make iron as a limiting factor to plant pathogens (Miethke and Marahiel 2007). H. indicus (L.) R.Br. is an important medicinal plant that is widely used in various systems of medicine and the plant has pharmacological, phytochemical and ethnobotanical importance (Nandy et al. 2020).
It has been recently reviewed by many researchers that the endophytic bacteria within a host plant have plant growth promoting properties (Jasim et al. 2013, Puri et al. 2017, Pawlik et al. 2017; Nandy et al. 2020). They are used as bioinoculants for sustainable agriculture (Kahtani et al. 2020). The endophytic bacterial community of H. indicus is less explored even though it is a medicinally significant plant. In this scenario, the present study aimed at the isolation, identification and analysis of the plant growth promoting potentials of the endophytic bacterial isolate SVH1 from H. indicus.
MATERIAL AND METHODS
According to the procedure of Jasim et al. (2013), isolation of the endophytic bacteria from H. indicus were done. All the nutrient agar plates inoculated with the root of the plant including the control plate were incubated at 28 0C for 5 days and observed daily for bacterial growth. The plates were examined regularly and distinct colonies developed were selected which were further purified by repeated streaking. The pure cultures thus obtained were regularly sub cultured and maintained. The endophytic bacterial isolates obtained from the culture plates were screened for the plant growth promoting traits. Using the procedure of Rahman et al. (2010), IAA production was tested.
The presence of IAA was confirmed using Reverse Phase HPLC. Using the method of Jasim et al. (2013), the ACC deaminase production and phosphate solubilization of the endophytic bacterial isolates from H. indicus were screened. The growth of bacteria in the media after 2 days of incubation was considered as positive result for ACC Deaminase production and the formation of yellow zone around the colony indicates phosphate solubilization.
Siderophore production was tested in CAS agar medium. Development of the yellowish halo around the colonies indicate the production of siderophore. The endophytic isolate which showed maximum plant growth promoting traits was subjected to morphological, biochemical and molecular characterization (Schwyn and Neilands 1987; Jasim et al. 2013).
Morphological characterisation of the colony of the endophytic bacterial isolate was done by observing the characters like colour, surface, margin, elevation, opacity, motility and Gram’s staining features of the colony. According to Cappuccino and Sherman (2014), biochemical characterization of the selected endophytic isolates was done. For molecular characterization, genomic DNA was isolated from the bacterial isolates and was used as template for Polymerase Chain Reaction (PCR). The PCR product was checked by agarose gel electrophoresis, purified further and was then subjected to sequencing. The sequence data thus obtained was checked by BLAST analysis (Zhang et al. 2000; Cappuccino and Sherman 2014).
RESULTS AND DISCUSSION
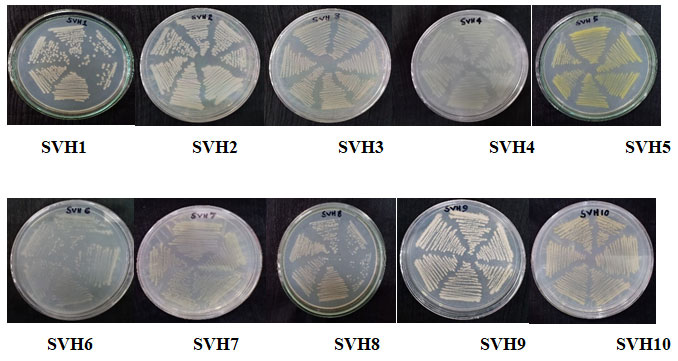
Isolation of the endophytic bacteria: Isolation of the endophytic bacteria was done by using fresh and cleaned tubers of H. indicus. The surface sterilization of the plant material was effective as no growth appeared on the control plates. Ten endophytic bacterial isolates were obtained from the culture plates by purification through continuous sub-culturing and were named as SVH1-SVH10 (Figure 1).
Figure 1: The isolated endophytic bacteria from H. indicus
Screening of the endophytic bacterial isolates were done for plant growth promoting traits. The results are given in Table 1.
Table 1. Screening of the endophytic bacteria for plant growth promoting traits
| Plant growth promoting traits |
Endophytic bacterial isolates |
|||||||||
| SVH1 | SVH2 | SVH3 | SVH4 | SVH5 | SVH6 | SVH7 | SVH8 | SVH9 | SVH10 | |
| IAA Production | +++ | + | + | + | + | _ | + | + | + | + |
| Phosphate solubilization | + | + | + | _ | _ | _ | _ | _ | + | + |
| ACC Deaminase Production | ++ | + | _ | _ | _ | _ | _ | _ | _ | + |
| Siderophore Production | + | _ | _ | _ | + | _ | _ | + | + | +
|
The screening studies revealed the potential of SVH1 in having plant growth promoting traits like IAA production, phosphate solubilization, ACC deaminase production and Siderophore production.
Identification of the isolate SVH1 by morphological, biochemical and molecular characterization: Morphological characterization: The morphological characterization of the endophytic bacteria isolate SVH1 was observed. (Figure 2) and tabulated (Table 2). The isolate was Gram positive and motile.
Figure 2: SVH1
Table 2. Morphological characterisation
Biochemical characterization: Biochemical characterization of the isolate was done using several tests. Indole Production test, Starch hydrolysis test, Catalase test, Voges-Proskauer’s test, Methyl Red test, Nitrate Reduction test and Citrate utilisation test was positive for the isolate SVH1. The isolate was negative for Urease test.
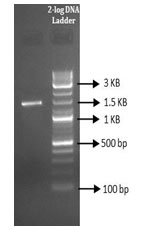
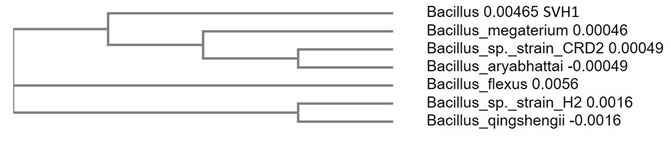
Molecular characterization of the isolate by 16s rRNA gene sequencing: Molecular characterization of the isolate was done by using 16s rRNA sequencing. The amplification of the 16s rRNA was confirmed by agarose gel electrophoresis (Figure 3). PCR product was gel eluted and sequenced and presented in FASTA format. The 16S rRNA sequence of the endophytic isolate was compared with that of other bacterial sequences by using BLAST. The sequence analysis of SVH1 showed maximum identity to Bacillus sp. The PCR amplified product of 16s rRNA gene sequence and the phylogenetic tree of SVH1 is given below.
Figure 3: PCR amplified product of SVH1
Following is the base pair sequence of SVH1.
>Bacillus SR1515-SVHI-RSF1_E05.ab1
GCAGTAATTCAGCTTGCTTCTATGACGTTAGCGGCGGACGGGTGAGTAACACGTGGGCAACCTGCCTGTAAGACTGGGATAACTTCGGGAAACCGAAGCTAATACCGGATAGGATCTTCTCCTTCATGGGAGATGATTGAAAGATGGTTTCGGCTATCACTTACAGATGGGCCCGCGGTGCATTAGCTAGTTGGTGAGGTAACGGCTCACCAAGGCAACGATGCATAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGACGAAAGTCTGACGGAGCAACGCCGCGTGAGTGATGAAGGCTTTCGGGTCGTAAAACTCTGTTGTTAGGGAAGAACTAGTACGAGAGTAACTGCTCGTACCTTGACGGTACCTAACCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTATCCGGAATTATTGGGCGTAAAGCGCGCGCAGGCGGTTTCTTAAGTCTGATGTGAAAGCCCACGGCTCAACCGTGGAGGGTCATTGGAAACTGGGGAACTTGAGTGCAGAAGAGAAAAGCGGAATTCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGGCGAAGGCGGGCTT
From analysing the base pair sequence of SVH1, the phylogenetic tree of the SVH1 was constructed (Figure 4).
Figure 4: Phylogenetic tree of the endophytic isolate SVH1
Screening of the isolates for plant growth promoting traits: Plant growth promotion by the endophytic microbes including fungi and bacteria makes them a better resource for modern agriculture (Zhao et al. 2017). In the present study we have isolated ten different endophytic bacteria from the roots of H. indicus. After the screening for plant growth promoting traits, SVH1 showed positive results for all the four screening tests of IAA production, Phosphate solubilization, Siderophore production and ACC Deaminase production.
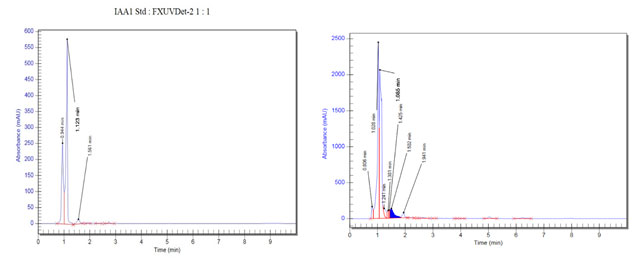
IAA production: From the screening of the selected endophytic isolates for the production of IAA, it was confirmed that the strain SVH1 is positive for IAA as it showed the development of red colour (Figure 5 a). Production of IAA was confirmed by RP-HPLC. The methanolic extract of SVH1 was subjected to HPLC analysis. Both the standard IAA and the extract of SVH1 showed the peak at the same retention time (Figure 6). The amount of IAA produced by each endophyte was also calculated using their absorbance at 530 nm.
Table 3: Quantification of IAA production
| Endophytic bacteria | Amount of IAA produced (µg/mL) |
| SVH1 | 23.48 |
| SVH2 | 15.24 |
| SVH3 | 16.64 |
| SVH4 | 16.08 |
| SVH5 | 7.99 |
| SVH7 | 11.44 |
| SVH8 | 7.30 |
| SVH9 | 1.8 |
| SVH10 | 3.63 |
IAA is an important phytohormone among the different auxins produced by plants. Some endophytic bacteria also have the ability to produce IAA. This may be the reason for the increased growth rate of plants when it is associated with endophytic bacteria. IAA performs different functions like stimulation of cell division, cell and tissue differentiation, cell elongation and lateral root formation. In bacterial cells, IAA has no specific function, but it can be speculated that this helps to maintain the interaction between plants and associated endophytic bacteria (Pattern and Glick 1996).
Etesami et al. (2015) suggested that bacterial IAA loosens the plant cell wall and the result of this reaction is increase in the release of the root exudates. This helps the rhizosphere bacteria to acquire more nutrients and colonization of this rhizosphere bacteria in to the plant will also be promoted (Etesami et al. 2015).
Although a large number of bacteria have been reported to produce IAA, this is the first report on bacterial endophytes producing IAA from H. indicus. Bacillus species isolated from the roots of Catleya walkeriyana, a Brazilian species of orchid, an endangered species, showed increase in all characters of the plant and prolonged the percentage of seedling survival (Galdiano et al. 2011; Chagas et al. 2015).
The genus Bacillus sp. was reported as one of the main genera of Plant Growth Promoting Rhizobacteria (Chagas et al. 2015). These PGPR can later colonize in to the plants and exist as endophytes. From table 3, it was understood that SVH1 showed maximum production of IAA in the medium supplemented with L-tryptophan. Gomes et al. (2017) reported the production of 4.12 µg/mL IAA by endophytic Bacillus sp. isolate EB.78 from Banana plant. Comparing to this result, Bacillus sp. SVH1 from H. indicus showed the production of 23.48 µg/mL IAA in the medium supplemented with L-tryptophan which is significantly greater.
Bhatt and Maheswari (2020) isolated a zinc solubilizing Bacillus megaterium CD25 and it showed the production of 13.8 µg/mL IAA. These bacteria also found to enhance growth in Capsicum annuum L. The IAA production by the endophytic bacteria in plants helps them for growth promotion and as well as it amplifies the further colonization of other beneficial bacteria. The strain SVH1 identified as Bacillus sp. in this study may have plant growth promoting role in H. indicus (Gomes et al. 2017; Bhatt and Maheswari 2020).
IAA is an important phytohormone among the different auxins produced by plants. Some endophytic bacteria also have the ability to produce IAA. This may be the reason for the increased growth rate of plants when it is associated with endophytic bacteria. IAA performs different functions like stimulation of cell division, cell and tissue differentiation, cell elongation and lateral root formation. In bacterial cells, IAA has no specific function, but it can be speculated that this helps to maintain the interaction between plants and associated endophytic bacteria (Pattern and Glick 1996). Etesami et al. (2015) suggested that bacterial IAA loosens the plant cell wall and the result of this reaction is increase in the release of the root exudates. This helps the rhizosphere bacteria to acquire more nutrients and colonization of this rhizosphere bacteria in to the plant will also be promoted (Etesami et al. 2015).
Although a large number of bacteria have been reported to produce IAA, this is the first report on bacterial endophytes producing IAA from H. indicus. Bacillus species isolated from the roots of Catleya walkeriyana, a Brazilian species of orchid, an endangered species, showed increase in all characters of the plant and prolonged the percentage of seedling survival (Galdiano et al. 2011; Chagas et al. 2015).
The genus Bacillus sp. was reported as one of the main genera of Plant Growth Promoting Rhizobacteria (Chagas et al. 2015). These PGPR can later colonize in to the plants and exist as endophytes. From table 3, it was understood that SVH1 showed maximum production of IAA in the medium supplemented with L-tryptophan. Gomes et al. (2017) reported the production of 4.12 µg/mL IAA by endophytic Bacillus sp. isolate EB.78 from Banana plant.
Comparing to this result, Bacillus sp. SVH1 from H. indicus showed the production of 23.48 µg/mL IAA in the medium supplemented with L-tryptophan which is significantly greater. Bhatt and Maheswari (2020) isolated a zinc solubilizing Bacillus megaterium CD25 and it showed the production of 13.8 µg/mL IAA. These bacteria also found to enhance growth in Capsicum annuum L. The IAA production by the endophytic bacteria in plants helps them for growth promotion and as well as it amplifies the further colonization of other beneficial bacteria. The strain SVH1 identified as Bacillus sp. in this study may have plant growth promoting role in H. indicus (Gomes et al. 2017; Bhatt and Maheswari 2020).
Figure 5: Plant growth promoting traits of SVH1 (a) – IAA production, (b)-phosphate solubilization, (c) – ACC deaminase production, (d) – Siderophore production.
Figure 6: HPLC Analysis of the standard IAA and the ethyl acetate extract of the endophytic isolate, SVH1. A. Indole 3 Acetic Acid B. Ethyl acetate extract of SVH1.
ACC deaminase Production: The endophytic bacterial isolate SVH1 was screened for the production of ACC deaminase on DF salt minimal medium containing 0.2% ammonium sulphate. The growth of the isolate on this media indicated that it is positive for the production of ACC deaminase (Figure 5 c). ACC deaminase is the enzyme that cleaves ACC into ammonia and α-ketobutyrate. The reduction in the level of ACC in plants resulted in the simultaneous reduction of plant ethylene levels due to the presence of ACC deaminase producing endophytic bacteria (Glick et al. 2007; Bhatt and Maheswari 2020).
The higher level of ethylene in plants is lethal to them. But the association with ACC deaminase producing endophytic bacteria enable the plants to tolerate the stressed conditions caused by ethylene. Different Bacillus species have been reported to produce ACC deaminase. ACC deaminase producing B. subtilis was reported from tomato seedlings by Xu et al. (2014). Kothari and Vyas (2015) reported that B. cereus brm, an endophyte from Vigna radiate (Mung beans) produced ACC deaminase and the plant could overcome inhibitory influence of salt on root and shoot elongation under salinity stress.
Similar results were found in the past study (Grobelak et al. 2018). The endophytic bacterial consortium with ACC deaminase production improved the growth of plants under the stress of heavy metal contaminated soil. By analysing these results, Bacillus sp. could be considered as a potent plant growth promoter. Thus, SVH1 could also be considered as a potent plant growth promoter (Xu et al. 2014; Kothari and Vyas 2015; Grobelak et al. 2018; Bhatt and Maheswari 2020).
Phosphate solubilization: Yellow zone around the bacterial colony indicated the phosphate solubilization efficiency of the isolate SVH1 (Figure 5 b). Phosphate solubilization index was found out using the following formula. Phosphate solubilization index =Total diameter of the colony (diameter of the colony + diameter of the yellow zone)/Diameter of the colony×100. The solubilization zone diameter of SVH1 was 2.2 cm and colony diameter were 1.6 cm. The phosphate solubilization index of SVH1 is 60. Nutrients available for plants determine the rate of growth in plants.
Macronutrients and micronutrients are the essential nutrients for plants. Among the macronutrients, phosphorus is an important one. The growth in plants may be limited at some point in their lifetime due to the non-availability of this phosphorus. Different phosphorus compounds are abundant in agricultural soils, but in the insoluble form. Several reports have shown that different rhizosphere bacteria have the ability to solubilize insoluble phosphates available in the soil (Khan et al. 2020).
Rhizosphere colonizing bacteria produce low molecular weight organic acids which in turn lead to the acidification of the soil or media. This contributes primarily towards the phosphate solubilizing mechanism (Puente et al. 2004). The potential of bacterial endophytes to solubilize mineral phosphates is a promising characteristic to the agricultural field (Quecine et al. 2012). Longfei et al. (2015) reported that endophytic Bacillus and Paenibacillus strains isolated from the medicinal plant Lonicera japonica showed higher rate of phosphate solubilization compared to that of other endophytic bacterial strains from this plant. From the study of Joe et al.
(2016), phosphate solubilizing endophytic Bacillus isolates from Phyllanthus amarus promoted plant growth and phosphorus content under salt stress. Saeid et al. (2018) reviewed from their studies that Bacillus species could be considered as the potent phosphate solubilizing group of microorganisms. Bacillus sp. SVH1 from H. indicus showed phosphate solubilization efficiency of 60 which is a significantly a greater value (Quecine et al. 2012; Longfei et al. 2015; Joe et al. 2016; Saeid et al. 2018; Khan et al. 2020).
Siderophore production: After 24 hours of incubation, the strain SVH1 showed the formation of yellowish hallow around the colony in the CAS agar medium. This indicated that this strain is capable of producing siderophore. Siderophores are the low molecular weight compounds that chelate iron with great affinity. The production of siderophores by the endophytic bacteria will help them to scavenge the iron from the extracellular environment. This payback the host plant directly and indirectly. They can promote the plant growth directly by making the iron in the surroundings easily available to the plant and indirectly by their biocontrol activities. The endophytic bacterial strain SVH1 showed siderophore production in CAS medium (Khan et al. 2020).
From the study of George et al. (2011) different Bacillus sp. isolated from the rhizosphere and roots of coconut showed siderophore production and they were antagonistic against Ganoderma applanatum and Theilaviopsis paradoxa. Rajkumar et al. (2009) reviewed that an endophytic siderophore producing Bacillus sp. isolated from the tissues of nickel hyperaccumulator plant Allysum bertoloni showed plant growth promotion under Ni stress. Siderophore producing endophytic bacteria thus can act as a promising source for plant growth promotion as well as biocontrol. Khan et al. (2020) reported the production of siderophores by endophytic bacteria from Lilium lancifolium. Bacillus SVH1 could also be considered as a potent plant growth promoter (Rajkumar et al. 2009; George et al. 2011; Khan et al. 2020).
CONCLUSION
The findings of the present study confirms that endophytic bacterial isolate SVH1 from H. indicus can be used as a potent plant growth promoter. The isolate SVH1 was identified as Bacillus sp. SVH1and was found to possess IAA production, ACC deaminase production, phosphate solubilization activity and siderophore production. The endophytic bacteria with plant growth promoting characteristics can be used as bioinoculants for sustainable agriculture.
Bacillus sp. SVH1 along with other potent plant growth promoting endophytic bacteria could be used for the preparation of a consortium to be used as an environment friendly replacement of chemical fertilizers after the quantitative studies in future. The secondary metabolite production by the endophytic bacteria is another significant trait. Production of the similar metabolites as that of the host plant by SVH1 and other endophytic bacteria from H.indicus would be investigated in the future studies.
ACKNOWLEDGEMENTS
This study was financially supported by the Council of Scientific and Industrial Research (CSIR) through its fellowship. Moreover, authors acknowledge the Principal of University College, Palayam, Thiruvananthapuram (University of Kerala) and Director of Department of Collegiate Education for all the facilities and assistance given during the period of work. Authors also thank Rajiv Gandhi Centre for Biotechnology (RGCB), Thiruvananthapuram for the bacterial genome sequencing.
Conflict of Interests: Authors declare no conflict of interests to disclose.
REFERENCES
ALKahtani, M.D., Fouda, A., Attia, K.A., et al. (2020). Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy, 10(9),1325.
Bhatt, K. and Maheshwari, D.K. (2020). Bacillus megaterium Strain CDK25, a Novel Plant Growth Promoting Bacterium Enhances Proximate Chemical and Nutritional Composition of Capsicum annuum L. Frontiers in Plant Science, 11, 1147.
Cappuccino, J.G. and Sherman, N. (1983). A laboratory manual. Addision Wesley Longman. Inc. Sydney, Australia, 477.
Chagas Junior, A.F., De Oliveira, A.G., De Oliveira, L.A., et al. (2015). Production of indole-3-acetic acid by Bacillus isolated from different soils. Bulgarian Journal of Agricultural Science, 21(2), 282-287.
Elbeltagy, A., Nishioka, K., Suzuki, H., et al. (2000). Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil science and plant nutrition, 46(3), 617-629.
Etesami, H., Alikhani, H.A. and Hosseini, H.M. (2015). Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX, 2, 72-78.


