Post Gragdute and Research Department of Biotechnology, Women’s Christian College, Chennai, Tamilnadu, India
Corresponding author email: anjo_64@yahoo.com
Article Publishing History
Received: 17/04/2020
Accepted After Revision: 18/06/2020
Medicinal plants play a predominant role in our E-mail ID: ecosystem due to the vast distribution of bioactive components such as alkaloids, phenols, tannins, flavanoids etc… within them and each with a specific function. A study on these bioactive compounds and their beneficial roles is of much interest and are of utmost importance these days due to their wide therapeutic properties. The present work has been carried out in order to investigate the presence of important bioactive components in different solvent extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L. leaf samples and thereby testing their antibacterial activity. Cold extraction method was employed for extraction of leaf samples using different solvents such as ethanol, chloroform, ethyl acetate and water (aqueous). The extracts were subjected to both qualitative and quantitative analysis to determine the presence and amount of bioactive compound in the sample. The extracts were also tested for their pathogen inhibitory activity (antibacterial activity) against both Gram +ve and Gram –ve bacteria such as Staphylococcus aureus, Streptococcus mutans, Klebsiella pneumoniae, Salmonella typhi and Enterobacter spp. Based on the results obtained ethanolic extract was found to be rich in majority of phytocomponents with increased bioactivity compared to other extracts. This further suggests that ethanolic extract of these samples could be used in future for isolation of various novel compounds for different pharmacological applications.
Antibacterial activity, Bioactive components, Cold extraction, Medicinal plants, Phytochemical screening, Solvent extracts
Leela K, Singh A. R. J. Phytochemical Screening and Antibacterial Activity of Three Medicinal Plants - Lawsonia inermis, Mangifera indica and Piper betel. Biosc.Biotech.Res.Comm. 2020;13(2).
Leela K, Singh A. R. J. Phytochemical Screening and Antibacterial Activity of Three Medicinal Plants – Lawsonia inermis, Mangifera indica and Piper betel. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3bLiNYC
Copyright © Leela and Singh This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nature has gifted us with enormous resources and one among them is the widely distributed, a therapeutically valuable source termed as “Medicinal Plants”. These medicinal plants contain numerous beneficially active compounds that are highly under research worldwide for treatment of many diseases. India has a rich diversity of plant species and is known for its traditional system of medicine such as Siddha, Ayurveda and Unani
(Pandey et al., 2013). Medicinal plants are used as a primary source in health care wherein all the parts such as root, leaf, flower, seed or even whole plant as such is used in the development of drug formulations. These natural or herbal drugs are widely preferred over synthetic medicines due to their easy availability, high efficiency and low toxicity (Patel 2016). Phytochemicals are also referred to as “Secondary metabolites” and these are naturally occurring compounds found in plants which play an important role in protecting plant cells against various environmental conditions and apart from it they also impart health benefits to human beings in the form of dietary supplements and as medications (Nyamai et al., 2016). The nature and concentration of secondary compounds present also varies within plant species and each compound exhibits a specific activity. Based on their structure, solubility and biosynthetic pathway these compounds are classified into three groups: Phenolic compounds, Alkaloids and Terpenes. These compounds exert various biological activities and also participate in both the primary as well as secondary metabolism of plants (Irina Francesca Gonzalez Mera et al., 2019).
Some of the pharmaceutical applications of secondary metabolites involve antiviral, antitumor, antioxidant, immunosuppressant, anti-ageing, anti-inflammatory, anti-hyperglycemic activities etc Vaishnav and Demain, 2011).
Lawsonia inermis L. is a medicinal plant commonly referred to as “Henna” belonging to the family Lythraceae. They contain various bioactive components such as Lawsone, quinone, xanthone, gallic acid, triterpenoids which are found to exhibit anti-neoplastic, anti-inflammatory, anti-haemorrhagic, hypotensive activity etc. They are also used as astringents, in treating leprosy, nervous disorders, also for treating boils and burns (Ghodekar et al., 2019).
Mangifera indica L. commonly referred to as “Mango” belongs to the family Anacardiaceae. Some of the phytochemical components reported in the plant involve Mangiferin, catechin, tannins, triterpenoids. Different parts of the plant are used in the treatment of rheumatism, dysentery, haemorrhage, asthma and anaemia They also possess anti-inflammatory, antiparasitic, anti-helmintic, anti-allergic, antipyretic, anti-HIV and hepatoprotective properties, ( Shah et al., 2010). Piper betel L. commonly referred to as “Betel/Paan” belongs to the family Piperaceae. It is grown extensively in India, Malaysia, Srilanka and Thailand….It is an evergreen herb which has been studied from past for its pharmacological activites and also used in many ayurvedic preparations. They are used as antiseptics, as healing agents, mouth freshener and are also reported to possess antimicrobial, antioxidant and antileishmanial properties, ( Datta et al., 2011). Due to the high prevalence and emergence of new dreadful diseases, there is a need for development of new drug formulations in order to overcome these circumstances. Plant based secondary metabolites has drawn the interest of many researchers worldwide due to their low toxicity, easy availability and minimal side effects compared to the synthetic ones. Plants contain abundant secondary compounds most of which are yet to be completely studied. This study deals with the phytochemical screening and antibacterial activity of different solvent extract of three important medicinal plants – Lawsonia inermis L., Mangifera indica L. and Piper betel L.
MATERIALS AND METHODS
Sample Preparation: The leaf samples of Lawsonia inermis L., Mangifera indica L. and Piper betel L. were collected from different localities. The collected samples were cleaned, washed thoroughly with distilled water and shade dried for about 3 weeks at room temperature. The samples were then powdered and stored in clean bottles until required.
Extraction & Determination of Percentage Yield: The powdered samples were extracted using solvents of different polarities and water (aqueous). For solvent extraction, about 10 grams of the powdered samples were kept immersed separately in 100ml of solvents such as ethanol, chloroform, ethyl acetate in the ratio of 1:10. The samples were covered and incubated in dark for about a week ( Sivareddy et al., 2019).
For aqueous extraction, about 10 grams of the powdered samples were added to 100ml of double distilled water and heated on water bath for about 30mins (Al-Manhel and Niamah., 2015). The samples were filtered using whatmann no.1 filter paper and the filtrate obtained was concentrated or dried to yield the crude extracts. The percentage (%) yield of crude extracts obtained was calculated based on the formula: (Terblanche et al., 2017). Percentage yield (%) = Dry weight of extract (g) / Dry weight of sample (g) × 100
Qualitative analysis: Ethanol, Chloroform, Ethyl acetate and Aqueous (water) extracts of all the three samples were subjected to qualitative phytochemical analysis in order to identify the presence of bioactive components in the extracts. The analysis was carried out for about 23 different phytocomponents such as Phenol (Ferric chloride test), Tannins, Glycosides (Keller-Killani test), Cardiac glycoside, Alkaloid (Hagers test), Flavanoids (Lead acetate test), Terpenoids (Salkowski test), Diterpenes (Copper acetate test), Steroids, Phytosterol, Quinones, Saponin, Resin (Acetone water test), Coumarins (Sodium hydroxide test), Volatile oil, Fixed oil and fats (Stain test), Carboxylic acid, Phlobotannins, Carbohydrate ( Benedicts and Fehlings test), Protein (Biuret and Xanthoproteic test), Aminoacid (Ninhydrin test), Starch (Iodine test) and Lipids according to the standard procedure (Godghate et al., 2012; George., 2017; Kumar et al., 2013).
Quantitative analysis: Phenol estimation: Phenol estimation was carried out according to Folin-Ciocalteau method. To 0.5 ml of extract, 2.5ml of 10% Folin-Ciocalteau reagent and 2.5ml of 7.5% Sodium carbonate was added. The samples were then incubated at 45º C for about 45 minutes and the absorbance was determined at 765nm using UV-Visible Spectrophotometer. Gallic acid was used as the standard. Samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The same procedure was repeated for gallic acid standard and calibration line was constructed. Total phenolic content was expressed in terms of Gallic acid equivalent (mg GAE/g extract),(Singleton et al., 1999).
Tannin estimation: To 0.5ml of extract, 8ml of distilled water was added followed by the addition of 0.5ml FC reagent and 1ml Sodium carbonate. The samples were then incubated for about 30 mins at room temperature and absorbance was measured at 725nm using UV-Visible Spectrophotometer. Tannic acid was used as the standard. Samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The same procedure was repeated for tannic acid standard and calibration line was constructed. Total tannin content was expressed as mg TAE/g extract, (Anbukkarasi et al., 2017).
Flavanoid estimation: 10 gram of plant sample was extracted twice with 100ml of 80% methanol at room temperature. The whole solution was filtered and the filtrate obtained was transferred into a crucible and allowed to dry over water bath and weighed to a constant weight. Percentage flavanoid content was calculated using the formula: % Flavanoid content = Weight of dried extract / Weight of Sample × 100 (Bohm and Kocipai 1994).
Alkaloid estimation: About 200ml of 10 % acetic acid in ethanol was added to 5gram of plant sample taken in a conical flask, covered and incubated for about 4 hours. It was filtered and the filtrate was concentrated on water bath to one quarter of original volume. Concentrated ammonium hydroxide was added drop wise to the extract until precipitation was complete. The solution was allowed to settle and the precipitate was collected, washed with dilute ammonium hydroxide and filtered. The residue is alkaloid which was dried and weighed. Percentage alkaloid content was calculated using the formula: % Alkaloid content = Weight of alkaloid (residue) / Weight of sample × 100 (Harborne, 1973).
Saponin estimation: About 20% of aqueous ethanol was added to 5gram of plant sample taken in a conical flask and heated at 55ºC with continuous stirring for about 4 hours. The residue was again extracted with 100ml of 20% ethanol and heated at 90 ºC in water bath. The concentrate was transferred into a separating funnel and to that 20 ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered and about 60ml of butanol was added and extracted twice with 5% sodium chloride. The remaining solution was heated on water bath and after evaporation the sample was dried to a constant weight. Percentage saponin content was calculated using the formula: % Saponin content = Weight of Saponin / Weight of sample × 100 (Obadoni and Ochuko 2001).
Terpenoid estimation: 10 gram of the powdered sample was kept soaked in 100ml of ethanol for about 24 hours. It was filtered and the filtrate was extracted with 40ml petroleum ether using the separating funnel. Ether extract was seperated and allowed to dry. After drying the percentage terpenoid content was calculated using the formula: % Terpenoid content = Weight of terpenoid extract / Weight of Sample × 100 (Ferguson, 1956).
Glycoside estimation: About 10ml of Baljets reagent (95ml of picric acid + 5ml of aqueous sodium hydroxide) was added to 1ml of extract and the sample was incubated for about 1hour at room temperature. It was then diluted with 20ml distilled water and mixed well. The absorbance was measured at 495nm using spectrophotometer. The samples were prepared in triplicate and mean value of absorbance was obtained. The values are expressed in Mean ± Standard deviation, (El-Olemy et al., 1994).
Cardiac glycoside estimation: 1gram sample was taken and to that 2.5ml of 15% lead acetate was added and the mixture was filtered. To the filtrate 2ml chloroform was added and shaken vigorously. 3ml glacial acetic acid was added followed by the addition of 0.5ml of 5% ferric chloride and 0.05ml of concentrated sulphuric acid. The reaction mixture was incubated in dark for about 2hours and the absorbance was measured at 530nm. The samples were prepared in triplicate for each analysis and mean value of absorbance was obtained. The values are expressed in Mean ± Standard deviation, (Ugwoke et al., 2017).
Coumarin estimation: To 0.5ml of extract, 2ml of distilled water and 0.5ml of lead acetate solution was added and shaken. To this 7ml distilled water was added and mixed well. 2ml of this solution was taken in a test tube and to that 8ml of 0.1M Hydrochloric acid solution was added and incubated at room temperature for about 30 minutes and the absorbance was measured at 320nm using UV-Visible spectrophotometer. The samples were prepared in triplicate for each analysis and mean value of absorbance was obtained. The values are expressed in Mean ± Standard deviation, (Osorio and Martins 2004).
Steroid estimation: About 0.5ml of extract was taken separately in a test tube and similarly different concentrations (20-100µg/ml) of standard were added to a series of test tubes labelled S1-S5. Cholesterol was used as the standard. To this 5ml of ferric chloride diluting reagent was added followed by the addition of 4ml of concentrated sulphuric acid. The reaction mixture was incubated at room temperature for about 30mins and the absorbance was measured at 540nm in colorimeter. Standard graph was plotted from which the steroid content was determined. The concentration of steroid was expressed in mg/100ml (Zak, 1954).
Analysis of Nutritional components
Carbohydrate estimation: It was performed according to Phenol-sulphuric acid method. Glucose was used as the standard. Different concentrations of standard glucose (20-100µg/ml) and about 0.5ml of extract were taken separately into a series of test tube. Volume in all test tubes were made upto 1ml using distilled water.1ml phenol and 5ml of sulphuric acid was added, shaken for 10 minutes and incubated in boiling water bath at 25 – 30ºC for 20 minutes. Green colour developed was read at 490nm in colorimeter. Standard graph was plotted from which the carbohydrate content was determined. The concentration was expressed in mg/100ml, (Dubois et al., 1956).
Protein estimation: It was performed according to Lowry’s method, Bovine serum albumin was used as the standard. Different concentrations of standard (BSA) (50-250µg/ml) and 0.5ml of extract were taken separately into a series of test tube. Volume was made upto 2.5ml in all test tubes with distilled water. 5ml of Reagent I (48ml of Reagent A- 2% sodium carbonate in 0.1N sodium hydroxide + 1ml of Reagent B – 1% Potassium sodium tartarate + 1ml of Reagent C – 0.5% Copper sulphate) was added to each tube and incubated for about 10minutes. After 10 minutes, 0.5ml of Reagent II (Folin – Ciocalteau in the ratio of 1:2) was added, mixed and incubated at room temperature for about 30minutes. Blue colour developed was read at 660nm in colorimeter. Standard graph was plotted from which the protein content was determined and concentration was expressed in mg/100ml (Lowry et al., 1951).
Aminoacid estimation: It was performed according to Ninhydrin method. Leucine was used as the standard. Different concentrations of standard Leucine (20-100µg/ml) and 0.5ml of extract was taken separately into a series of test tube. Volume was made upto 2ml in all tubes with distilled water. To this 2ml of ninhydrin reagent was added, vortexed and incubated in water bath for about 15 minutes and cooled. About 3ml of 50% ethanol was added to all tubes and mixed well. The absorbance was measured at 570nm in colorimeter. Standard graph was plotted from which the aminoacid content was determined and the concentration was expressed in mg/100ml. (Moore, S and Stein, W.H., 1948)
Lipid estimation: It was carried out according to Folch method. 1gram of sample was kept immersed in 20ml solvent mixture comprising Chloroform: methanol in the ratio of 2:1. It was agitated for about 15-20 minutes in shaker and the solution was filtered. About 0.9% sodium chloride solution was added to the filtrate and centrifuged at 2,000 rpm for 10 minutes. Upon centrifugation 2 layers got seperated wherein the upper phase is methanol while lower chloroform phase contains lipid. Lipid was collected, allowed to dry and weighed. Weight of lipid is expressed as mg lipid per 100mg of dry material, (Folch, 1957).
Antibacterial activity: The extracts were screened for their antibacterial activity against both Gram positive and Gram negative bacteria such as Staphylococcus aureus, Streptococcus mutans, Klebsiella pneumoniae, Salmonella typhi and Enterobacter spp by means of Agar well diffusion method. About 150ml of Muller Hinton agar medium was prepared, poured into the petriplates and allowed to solidify. Once solidified the bacterial cultures were swabbed onto the agar medium using a sterile cotton swab .The wells were punctured using a sterile cork borer and the extracts were dispensed into each well using a micropipette. The petriplates were then incubated at 37°C for 24 hours and observed for the zone of inhibition. The diameter was measured in mm ( Saif et al., 2017).
RESULTS AND DISCUSSION
Extraction: In this study, cold extraction method was employed for extraction of plant leaf samples (Figure 1-3) using various solvents such as ethanol, ethyl acetate, chloroform and aqueous (Figure 4).This method of extraction is found to be advantageous over hot extraction method wherein the chance for loss of required volatile compounds in the plant is low.
Figure

Organic solvents are generally used for extraction of bioactive components from plant sample. However, among the solvents ethanol is most commonly used in medicinal preparations due to its safety and low toxic nature ( Wendakoon et al., 2012). The choice of solvent used for extraction also varies depending on the type of compound to be isolated. Ethanol can be used for extraction of Polyphenol, flavanols, tannins, alkaloids, steroids etc…Water can extract compounds such as saponins, tannins, starch, polypeptides, while chloroform is used for extracting flavanoids and terpenoids (Pandey and Tripathi., 2014).
Figure

Figure 4: Extraction of Lawsonia inermis l., Mangifera indica l. and Piper betel l. using different solvents and water
Percentage Yield of extracts
Figure 5: Shows percentage yield of extracts in different solvents
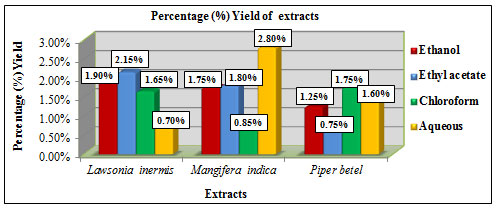
The percentage yield of extracts obtained using different solvents like ethanol, chloroform, ethyl acetate and aqueous were calculated using the formula: % Yield = Dry weight of extract (g) / Dry weight of sample (g) × 100. Lawsonia inermis L. showed higher yield in ethyl acetate extract with 2.15% and lower yield in aqueous extract with 0.7%. Mangifera indica L. showed higher yield in aqueous extract with 2.8 % and lower yield in chloroform extract with 0.85%. Piper betel L. showed higher yield in chloroform extract with 1.75% and lower yield in ethyl acetate extract with 0.75% (Figure 5). Among the solvents ethanol showed considerably better % yield in all the three samples. A study by Mohammed et al., 2016 has reported the use of about 250ml of ethanol, chloroform and 500ml water for extraction of 50 grams of Mangifera indica L. leaf by percolation method. The % yield reported was 13.24% for aqueous extract, 6.76% for ethanolic extract and 2.46% for chloroform extract. The extract yield also varies depending upon the factors such as solvent choice, pH, solvent to sample ratio, temperature, time and the technique followed (Terblanche et al., 2017).
Qualitative phytochemical analysis: Preliminary phytochemical screening was carried out for the extracts of all three samples and the results obtained were tabulated (Table 1).
Table 1: Shows qualitative phytochemical results for Ethanol (ET), Ethyl acetate (EA), Chloroform (CH) and Aqueous (AQ) extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L.
| S.no | Phytochemicals | Results | |||||||||||
| Lawsonia inermis L. | Mangifera indica L. | Piper betel L. | |||||||||||
| ET | EA | CH | AQ | ET | EA | CH | AQ | ET | EA | CH | AQ | ||
| 1 | Alkaloid | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | Phenol | + | – | – | + | + | + | – | + | + | – | – | + |
| 3 | Tannin | + | + | – | + | + | + | – | + | + | – | – | + |
| 4 | Flavanoid | + | + | – | + | + | + | + | + | + | + | + | + |
| 5 | Glycoside | + | + | + | + | + | + | + | + | + | + | + | + |
| 6 | Cardiac glycoside | + | + | + | + | + | + | + | + | + | + | + | + |
| 7 | Terpenoids | + | + | + | + | + | + | + | + | + | + | + | + |
| 8 | Diterpenes | + | + | + | + | + | + | – | + | + | + | + | + |
| 9 | Coumarins | + | + | – | + | + | + | – | + | + | + | – | + |
| 10 | Lipids | + | + | + | + | + | + | + | + | + | + | + | + |
| 11 | Saponin | + | + | – | + | + | – | – | + | + | – | – | + |
| 12 | Steroid | + | + | + | + | + | + | + | + | + | + | + | + |
| 13 | Phytosterol | + | + | + | + | + | – | + | + | + | + | + | + |
| 14 | Quinones | + | – | – | + | + | – | – | – | + | + | + | + |
| 15 | Resin | + | – | + | – | + | – | + | + | – | – | + | + |
| 16 | Volatile oil | + | + | – | – | + | + | – | + | + | + | – | – |
| 17 | Fixed oil and Fats | + | + | + | – | + | + | + | – | + | + | + | – |
| 18 | Carboxylic acid | + | + | + | + | + | + | + | + | + | + | + | + |
| 19 | Phlobotannins | + | – | – | + | + | – | – | + | + | – | – | + |
| 20 | Carbohydrate | + | + | + | + | + | + | + | + | + | + | + | + |
| 21 | Protein | + | – | – | + | + | – | – | + | + | – | – | + |
| 22 | Aminoacid | + | – | – | + | + | – | – | + | + | – | – | + |
| 23 | Starch | – | + | + | – | – | + | + | – | – | – | – | – |
Among the extracts, ethanolic and aqueous extract of all the three samples showed positive result for majority of the phytochemicals such as phenol, tannin, flavanoid, alkaloid, saponin, terpenoids, diterpenes, glycoside, cardiac glycoside, coumarins, steroids, phlobotannins, carbohydrate, protein, aminoacid and lipids etc… compared to ethyl acetate and chloroform extracts. Similar results were found to have been reported by Sharma et al., (2018) for ethyl acetate extracts of Lawsonia inermis L. and by Forhad Uddin et al., (2015) for ethanol extract of Piper betel L.
Quantitative phytochemical analysis: Based on the qualitative results, ethanolic and aqueous extracts of all the three samples were chosen for further quantification studies.
Figure 6: Total phenolic content (mg GAE/g extract)
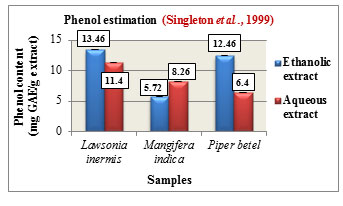
Figure 7: Total tannin content (mg TAE/g extract)
Phenol estimation was carried out according to Folin-Ciocalteau method and the total phenolic content is expressed in mg GAE/ g extract. The phenolic content was found to be higher in the ethanolic extracts of Lawsonia inermis L. with 13.46 mg GAE/g and Piper betel L. with 12.46 mg GAE/g while Mangifera indica L. showed higher phenolic content in its aqueous extract with 8.26 mg GAE/g. Comparatively among the three samples, Lawsonia inermis L. showed higher phenolic content in both its ethanolic and aqueous extracts followed by Piper betel L. and Mangifera indica L. (Figure 6). Phenols are reported to possess anti-cancer, antioxidant as well as antimutagenic properties. They are also used in the treatment of ailments such as cardiovascular problems, inflammation, stroke ,(Ozcan et al., 2014).
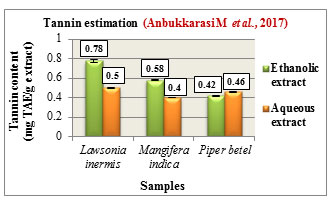
Tannin estimation was carried out according to Folins method and the total tannin content was expressed in mg TAE/ g extract. The tannin content was found to be higher in the ethanolic extracts of Lawsonia inermis L. with 0.78 mg TAE/g and Mangifera indica L. with 0.58 mg TAE/g while Piper betel L. showed higher tannin content in its aqueous extract with 0.46 mg TAE/g. Comparatively Lawsonia inermis L. showed higher tannin content in both its ethanolic and aqueous extracts followed by Mangifera indica L.and Piper betel L. (Figure 7). Tannins are polyphenolic compounds which are reported to possess antiviral, antiparasitic, anti-oxidant, anti-inflammatory activities. They are also used in the treatment of hemorrhoids, infections,skin ulcer, (Praveen and Upadhyaya., 2012).
Figure 8: Flavanoid estimation
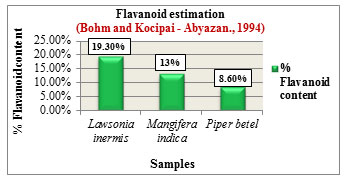
Figure 9: Saponin estimation
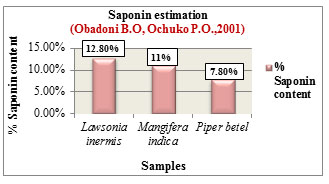
The % Flavanoid content was determined based on the method by Bohm and Kocipai-Abyazan.,1994. Among the samples, Lawsonia inermis L. showed higher flavanoid content of 19.30% followed by Mangifera indica L. with 13% and Piper betel L. with 8.60% (Figure 8). Flavanoids are pharmacologically active compounds that are loaded with numerous health benefits and are found to be potential candidates in drug production with reported activities such as antioxidant, antiageing & anticancer properties, (Hayat et al., 2017). The % Saponin content was determined based on the method by Obadoni B.O and Ochuko P.O., 2001. Among the samples, Lawsonia inermis L. showed higher saponin content of 12.80% followed by Mangifera indica L. with 11% and Piper betel L. with 7.80% (Figure 9). Saponins exhibit numerous medicinal properties and are widely used in the treatment of cancer, against microbial infections, as anti-inflammatory and hepatoprotective drugs. Apart from health care they are also used in industries for preparation of detergents, cosmetics, soap due to their surfactant properties (Moghimipour and Handali, 2015).
Figure 10: Alkaloid estimation
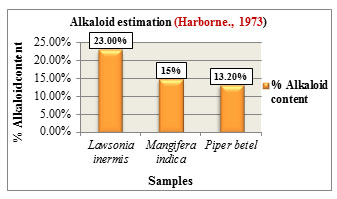
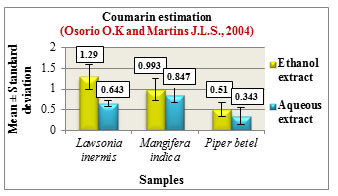
Figure 11: Coumarin estimation
Alkaloid estimation was carried out according to the method given by Harborne., 1973. Comparatively Lawsonia inermis L. showed higher alkaloid content with 23% followed by Mangifera indica L. with 15% and Piper betel L. with 13.20% (Figure 10). Alkaloids play a protective role in plants against insects and herbivores due to their bitter tasting property. They exhibit antiarrhythmic, antihypertensive activities and are also used as analgesic & antimalarial drugs due to their stimulant properties, (Saxena et al., 2013).
Coumarin estimation was carried out based on the method by Osorio O.K and Martins J.LS., 2004. The results are expressed in Mean ± Standard deviation. The ethanolic extracts of all the three samples showed higher coumarin content compared to the aqueous extracts.Among the samples, Lawsonia inermis L. showed increased coumarin content followed by Mangifera indica L. and Piper betel L. (Figure 11). Coumarins are reported to possess anticancer, anti-aging as well as anticoagulant properties and are also used as enhancing agents in cosmetic products and as food additives, (Rohini and Srikumar 2014).
Figure 12: Glycoside estimation
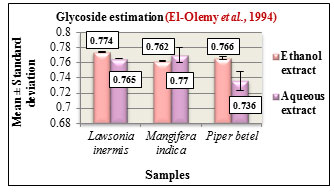
Figure 13: Cardiac glycoside estimation
Glycoside estimation was carried out according to Baljets method. The results are expressed in Mean ± Standard deviation. The glycoside content in the ethanolic and aqueous extracts of all the three samples were determined. Based on the estimation, Lawsonia inermis L. and Piper betel L. showed higher glycoside content in the ethanolic extracts while Mangifera indica L. showed higher glycoside content in its aqueous extract (Figure 12). Glycosides play a predominant role in pharmaceutical industry due to their innate biocompatibility and are also used as analgesic, antipyretic, anti-inflammatory agents, (Saawarn et al., 2015).
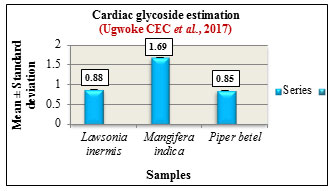
Cardiac glycoside estimation was carried out according to method by Ugwoke CEC et al., 2017. The results are expressed in Mean ± Standard deviation. Among the samples, Mangifera indica L. showed higher cardiac glycoside content followed by Lawsonia inermis L. and Piper betel L. (Figure 13). Cardiac glycosides are compounds produced by plants in very small amounts. Cardiac glycoside such as peruvoside are used as a promising source in the control of cancer mainly ovarian cancer and leukemia ( Patel., 2016). They are also used in the treatment of congestive heart failure ( Morsy 2017).
Figure 14: Steroid estimation
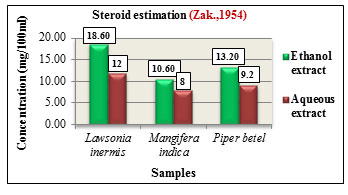
Figure 15: Terpenoid estimation
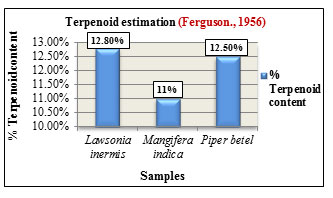
Steroid estimation was carried out according to method by Zak.,1954. The total steroid content was expressed in concentration(mg/100ml). Lawsonia inermis L. showed higher steroid content in its ethanolic extract with 18.60mg than aqueous extracts with 12mg. Mangifera indica L. also showed increased steroid content in its ethanolic extract with 10.60mg than aqueous extract with 8mg while Piper betel L. showed higher steroid content in its ethanolic extract with 13.20mg than aqueous extract with 9.2mg. Comparatively among the three samples, Lawsonia inermis L. showed higher steroid concentration followed by Piper betel L. and Mangifera indica L. (Figure 14). Steroids exhibit different biological activities such as regulation of cell proliferation, tissue differentiation and in cell membrane stabilization. They are also utilized as carbon and energy sources by various bacteria and also participate in cellular signalling mechanisms ( Cabezon et al., 2018). Terpenoid estimation was carried out based on method by Ferguson.,1956. Among the samples, Lawsonia inermis L. showed higher terpenoid content with 12.80% followed by Piper betel L. with 12.50% and Mangifera indica L. with 11% (Figure 15). Terpenoids are the largest class of compounds produced by plants. They exhibit antiviral, anti-inflammatory, antimicrobial, antioxidant activities and are also used as food additives and as fragrances in perfumes, (Brahmkshatriya and Brahmkshatriya 2013).
Analysis of nutritional components: Essential nutritional components such as carbohydrate, protein, aminoacid and lipid content in the ethanolic and aqueous extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L. were determined.
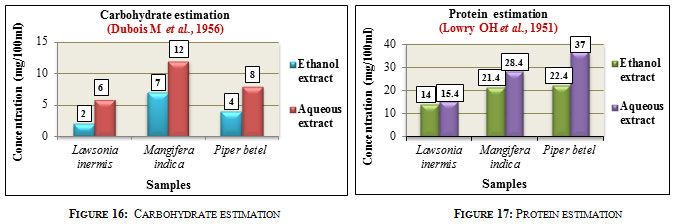
Carbohydrate estimation was carried out according to Phenol sulphuric acid method. The results were expressed in mg/100ml. Carbohydrate concentration was found to be higher in the aqueous extracts of all the three samples compared to that of the ethanolic extracts. Among the three samples, Mangifera indica L. showed higher carbohydrate content with 12mg in aqueous and 7mg in ethanolic extract followed by Piper betel L. with 8mg in aqueous and 4mg in ethanolic extract and Lawsonia inermis L. with 6mg in aqueous and 2mg in ethanolic extract (Figure 16). Carbohydrates are essential compounds produced by plants which serve as energy reserves and are widely used in pharmaceutical industry, textile, paper, food, agricultural as well as in chemical industries, (Bemiller 2004).
Protein estimation was carried out according to Lowry’s method. The results were expressed in mg/100ml. The aqueous extracts showed higher protein concentration than the ethanolic extracts in all the three samples. Comparatively among the three samples, Piper betel L. showed higher protein content with 37mg in aqueous and 22.4mg in ethanolic extracts followed by Mangifera indica L. with 28.4mg in aqueous and 21.4mg in ethanolic extract and Lawsonia inermis L. with 15.4mg in aqueous and 14mg in ethanolic extract (Figure 17). Proteins are organic compounds distributed in plant tissues and are widely used in clarification of wine, in vaccines, tissue engineering and controlled drug delivery (Wadhave et al., 2014).
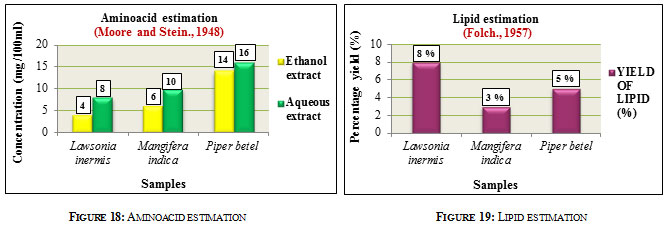
Aminoacid estimation was carried out according to Ninhydrin method. Results were expressed in mg/100ml. The aqueous extracts showed higher aminoacid concentration than ethanolic extracts in all the three samples. Aminoacid content in Lawsonia inermis L. was found to be 8mg in aqueous and 4mg in ethanolic extracts. Mangifera indica L. had aminoacid content of about 10mg in the aqueous and 6mg in ethanolic extracts while Piper betel L. showed aminoacid content of 16mg in aqueous and 14mg in ethanolic extracts. Comparatively Piper betel L. showed higher aminoacid content followed by Mangifera indica L. and Lawsonia inermis L. (Figure 18). Amino acids play an important role in Pharma industry in the form of precursor in antibiotic production, in transfusion, in the treatment of heart failure, peptic ulcer etc. They are also utilized as flavor enhancers, food additives, in the manufacture of artificial sweetners (Ivanov 2016). Lipid estimation was carried out according to method given by Folch, 1957. Among the samples, Lawsonia inermis L. showed higher lipid content of 8% followed by Piper betel L. with 5% and Mangifera indica L. with 3% (Figure 19). Lipids are widely utilized in the preparation of cosmetics, in paints and varnishes, in detergents, also in pharmaceuticals and diagnosis,(Alvarez and Rodriguez., 2000).
Antibacterial activity of extracts
Table 2. Antibacterial activity of Ethanol (ET), Ethyl acetate (EA), Chloroform (CH) and Aqueous (AQ) extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L
| S.no | Organisms | Extracts – Zone of inhibition (in mm) | |||||||||||
| Lawsonia inermis L. | Mangifera indica L. | Piper betel L. | |||||||||||
| ET | EA | CH | AQ | ET | EA | CH | AQ | ET | EA | CH | AQ | ||
| 1 | Staphylococcus aureus | 8 | 5 | 4 | 2 | 16 | 15 | – | 3 | 9 | 7 | 3 | 1 |
| 2 | Streptococcus mutans | 5 | 3 | 7 | 1 | 2 | – | 1 | 6 | 6 | 5 | 2 | 1 |
| 3 | Klebsiella pneumoniae | 6 | 3 | 3 | 3 | 3 | 5 | 2 | 5 | 6 | 3 | 2 | 1 |
| 4 | Salmonella typhi | 6 | 4 | 4 | 2 | 10 | 9 | 6 | 5 | 7 | 8 | 3 | – |
| 5 | Enterobacter spp. | 8 | 3 | 4 | 1 | 7 | 5 | – | 10 | 4 | 5 | 2 | 1 |
Figure 20: Antibacterial activity of Lawsonia inermis L., Mangifera indica L. and Piper betel L. extracts
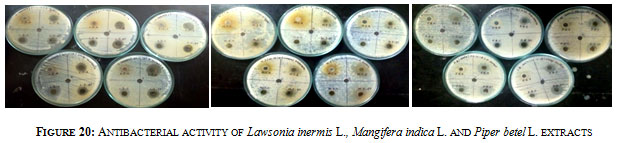
Antibacterial activity was determined for the ethanol, ethyl acetate, chloroform and aqueous extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L. against 5 Pathogenic organisms such as Staphylococcus aureus, Streptococcus mutans, Salmonella typhi, Klebsiella pneumoniae and Enterobacter spp (Figure 20). Among the three samples Lawsonia inermis L. showed good inhibition against all organisms followed by Piper betel L. and Mangifera indica L. The ethanolic extract from all the three samples were found to be more active in inhibiting bacterial pathogens while aqueous extracts from Lawsonia inermis L. & Piper betel L. and chloroform extract from Mangifera indica L. displayed less inhibition activity. Chloroform extract of Mangifera indica L. displayed no inhibition against Staphylococcus aureus and Enterobacter spp while ethyl acetate extracts didn’t show any inhibition against Streptococcus mutans. In case of Piper betel L. only aqueous extract was found not to be active against Salmonella typhi (Table 2). Bangash et al., (2012) have also reported that ethanolic extract was the most effective against bacterial pathogens than chloroform extract which is least effective.
CONCLUSION
The present work deals with the phytochemical studies and antibacterial activity of leaf samples of Medicinal plants Lawsonia inermis L., Mangifera indica L. and Piper betel L. Phytochemical analysis is generally carried out in order to analyze the presence or absence as well to determine the quantity of a specific compound in the plant extract. The nature and distribution of bioactive compound varies in different plant species based on their growth and environmental conditions. In this study phytochemical analysis (Qualitative & Quantitative study) was carried out for the leaf extracts of Lawsonia inermis L., Mangifera indica L. and Piper betel L. obtained using different solvents and their antibacterial activity was also determined against 5 bacterial pathogens such as Staphylococcus aureus, Streptococcus mutans, Klebsiella pneumoniae, Salmonella typhi and Enterobacter spp. Among the tested extracts, ethanolic extract showed good yield as well as increased activity against all bacterial pathogens and based on the results obtained further studies will be carried out in future on the isolation and purification of compounds for different pharmacological applications.
ACKNOWLEDGEMENTS
This work has been catalyzed and financially supported by Tamilnadu State Council for Science and Technology, Dept of Higher Education, Government of Tamilnadu through RFRS (Research funding for Research Scholars) Scheme 2018-2019.
Conflict of interest: None
REFERENCES
Alaa Jabbar Al-Manhel and Alaa Kareem Niamah.(2015).Effect of aqueous and alcoholic plant extracts on inhibition of some types of microbes and causing spoilage of food. Journal of Nutrition and Food Sciences.S5:006.
Amita Pandey and Shalini Tripathi. (2014). Concept of standardization, extraction and pre phytochemical screening, strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry. 2(5), pp: 115-119.
Amol A.Wadhave, Amrita I. Jadhav and Vilas A. Arsul. (2014). Plant proteins applications: A Review. World Journal of Pharmacy and Pharmaceutical sciences. Vol3, Issue 3, pp: 702-712.
Anbukkarasi M, Dhamotharan R and Janarthanam B. (2017). Studies on Phytochemical screening, Tannins content and antibacterial activity from leaf and callus extracts of Memecylon umbellatum. Asian Journal of Pharmaceutical and Clinical Research.Volume 10, Issue 5, pp: 265-269.
Antonio M. Rabasco Alvarez and Maria Luisa Gonzalez Rodriguez. (2000). Lipids in pharmaceutical and cosmetic preparations. Grasas y Aceites. Volume 51, Fasc.1-2, pp: 74-96.
Arani Datta, Shreya Ghoshdastidar and Mukesh Singh. (2011). Antimicrobial property of Piper betel Leaf against clinical isolates of bacteria. International Journal of Pharma sciences and Research. Vol. 2(3), pp: 104-109.
Ashvin Godghate, Rajaram Sawant and Ashok Sutar. (2012). Phytochemical analysis of ethanolic extract of roots of Carrisa carandus Linn. Rasayan Journal of Chemistry. 5(4), pp: 456-459.
Basireddy Sivareddy, Bernard Ajay Reginald, Sireesha D., Meda Samatha, Himakar Reddy K. and Subrahamanyam G. (2019). Antifungal activity of solvent extracts of Piper betel and Ocimum sanctum Linn on Candida albicans: An In vitro comparative study. Journal of Oral and Maxillofacial Pathology. Volume 23, Issue 3, pp: 333-337.
Bemiller, J.N. (2004). “Carbohydrates”. Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley and Sons, Inc., New York. Vol 4, pp: 696-733.
Bohm B.A, Kocipai-Abyazan R. (1994). Flavanoids and condensed tannins from leaves of Hawaiin Vaccinium vaticulatum and V.Calycinium. Pacific science. 48, pp: 458-468.
Chitra Wendakoon, Peter Calderon and Daniel Gagnon. (2012). Evaluation of selected medicinal plant extracted in different ethanol concentrations for antibacterial activity against human pathogens. Journal of medicinally active plants. 1(2), pp: 60-68.
El-Olemy, M.M., Al-Muhtadi, F.J., Afifi, A.F.A. (1994). Experimental Phytochemistry; A Laboratory manual. King Saud University press, Saudi Arabia, pp: 21-27.
Eskandar Moghimipour and Somayeh Handali. (2015). Saponin: Properties, Methods of Evaluation and Applications. Annual Research and Review in Biology. 5(3), pp: 207-220.
Fawad Ali Bangash, Hashmi A.N, Mahboob A, Zahid M, Hamid B, Muhammad S.A,Shah Z.U, Afzaal H. (2012). In vitro antibacterial activity of Piper betel leaf extracts. Journal of applied Pharmacy. 03(04), pp: 639-646.
Ferguson NM. (1956). A Textbook of Pharmacognosy. Macmillan Company, New York. 191.
Folch J, Lees M and Stanley G.H.S. (1957). A Simple method for the isolation and purification of total lipids from animal tissues. J.Biol.Chem. 226, pp: 497-509.
Ghodekar Anuradha S, Jarande Kajal S, Satav Pallavi S, Patil Neha and Waghmode Meghmala. (2019). Bioactive potential of Lawsonia inermis Linn. International Journal of Pharmacy and Biological sciences. 9(3), pp: 256-266.
Harborne, J.B. (1973). Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall Ltd, London. pp: 279.
Irina Francesca Gonzalez Mera, Daniela Estefania Gonzalez Falconi and Vivian Morera Cordova. (2019). Secondary metabolites in plants: main classes, phytochemical analysis and pharmacological activities. Revista Bionatura. Volume 4, Issue 4, pp: 1000-1009.
Kalin Ivanov, Assena Stoimenova, Danka Obreshkova, Luciano Saso. (2013). Biotechnology in the production of Pharmaceutical industry ingredients: Aminoacids. Biotechnology and Biotechnological equipment. 27:2, pp: 3620-3626.
Lorena Fernandez Cabezon, Beatriz Galan and Jose L. Garcia. (2018). New insights on Steroid Biotechnology. Frontier Microbiology, 9:958.
Lowry O.H, Rosenbrough N.J, Farr, A.L, Randall, R.J. (1951). Protein measurement with the Folin Phenol reagent. J Biol Chem. 193, pp: 265-275.
Mamta Saxena, Jyoti Saxena, Rajeev Nema, Dharmendra Singh and Abhishek Gupta. (2013). Phytochemistry of Medicinal plants. Journal of Pharmacognosy and Phytochemistry. Volume 1, Issue 6, pp: 168-182
Mathew George. (2017). Qualitative and Quantitative Phytochemical analysis on the leaves and fruits of Passiflora foetida. International Journal of Pharmaceutical Science Invention. Volume 6, Issue 3, pp: 26-30.
Dubois M, Gilles K.A., Hamilton J.K., Rebers P.A. and Fred.Smith. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry. Vol.28 (3), pp: 350-356.
Mohammed A.H, Nainna S.Z, Yusha’u M, Salisu B, Adamu U and Garba S.A. (2016). Phytochemical screening and antibacterial activity of Mangifera indica extracts. UMYU Journal of Microbiology Research. Volume 1, No 1, pp: 23-28.
Mohammed Farhad Uddin, Sheikh Aftab Uddin, Mohammed Delwar Hussain, Mohammed Abdul Manchur. (2015). Antioxidant, cytotoxic and phytochemical properties of ethanol extract of Piper betel leaf. International Journal of Pharmaceutical Sciences and Research. 6(10), pp: 4252-4258.
Mohammed Mansour Saleh Saif, Abdulkawi Ali Al-Fakih and Maik Abdu M Hassan. (2017).Antibacterial activity of selected plant (Aqueous and methanolic) extracts against some pathogenic bacteria. Journal of Pharmacognosy and Phytochemistry. 6(6), pp: 1929-1935.
Momina Hayat, Marya Abbas, Faiza Munir, Muhammad Qasim Hayat, Rumana Keyani and Rabia Amir. (2017). Potential of plant flavanoids in pharmaceutics and nutraceutics. Journal of Biomolecules and Biochemistry. 1(1), pp: 12-17.
Moore, S and Stein, W.H. (1948). Photometric methods for use in the chromatography of amino acids. J Bio Chem. 176: 367-388.
Nagy Morsy. (2017). Cardiac glycosides in medicinal plants. Aromatic and Medicinal plants- Back to nature, edited by Hany A. El-Shemy.
Nyamai DW, Arika W, Ogola PE, Njagi ENM, Ngugi MP. (2016).Medicinally important Phytochemicals: An Untapped Research Avenue. Research and Reviews: Journal of Pharmacognosy and Phytochemistry. Volume 4, Issue 1, pp: 35-49.
Obadoni B.O, Ochuko P.O. (2001). Phytochemical studies and comparative efficacy of the crude extract of some homeostatic plant in Edo and delta states of Nigeria. Global J.Pure appl.sci. 8, pp: 203-208.
Osorio O.K, Martins J.L.S. (2004). Determinacao de cumarina en extrato fluido tintura de guaco par espectrofotometria derivada de primeira ordem. Braz.J.Pharm.Sci. 40(4), pp: 481-486.
Ozcan T., Akpinar-Bayizit A., Yilmaz-Ersan L., and Delikanli B. (2014). Phenolics in human health. International Journal of Chemical engineering and applications.Vol 5, No 5, pp: 393-396.
Pandey M.M., Subha Rastogi and Rawat A.K.S. (2013). Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evidence based complementary and alternative medicine. pp: 1-12.
Patel DK. (2016). Medicinal and Aromatic plants: Role in Human society. Medicinal and Aromatic plants. 5(3), pp: 175.
Praveen Kumar Ashok and Kumud Upadhyaya. (2012).Tannins are astringent. Journal of Pharmacognosy and Phytochemistry. Volume 1, Issue 3, pp: 45-50.
Preeti Vaishnav and Arnold L. Demain. (2011).Unexpected applications of secondary metabolites. Biotechnology Advances. Volume 29, Issue 2, pp: 223-229.
Priyanka P.Brahmkshatriya and Pathik S.Brahmkshatriya. (2013).Terpenes: Chemistry, Biological role and Therapeutic applications. In: Ramawat K, Merilon JM. (eds) Natural products. Springer, Berlin, Heidelberg. pp: 2665-2691.
Ritesh Kumar Sharma, Anjana Goel. (2018). Identification of phytoconstituents in Lawsonia inermis Linn. Leaves extract by GC-MS and their antibacterial potential. Pharmacogn, J. 10(6), pp: 1101-1108.
Rohini.K and Srikumar PS. (2014). Therapeutic role of Coumarins and Coumarin related compounds. Journal of Thermodynamics and Catalysis.Vol5, Issue 2:130.
Seema Patel. (2016). Plant derived cardiac glycosides: Role in heart ailment and cancer management. Biomedicine and Pharmacotherapy. 84:1036-1041.
Shah K.A., Patel M.B., Patel R.J. and Parmar P.K. (2010).Mangifera indica (Mango). Pharmacognosy reviews. 4(7), pp: 42-48.
Singleton, V.L, Orthofer R, Lamuela Raventos, R.M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteau reagent. Methods Enzymol. 299, pp: 152-178.
Suman Kumar R., Venkateshwar C., Samuel G., Gangadhar Rao S. (2013). Phytochemical screening of some compounds from plant leaf extracts of Holoptelea integrifolia (Planch.) and Celestrus emarginata (Grah.) used by Gondu tribes at Adilabad District, Andhrapradesh, India. International Journal of Engineering Science Invention. Volume 2, Issue 8, pp: 65-70.
Swati Saawarn, Nisheeth Saawarn, Madhusudan Astekar, Megha Jain and Anish Gupta. (2015).New perspectives in exploiting secondary metabolite glycosides as drug leads. Journal of Molecular Biomarkers and Diagnosis. 6:260
Terblanche U, S.Semakalu CC, Mtunzi F, Pillay M. (2017). Screening of variables influencing extraction yield of Cotyledon orbiculata: 23 Full Factorial design. International Journal of Pharmacognosy and Phytochemical Research. 9(3), pp: 303-312.
Ugwoke CEC, Orji J, Anze SPG, Ilodibia C.V. (2017). Quantitative phytochemical analysis and antimicrobial potential of ethanol and aqueous extracts of leaf, stem and root of Chromdaena odorata (Asteraceae). International Journal of Pharmacognosy and Phytochemical research. 9(2), pp: 207-214.
Zak, B., Dickenman RC, White, EG, Burnett H., and Cherney, PJ. (1954). Rapid estimation of free and total cholesterol.Am.J.Clin.Path.24:1307


