1Department of Botany, KR College, Gopalganj, Bihar and
2Department of Botany, Gopeshwar College, Hathwa, Gopalganj,
Bihar.(Jai Prakash Vishvidyalay, Chapra, Bihar, India)
Corresponding author email: manojkumarsingh12672@gmail.com
Article Publishing History
Received: 11/07/2020
Accepted After Revision: 15/09/2020
Phyllanthus niruriL. is one of the highly used medicinal plants both traditionally and scientifically. Phytochemical screening of such plants is of high importance to establish the claims of medicinal uses by traditional and folk medicine practitioners. The phytochemical screening reveals the presence of potent bioactive compounds that can be effectively used for the preparation of better herbal drugs. The study aims at determining the potent bioactive compounds present in the leaf extract of Phyllanthus niruri L. utilizing qualitative analysis and HPLC analysis with standard as well as evaluate the antimicrobial potential of such extract. This done means of Soxhlet extraction of dried plant leaves. The extracts are screened by standard protocol to determine bioactive compounds. Further selective and confirmed detection of bioactive compounds is done employing HPLC using standard compounds of phenol, flavonoid, lignans, and alkaloid. The extracts were also evaluated for their antimicrobial potential against bacteria (Staphylococcus aureus and Bacillus subtilis) and fungi (Aspergillus niger and Candida albicans).
The overall result obtained from the study showed the presence of potent bioactive compounds in extracts of Phyllanthus niruri L., such as quercetine, rutin, phyllanthin, and ellagic acid. The extract also showed the effective antimicrobial potential against both bacteria and fungi. The study derives the conclusion that the plant Phyllanthus niruri L. is a rich source of potent bioactive compounds and hence carries out several important biological activities. The presence of such essential phytochemicals in its extract is the reason behind its historical and traditional use in medicines.
Phyllanthus niruri Phytochemical Screening, HPLC, Antimicrobial Activity.
Singh M. K, Ahmad S. Phytochemical Profile of Phyllanthus niruri and Evaluation of its Potent Bioactive Compounds. Biosc.Biotech.Res.Comm. 2020;13(3).
Singh M. K, Ahmad S. Phytochemical Profile of Phyllanthus niruri and Evaluation of its Potent Bioactive Compounds. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/2WVsGOP
Copyright © Singh and Ahmad This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Traditional medicine using plant extracts continues to provide health coverage for over 80% of the world’s population, especially in the developing world (Igbinosa et al., 2009). In the Democratic Republic of Congo (DRC), among the species used in the treatment against malaria, Phyllanthus niruri L.is well-positioned for different previous studies on this plant (Pauwels, 1993; Tona et al., 1999 and Cimanga et al., 2004). Medicinal plants are now getting more attention than ever because they have the potential of myriad benefits to society or indeed to all mankind, especially in the lien of medicine and pharmacological studies. The medicinal value of these plants lies in bioactive phytochemical constituents that produce definite physiological action on the human body (Akinmoladun et al., 2007 Gupta and Vaghela (2019 Meselhy et al 2020).
Some of the most important bioactive phytochemical constituents are alkaloids, essential oils, flavonoids, tannins, terpenoids, saponins, phenolic compounds and many more (Edeoga et al., 2005). Due to their specialized biochemical capabilities, plants can synthesize and accumulate a vast array of primary and secondary chemicals useful for the plant itself as protecting against environmental stress factors. These compounds have made many plants useful also for humans, for instance, spices and medicines, etc. (Akinmoladun et al., 2007 and Edeoga et al., 2005).P. niruri L. is one of the most important medicinal plants used in different regions in the world for the treatment of various diseases such as jaundice, asthma, hepatitis, flu, dropsy, diabetes, fever causing by malaria (Kerharo and Adam, 1974; Ishimari et al., 1999; and Paran-jape, 2001) but its availability is drastically decreasing because of numerous harvests.
Phyllanthus is a large genus of shrubs, trees and rare herbs of the family Euphorbiaceae, comprising more than 600 species. The genus is found in almost overall warmer parts of the world (Burkil, 1966). Among the Phyllanthus species, P. niruri is a small erect annual herb growing up to 30–40 cm in height and is indigenous to the Amazon rainforest and other tropical areas, including South East Asia, Southern India and China (Girach et al., 1994).P. niruri L.has been the subject of much research to investigate the active constituents and their pharmacological activity, beginning in the mid-1960s. Row et al., 1964 was the first to work on P. niruri L. and reported the isolation of phyllanthin. It has a rich source of phytochemicals, many of which have been found only in P. niruri L.(Dhar et al., 1968 Nisar et al 2018, Mehta et al 2019, Meselhy et al 2020).
The aerial part of Phyllanthus niruri L. has been used by many countries in folk medicine for the treatment of various disease conditions such as increase libido or fertility in men. In India, the plant is usually used by traditional medicine practitioners for the treatment of asthma, bronchial infection, liver diseases, diabetes, gonorrhea, inducing labor and treatment of edema, feverish pain, sore throat, female sterility, oliguria, and vaginitis. They also used the plant to manage irregular menstruation, tachycardia, dysentery, spasmodic cough, itchiness, arthritis, otitis, swelling, skin ulcer and weakness of male organ (Obianime and Uche, 2009). In Brazil, the tea of Phyllanthus niruri L. is used to treat renal calculi (Nishiura et al., 2004). In South Africa, it is used in folk medicine to treat hyperuricemia (Murugaiya et al., 2009 Gupta and Vaghela (2019).
The extract of Phyllanthus niruriL. has shown several pharmacological activities. Ethanolic extract of Phyllanthus niruri L. was found to have significant antidiabetic activity in insulin-dependent diabetes mellitus rats but showed no effect on non-insulin-dependent diabetes mellitus rat (Bavarva and Narasimhacharya, 2007). Phyllanthus niruriL.has shown an inhibitory effect against calcium oxalate crystal growth and aggregation in human urine. This medicinal plant exhibited antiurolithic activity in both in vitro and in vivo studies (Barros et al., 2003). Scientific studies have shown that Phyllanthus niruri L.has an antihyperlipidemic effect. It was also reported that the aqueous extract exhibited antihyperlipidemic activity (Nwanjo et al., 2007 Mehta et al 2029, Meselhy et al 2020).
Meselhy et al. (2020) highlighted the direct methods used for extraction of lignans from aerial parts of P. niruri L. Identified lignans were phyllanthi, hypophyllanthin, phylltetralin, nirtetralin, and niranthin. Different solvents gave yield based on extraction concentrations. At 18.10 g % (w/w), aqueous extract yielded phyllanthin of 0.33 ± 0.10 mg/g extract), while at 3.6 g% w/w, methanolic extract yielded comparatively lower phyllanthin content of 3.1 mg/g extract. Soxhlet method of extraction also yielded at concentrations of 0.82, 1.12, and 3.40 g% w/w of hexane, dicholoromethane or acetone yielded higher phyllanthin contents of 36.2 ± 2.6, 11.7 ± 1.68, and 11.7 ± 1.10 mg/g.
Alkaline digestion was employed to obtain high phyllanthin content 22.34 ± 0.13 mg/g at 3.1 g% w/w, Microwave-assisted extraction method yielded 21.2 ± 1.30 mg/g content and plant material treatment at 50˚C with two hydrolytic enzymes, cellulase (9 U/g for 12 h) and protease (4 U/g up to 72 h) yielded phyllanthin content 25.9 mg/g extract. Ethanolic extract of Phyllanthus niruri L. was found to have potential antiplasmodial activity in vitro by inhibition of the developmental stage of a trophozoite to schizonts. Another study showed that P. niruri exhibited potent systemic antinociceptive actions against two models of neurogenic pain (Santos et al., 1995 Meselhy et al 2020).
In recent years, excessive use of drugs has made most of them resistant against popular antibiotics. Plants have a rich source of active components and have upper hand over chemical compounds owing to their severe side effects. In consideration of the present scenario, this study highlights the active components analysis of Phyllanthus niruri l. It would help to identify its antibacterial and antifungal potency and thus used to curb their growth. The aim of the current study was to carry out the phytochemical profiling of Phyllanthus niruri L. through Liquid chromatography and to obtain potent bioactive components from the work.
MATERIAL AND METHODS
Sample Preparation: Phyllanthus niruri L. leaves were collected from the Gopalganj district in Bihar. The leaves were washed with water to remove dust particles. The washed leaves were dried at room temperature and then powdered mechanically. The powdered leaves were extracted with the help of Soxhlet apparatus with methanol and petroleum ether as a solvent at 75ºC for 6 hours. The extracts were dried and refrigerated at 4ºC for further usage.
Phytochemical Screening: The phytochemical screenings were performed by following the standard procedures mentioned in Harbone (Harbone, 1998). Screenings for the presence of saponin, alkaloid, tannin, glycoside, flavonoid, phenol, terpenoid and carbohydrates were performed for both the extracts.
Antimicrobial Assay: The antimicrobial activity of both the extracts of Phyllanthus niruriL. was assessed by the Kirby Bauer Disc Diffusion Method. The microbial strains used include Bacillus subtilis, Staphylococcus aureus, Aspergillus niger, and Candida albicans. For the test, Muller Hinton Agar Media was prepared and poured in Petri-dishes. After the solidification of agar media, 0.1 ml of each microbial strain was spread over the media. The discs were prepared with Whatman filter paper.Then the discs were saturated with the plant extract and left for drying. For sample preparation, 100μg/ml extract of P. niruri L. in the respective solvent was deposited onto the disc. After the disc was dry completely they were placed on the inoculated medium. The plates were incubated for 24 hours at 37ºC for bacteria and 27ºC for fungi. Levofloxacin for bacteria and Fluconazole for fungus were used as controls. After incubation, the zone of inhibition around the wells was detected, and the diameter of these inhibition zones was measured and recorded.
HPLC Analysis: The methanolic and petroleum ether extracts of Phyllanthus niruri L. were subjected to HPLC analysis of the instrument HPLC (Shimadzu VP 1605)with HiQSil C18-HS column (column size:4.6 mm × 250 mm × 5µM; 25°C), isocratic pump. The HPLC analysis of the extract was done to determine the presence of some specific phytochemical compounds in the extract. Acetonitrile was used as a solvent system for the elution of the sample. HPLC chromatograms were detected using a photodiode array UV detector at the scan range of 255nm-370nm phytochemical scan range according to the absorption maxima of analyzed compounds. Each compound was identified by its retention time and by spiking with standards under the same conditions.
The standard phytochemical compounds used, the mobile phase used for them and the detection parameter used is summarized in table 1. The stock solution of concentration 10µg/ml was prepared by dissolving 10µg standard in 0.5ml HPLC-grade methanol followed by sonication for 10 minutes and the resulting volume was made up to 1ml with the methanol. The standard and sample solutions were filtered through a 0.22μm PVDF-syringe filter and the mobile phase was degassed before the injection of the solutions.
Table 1. Showing the standard and the condition used for the standard phytochemical compounds during the HPLC analysis
| S. No. | Standard | Mobile Phase | Mobile Phase ratio |
Detector | Flow rate | Run Time |
| 1 | Quercetin | Acetonitrile: Methanol | 75:25 | 355nm | 1.5ml/min | 15min. |
| 2 | Rutin | Methanol | – | 270nm | 1.5ml/min | 15min. |
| 3 | Ellagic acid | Methanol: Acetone | 50:50 | 210nm | 1.5ml/min | 15min. |
| 4 | Gallic acid | Acetone: Methanol | 55:65 | 240nm | 1.5ml/min | 15min. |
| 5 | Phyllanthus | Methanol:Acetone: Acetonitrile | 25:50:25 | 210nm | 1.5ml/min | 15min. |
RESULTS AND DISCUSSION
Herbal medicine has been used for centuries for the treatment of various diseases. It is an important part of Ayurveda, Siddha, and Unani medicine. Different parts of various plants are used by indigenous people across the world to cure wounds, snakebites, abdominal pain, skin infections, and several other diseases. In a study by WHO, it was estimated that 80% of the world population still depend on herbs and plants as medicine (WHO, 1991). Several phytochemicals such as vincristine, artemisinin, quinine, and digoxin have been isolated from plants which have shown a broad range of pharmacological activities (Eslami et al., 2017; Adedinsewo et al., 2017). This study aimed at phytochemical screening and antimicrobial activity of Phyllanthusniruri L.
Phytochemical Screening: The phytochemical screening of methanolic and petroleum ether extract of the plant was carried out by standard procedure. The result obtained is summarized in table 2. It showed the presence of phenol, flavonoid, saponin, alkaloid, and terpenoid. These compounds are responsible for antioxidant activity and antibacterial activity.
Table 2. Showing the result for the phytochemical analysis of the plant extract for both solvents, where (+) indicates the presence of the compound and (-) indicates the absence of compound.
| S. No. | Phytochemical compound | Methanolic extract | Petroleum ether extract |
| 1 | Alkaloid | + | + |
| 2 | Saponin | + | – |
| 3 | Tanin | + | + |
| 4 | Glycosides | – | + |
| 5 | Terpenoids | + | + |
| 6 | Carbohydrates | – | – |
| 7 | Flavanoids | + | + |
| 8 | Phenols | + | – |
Antimicrobial activity: The extracts of Phyllanthus niruri showed good antimicrobial activity against the selected microbial strains. The extract carried out potential antimicrobial activity due to the presence of bioactive compounds like flavonoids and phenols in them. The result of the antimicrobial activity of both extracts is summarized in table 3.
Figure 1: Antibacterial activity of extract of Phyllanthus niruri L. against B. subtilis and S. aureus
Figure 2: Antifungal activity of extract of Phyllanthus niruri L. against A. niger and C. albicans In the figure above disc 1 denotes methanolic extract and disc 2 denotes petroleum ether extract of Phyllanthus niruri L. and +C is for antibiotic positive control.
Table 3. Showing the result for the antimicrobial activity of the plant extract for both solvents against the respective microbes, the table depicts the Zone of Inhibition obtained against each extract
| S. No. | Microbe | Zone of Inhibition (mm) | ||
| Methanolic extract | Petroleum ether extract | Positive control | ||
| 1 | Bacillus subtilis | 17 | 16 | 26 |
| 2 | Staphylococcus aureus | 14 | 16 | 27 |
| 3 | Aspergillus niger | 11 | 13 | 18 |
| 4 | Candida albicans | 10 | 11 | 17 |
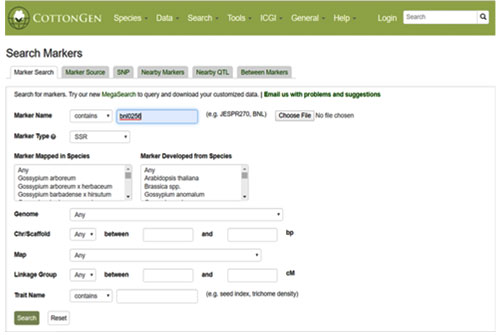
HPLC Analysis: The HPLC analysis of both the extracts revealed the presence of some potent phytochemical compounds in the plant extract. The phytochemical compound confirmation was done with the help of standard. The confirmation basis is the retention time at which the standard phytochemical compound gave the peak. The similarity in peak formation time between sample and standard is indicative of the presence of that phytochemical compound in sample extract. The result depicted the presence of flavonoids like quercetin and rutin, phenols like ellagic acid, alkaloid like securinine and lignan like phyllanthin; in both the extracts. The chromatograms and results of HPLC analysis of the sample and standard obtained are given below. The retention time peak shown by the entire standard compound is also found in the sample extract.
Figure 3: HPLC chromatogram of Phyllanthus niruri L. [A] Methanolic Extract [B] Petroleum ether extract
The modern sedentary lifestyle, stress, pollution, junk food, and alcohol have exacerbated the harms caused by free radicals. The free radicals are associated with diseases such as diabetes, arthritis, cancer, Parkinson’s disease, and Alzheimer’s disease (Kaur and Kumar, 2016; Meena et al., 2018). The presence of phytochemical compounds in plant extract of Phyllanthus niruri L. was determined by qualitative analysis as well as by HPLC. The analysis revealed the presence of phytochemicals like phenol, flavonoid, alkaloid, and lignans the extracts can scavenge free radicals. These compounds have previously shown strong anticancer, antidiabetic, anti-inflammatory and antimicrobial activity (Nyamai et al., 2016).
The bioactive compounds present in the extract are full of potential medicinal properties and hence gain a lot of attention for use in the herbal medication process. Phenols and flavonoids have significant antioxidant properties. Phenols are also associated with the ability to inhibit the growth of bacteria (Chan et al., 2011). Rutin is important because it strengthens capillaries and so can help people suffering from arteriosclerosis or high blood pressure (Becker et al., 1985) while quercetin has anti-aggregant, anticancer, anti-fungal (especially anti-dermatophytes), antifeedant, anti-glaucomic, anti-inflammatory, anti-oxidant, antiseptic and anti-spasmodic activity (Saija et al., 2003).
Figure 4: HPLC Chromatograms of standard compounds showing single peak (a) Quercetin chromatogram (Rt – 7.358 min) (b) Rutin chromatogram (Rt – 3.833 min) (c) Ellagic acid chromatogram (Rt – 6.225 min) (d) Securinine chromatogram (Rt – 4.360 min) (e) Phyllanthin chromatogram (Rt – 6.642 min)
Table 4. Showing the summarized result of the HPLC chromatogram analysis of the standard phytochemical compounds
| S.No. | Standard name | Retention Time | Area | Area % | Height | Height % |
| 1 | Quercetine | 7.358 | 144977 | 100 | 21456 | 9.076 |
| 2 | Rutin | 3.833 | 244977 | 100 | 21456 | 9.076 |
| 3 | Ellagic Acid | 6.225 | 144977 | 100 | 21456 | 9.076 |
| 4 | Securinine | 4.360 | 2135442 | 97.133 | 203445 | 94% |
| 5 | Phyllanthin | 6.642 | 942696 | 94 | 45062 | 943 |
Furthermore, these compounds have shown anti-inflammatory, anticancer and antidiabetic activity. The presence of these compounds forms the basis of antioxidant and antimicrobial properties of the P. niruriL. extract. Phenolic compounds may play an important role in preventing chronic illnesses such as cardiovascular disease, a certain type of cancers, neurodegenerative disease, and diabetes (Verma et al., 2010). Flavonoids have been shown to possess many pharmacological properties such as anti-oxidant activities, anti-inflammatory activities, anti-cancer activities, and anti-microbial effects, hence, flavonoids may have a contributory effect to its fertility properties and other pharmacological effects the plant possesses (Verma et al., 2011 and Harbone, 1998). The study was aimed to perform the phytochemical analysis and determine antioxidant potential of P. niruri. Mehta et al. (2019) in their study considered qualitative and quantitative characterization of phytochemicals and antioxidants present in P. niruri extract. It was observed from the results that aqueous extract of the plant possessed high antiodixant activity that could be by virtue of flavonoids present in it.
Phyllanthin belongs to the lignanscategory and has been shown to possess hepatoprotectiveand anti-genotoxic activities (Row et al., 1964). Phyltetralin, nirtetralin, and niranthinextracted from Phyllanthus niruriL. exhibited anti-inflammatory activity by inhibiting carrageenan-induced paw edema and neutrophil influx (Kassuya et al., 2005).The HPLC analysis of the sample is revealing the presence of potent phytochemicals compounds in both plant extracts indicating that the plant extracts have good medicinal value as reported by Meselhy et al., (2020). The emergence of antibiotic-resistant bacteria is another major health concern globally. The search for novel antioxidant and antimicrobial compounds is carried out throughout the globe by scientists meticulously. Phenol, flavonoids, and tannins are a major class of phytochemicals that have antimicrobial activity (Cowan et al., 1999; Gupta and Vaghela, 2019). The plant extract of Phyllanthus niruri L. has shown the good antimicrobial property as well hence their introduction in herbal medicine preparation might be an effective approach (Nisar et al., 2018).
CONCLUSION
The overall conclusion derived from the study is that Phyllanthus niruriL. is a source of potent bioactive compounds having essential and effective biological properties. Phyllanthus niruri L. is an important medicinal plant. The plant is widely used for the treatment of hepatic disease, edema, dropsical condition, and urinary troubles. P. niruriL.has many effective traditional uses for a wide variety of diseases. Some of the medicinal uses have been proven in experimental models, which suggest that the extracts of the plant possess various pharmacological actions. All the pharmacological properties shown by the extract of the plant is due to the presence of such bioactive compounds. The study reveals the presence of such effective compounds.
The study from the HPLC analysis has shown the presence of such bioactive compounds which carry out altogether a wide variety of biological functions. The study also concludes that these bioactive compounds impart good antimicrobial properties in the extracts as well. So the introduction of such an important plant in herbal drug formulation could lead to betterment in the effectiveness of drugs. Further studies could be carried out on various other solvent extracts of P. niruriL. as well as on determining another biological potential of extract than antimicrobial. Purification and incorporation of these bioactive compounds from P. niruriL. into medicinal use could a better idea for further study.
REFERENCES
Adedinsewo, Demilade, Junjun Xu, PradyumnaAgasthi, AdesojiOderinde, OluwatoyosiAdekeye, Rajesh Sachdeva, George Rust, and Anekwe Onwuanyi (2017). Effect of digoxin use among Medicaid enrollees with atrial fibrillation. Circulation: Arrhythmia and Electrophysiology 10, no. 5: e004573.
Akinmoladun, Afolabi C., E. O. Ibukun, Emmanuel Afor, Efere Martins Obuotor, and E. O. Farombi (2007). Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum.Scientific Research and Essays 2, no. 5: 163-166.
Barros, Marcio E., N. Schor, and M. A. Boim (2003). Effects of an aqueous extract from Phyllantus niruri on calcium oxalate crystallization in vitro. Urological research 30, no. 6: 374-379.
Bavarva, Jasmin H., and A. V. R. L. Narasimhacharya (2007). Comparative Antidiabetic, Hypolipidemic, and Antioxidant Properties of Phyllanthus niruri. in Normal and Diabetic Rats. Pharmaceutical biology 45, no. 7: 569-574.
Becker, C. G., D. P. Hajjar, and J. M. Hefton (1985). Tobacco constituents are mitogenic for arterial smooth-muscle cells.The American journal of pathology 120, no. 1: 1-5.
Burkil, H (1966). A Dictionary of the economic products of Malay Peninsula, Governments of Malaysia & Singapore by ministry of Agriculture and cooperatives; Kualalampur, Malaysia. Vol-I (AH) and pp 1748-1749.
Chan, Eric WC, Eu Ying Soh, Pei Pei Tie, and Yon Peng Law (2011). Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis Pharmacognosy research 3, no. 4: 266-272.
Cimanga, R. K., L. Tona, N. Luyindula, K. Mesia, M. Lusakibanza, C. T. Musuamba, S. Apers et al. (2004). In vitro antiplasmodial activity of callus culture extracts and fractions from fresh apical stems of Phyllanthus niruri L.(Euphorbiaceae): part 2. Journal of ethnopharmacology 95, no. 2-3: 399-404.
Cowan, Marjorie Murphy(1999). Plant products as antimicrobial agents.Clinical microbiology reviews 12, no. 4: 564-582.
Dhar, M. L., M. M. Dhar, B. N. Dhawan, B. N. Mehrotra, and C. Ray. Screening of Indian plants for biological activity: I. Indian journal of experimental biology 6, no. 4 (1968): 232-247.
Edeoga, H. Okwu, D. E. Okwu, and B. O. Mbaebie (2005). Phytochemical constituents of some Nigerian medicinal plants. African journal of biotechnology 4, no. 7: 685-688.
Eslami, Habib, Seyed Kaveh Mohtashami, Maryam TaghaviBasmanj, Maryam Rahati, and HamzehRahimi (2017). An in-silico insight into the substrate binding characteristics of the active site of amorpha-4, 11-diene synthase, a key enzyme in artemisinin biosynthesis. Journal of molecular modeling 23, no. 7: 202.
Girach, R. D., Aminuddin, P. A. Siddiqui, and Subhan A. Khan (1994). Traditional plant remedies among the Kondh of District Dhenkanal (Orissa). International journal of pharmacognosy 32, no. 3: 274-283.
Gupta, M., &Vaghela, J. S. (2019). Recent advances in pharmacological and phytochemistry studies on Phyllanthus amarus. Pharmaceutical and Biosciences Journal, 7(1), 01-08.
Harborne, A. J. (1998). Phytochemical methods a guide to modern techniques of plant analysis. Springer science & business media.
Igbinosa, O. O., E. O. Igbinosa, and O. A. Aiyegoro (2009). Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). African journal of pharmacy and pharmacology 3, no. 2: 058-062.
Ishimari, K., K. Yoshimatsu, T. Yamakawa, H. Kamada, and K. Shimomura (1999). Genetic transformation of Phyllanthus niruri L.(P. amarus). In Transgenic Medicinal Plants, For:45 pp. 237-248. Springer, Berlin, Heidelberg.
Kassuya, Cândida AL, Daniela FP Leite, LuciliaVilela de Melo, Vera Lúcia G. Rehder, and João B. Calixto (2005). Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta medica. 71, no. 08: 721-726.
Kerharo, G and Adam, JG.(1974), Senegalese traditional pharmacopeia. Medicinal Plants and Toxic, Vigot Frèrespp.427-428.
Meena, J., Sharma, R. A., & Rolania, R. (2018). A review on phytochemical and pharmacological properties of Phyllanthus amarus Schum. And Thonn. International journal of Pharmaceutical sciences and Research, 9(4), 1377-1386.
Mehta, M., Gupta, S., Duseja, A., & Goyal, S. (2019). Phytochemical and antioxidants profiling of Phyllanthus niruri: A hepatoprotective plant. World J Pharm Pharm Sci, 8, 1117.
Meselhy, M. R., Abdel-Sattar, O. E., El-Mekkawy, S., El-Desoky, A. M., Mohamed, S. O., Mohsen, S. M, & El-Halawany, A. (2020). Preparation of Lignan-Rich Extract from the Aerial Parts of Phyllanthus niruri Using Nonconventional Methods. Molecules, 25(5), 1179.
Nisar, M. F., He, J., Ahmed, A., Yang, Y., Li, M., & Wan, C. (2018). Chemical components and biological activities of the genus phyllanthus: A review of the recent literature. Molecules, 23(10), 2567.
Nishiura, J. L., A. H. Campos, M. A. Boim, I. P. Heilberg, and N. Schor (2004). Phyllanthus niruri normalizes elevated urinary calcium levels in calcium stone forming (CSF) patients. Urological research 32, no. 5 (2004): 362-366.
Nwanjo, H. U., G. Oze, M. C. Okafor, D. Nwosu, and P. Nwankpa (2007). Protective role of Phyllantus niruri extract on serum lipid profiles and oxidative stress in hepatocytes of diabetic rats. African Journal of Biotechnology 6, no. 15:1744-1749.
Nyamai, Dorothy Wavinya, W. Arika, P. E. Ogola, E. N. M. Njagi, and M. P. Ngugi (2016). Medicinally important phytochemicals: An untapped research avenue. Journal of Pharmacognosy and Phytochemistry 4, no. 1: 35-49.
Obianime, A. W., and F. I. Uche (2009). The Phytochemical constituents and the effects of methanol extracts of Phyllanthus amarus leaves (kidney stone plant) on the hormonal parameters of Male guinea pigs. Journal of Applied Sciences and Environmental Management 13, no. 1:5-9.
Paran-jape, P (2001). Indian Medicinal Plants: Forgotten Healers. Chaukhamba Sanskrit Pratisthan, Dheli pp.48
Pauwels, L (1993).NzayiluN’ti. Guide trees and shrubs in the area of Kinshasa- Brazzaville. JBNB 4:459.
Row, LR.,Srinivasulu, C., Smith, M and Subba Rao, GSR (1964). New lignins from Phyllanthus niruri linn. Tetrahedron Lett. 5:1557-1567.
Saija, Antonella, Antonio Tomaino, Domenico Trombetta, Maria Luisa Pellegrino, Beatrice Tita, Chiara Messina, Francesco P. Bonina, Concetta Rocco, Giovanni Nicolosi, and Francesco Castelli (2003). In vitro’antioxidant and photoprotective properties and interaction with model membranes of three new quercetin esters. European Journal of Pharmaceutics and Biopharmaceutics 56, no. 2: 167-174.
Santos, Adair RS, C. ValdirFilho, RosendoA. Yunes, and João B. Calixto (1995). Analysis of the mechanisms underlying the antinociceptive effect of the extracts of plants from the genus Phyllanthus. General Pharmacology: The Vascular System 26, no. 7 : 1499-1506.
Tona, L., N. P. Ngimbi, M. Tsakala, K. Mesia, K. Cimanga, S. Apers, T. De Bruyne, L. Pieters, J. Totte, and A. J. Vlietinck (1999). Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. Journal of Ethnopharmacology 68, no. 1-3: 193-203.
Verma, K. S., Trivedi Deepika, Awasthi Aparna, and Sangeeta Dolly Juda (2011). Indigenous Knowledge and Conservation of Endangered Angiosperm flora of Jabalpur with special emphasis of Herbs, Shrubs and Climbers. Anusandhan 5: 60-63.
Verma, KS., Dixit, V., Khare, D and Awasthi, A. (2010). Phytochemical screening and antibacterial activity of Buchananialanzan Spreng. J Phytol Res 23, no. 2 : 339-342.
World Health Organization (1991). Guidelines for the assessment of herbal medicines. No. WHO/TRM/91.4. Unpublished. Geneva: World Health Organization, 1991.


![Fig 3: HPLC chromatogram of Phyllanthus niruri L. [A] Methanolic Extract [B] Petroleum ether extract](http://bbrc.in/wp-content/uploads/2020/11/Vol_13No_3_DES_HEN_Fig3.jpg)