1Department of Zoology, Faculty of Life Sciences, Federal
University of Lafia, Nasarawa State, Nigeria.
2Applied Entomology & Parasitology Unit, Department of Zoology, Faculty
of Natural Sciences, University of Jos, Nigeria.
3Department of Chemistry, Faculty of Physical Sciences, Federal
University of Lafia, Nasarawa State, Nigeria.
Corresponding author email: affikuisaacjoshua1@gmail.com
Article Publishing History
Received: 15/06/2025
Accepted After Revision: 04/08/2025
Mosquito-borne diseases remain a major public health challenge globally, necessitating the search for eco-friendly alternatives to synthetic insecticides. Thus, this study investigated the phytochemical composition and larvicidal activity of Sida acuta leaf oil extract against Aedes aegypti larvae under varying treatments. Whole plant samples were collected in the morning (0600–0700 hours) and evening (1800–1900 hours), thereafter, identified, air-dried, then ground into fine powder, and extracted using n-hexane for a standing period of three days. Phytochemical constituents were determined using standard qualitative and quantitative methods. Eggs of Ae. aegypti mosquito were hatched in the laboratory during which the reared first-instar larvae of Ae. aegypti were exposed to varying concentrations of the oil extract at 12.5, 25, 37.5, 50, 62.5, and 125 ppb, respectively.
Larvae knocked-down within the first hour exposure period were recorded immediately, and thereafter mortality was monitored at 24, 48, and 72 hours, respectively, post-exposure. Our qualitative analysis indicated high levels of alkaloids, steroids, and phenols, while flavonoids, tannins, and glycosides were present in low concentrations. Terpenoids were detected at low level in morning collection extract but were absent in evening extract. Anthraquinones and saponins were not detected in both time of day extracts. Quantitative screening confirmed the presence of flavonoids, phenols, tannins, alkaloids, saponins, terpenoids, cyanides, oxalates, and phytates. Larvicidal testing revealed lowest mortality rate of 1% for the morning extract in 50 ppb treatment and highest mortality rate of 3% for the evening extract in 62.5 ppb treatments after 72 hours exposure period. No significant difference (P > 0.05) was observed in the mortality rate in relation to treatments as well as between times of day. These findings demonstrate that Sida acuta leaf oil extract contains various bioactive phytochemicals. The larvicidal efficacy of Sida acuta against Ae. aegypti under the tested conditions was quite low which implies resistance but a more efficacious larvicidal activity maybe observed in the near future with increase in concentrations. In conclusion, Sida acuta leaf oil extract is a potential plant-based biopesticide for use in mosquito vector control.
Aedes aegypti, Sida acuta, Phytochemicals, Plant oil extract, Larvicide, Biosolution, Susceptibility profile
Joshua I.A, Ombugadu A,Ashigar M.A, Maikenti J.I, Ahmed H.O, Polycarp I.A, Otakpa E.O, Nwokocha C.E, Judah S.J, Pam V.A. Phytochemical Composition and Larvicidal Activity of Sida Acuta Oil Extract Against Aedes Aegypti Larvae. Biosc.Biotech.Res.Comm. 2025;18(3).
Joshua I.A, Ombugadu A,Ashigar M.A, Maikenti J.I, Ahmed H.O, Polycarp I.A, Otakpa E.O, Nwokocha C.E, Judah S.J, Pam V.A. Phytochemical Composition and Larvicidal Activity of Sida Acuta Oil Extract Against Aedes Aegypti Larvae. Biosc.Biotech.Res.Comm. 2024;18(3). Available from: <a href=”https://shorturl.at/tfZ03“>https://shorturl.at/tfZ03</a>
INTRODUCTION
Plant-based pesticides are one of the most widely used biological mosquitoes and other vectors control methods because of their low cost, ease of availability, and eco-friendliness. They can also cause changes in the morphology, physiology, biochemical processes, and behaviour of different mosquitoes and other vectors life stages, indicating their significance in insect population control (Three thousand years ago, extracts from aromatic plants were employed as ectoparasite and anthelmintic repellents as well as to preserve harvested goods from pests, beginning a long history of the use of plant extracts for insect control. Based on their antibacterial properties and application in pharmaceutical goods, plant essential oils, particularly leaf oil extracts, have been employed, extensively (Scalvenzi et al., 2019, Selvakumaran et al., 2024).
Phyto-chemicals can be obtained from the entire plant or a specific part of it by extraction with various types of solvents such as methanol, ethanol, petroleum ether, water, and chloroform, depending on the polarity of the phyto-chemicals. They are also advantageous due to their ecosafety, target specificity, lack of record of insect resistance development, higher acceptability, and suitability for rural areas (Ngwamah & Naphtali, 2019). They are inexpensive, readily available, and environmentally beneficial, plant-based pesticides are a favourite among biological mosquito control techniques (Mwingira et al., 2020).
Alkaloids, flavonoids, tannins, essential oils, and phenols are just a few of the phyto-chemicals that have been reported to have insecticidal properties, whose effects vary depending on the plant species, mosquito species, geographical varieties and parts used, the extraction technique employed, and the polarity of the solvents utilized during extraction (Rodrigues et al., 2020; Souheila et al., 2020).
Diverse researchers (Ullah et al., 2018; Scalvenzi et al., 2019; Balboné et al., 2022) have been focusing their efforts in recent years on finding plant-based natural remedies as an alternative to conventional pesticides used to control vectors for which resistance was discovered. Essential oils (EOs) and their constituents have drawn a lot of attention in the hunt for new pesticides because it has been discovered that they have insecticidal potential (Balboné et al., 2022). Through the development of standards for their use and registration, the World Health Organization has facilitated the replacement of conventional insecticides with bacterial and phytochemicals. Pyrethrin was the first phytochemical to be extracted from chrysanthemums and is known to be an oviposition deterrent and female irritant (Ullah et al., 2018).
Potential sources of commercially available anti-mosquito bioactive substances include plant extracts or phytochemicals. According to Ninditya et al. (2020), some phytochemical compounds have a general toxicant effect on the adult and larval stages, while others have an attractant or repellant effect that hinders growth and development by causing olfactory stimuli. Natural product pesticides quickly degrade in the environment, are less toxic to other organisms, and have a synergistic combination of active compounds in extracts that induce multiple mechanisms of action and reduce pest resistance, they are preferred over conventional pesticides for controlling vectors (Silverio et al., 2020).
According to Aminah et al. (2021), Sida species possess a number of secondary metabolites, including those for alkaloids, flavonoids, coumarins, ecdysteroids, triterpenes, and tocopherols. Glycosides, steroids, and saponins are present in the ethanolic leaf extract of Sida acuta and amino acids, glycosides, terpenoids, steroids, flavonoids, and saponins are present in the petroleum ether leaf extract of Sida acuta in the study of the phytochemical screening of ethanol and petroleum ether leaf extract of Sida acuta, Burm. F. carried out to identify the secondary metabolites; however, neither of the Sida acuta leaf extracts contained either carbohydrates or tannins (Amitha & Joseph, 2019). Sida acuta is a powerful medication that has been used for centuries to treat a variety of conditions.
A review of the potential effects of the plant on the central nervous system and other diseases revealed that it contains phytochemicals with a variety of pharmacological actions, including terpenoids, β-phenethylamine, glutathione peroxidase, choline, vasicine, ephedrine, and cryptolepine (the main alkaloid of the plant), saponosides, coumarins, steroids (ecdysterone, β-sistosterol, stigmaterol, ampesterol), tannins, phenolic compounds (evofolin-A and B, scopoletin, loliolid, and 4-ketopinoresinol), polyphenol, sesquiterpene, and flavonoids (Tcheghebe et al., 2017). Consequently, based on the findings, it can be said that aqueous extract of S. acuta may have a wealth of medicinal qualities and may even be a unique medication (Abhishek et al., 2023).
Sida species, including Sida acuta, have been found to contain a variety of phytochemicals, including tannins, cardiac glycosides, and saponins, which may contribute to their potential biological activities. Research has shown that extracts from Sida acuta have larvicidal activity against Aedes mosquitoes, which are vectors of diseases like dengue and yellow fever. The plant’s essential oil and hydrosol have also demonstrated strong larvicidal properties, and the plant’s oil extract has been used to create silver nanoparticles, which showed promise as an environmentally friendly way to control vector mosquitoes (Krishnaveni et al., 2018; Ullah et al., 2018; Lwande et al., 2020; Shittu & Alagbe, 2020; Bassey et al., 2021; Usman & Abdulkarim, 2023).
The phytochemical composition and mineral content of the plant suggest that it may have medicinal properties and potential uses. There is an urgent need to update the information status of the phytochemical screening of Sida acuta oil extracts and their larvicidal activity against Aedes aegypti, as there are not many studies online on that aspect. Therefore, this research evaluated the phytochemical composition and larvicidal activity of Sida acuta plant oil extract against Aedes aegypti.
MATERIAL AND METHODS
Study Area: This study was conducted in Lafia, Nasarawa State (Figure 1). Lafia is the State capital of Nasarawa, which also serves as one of the Local Government Districts in the State. Lafia lies between 8°29’N and 8°30’E latitude and longitude. The City has 330,712 residents overall, according to the 2006 population census (National Bureau of Statistics, 2006; Michael et al., 2016). The metro area population of Lafia in 2025 was 403,000, a 3.87% increase from 2024 (Abiodun, 2022).
The majority of the workforce of the state is employed in farming, and the products produced include cassava, yam, rice, corn, soybeans, asha, groundnuts, vegetables, sugar cane, and millet (Abiodun, 2022). The state is endowed with valuable mineral resources, including aquamarine, columbite and coal. Because most of the population works as farmers and cultivators of cash crops, they are more susceptible to diseases spread by mosquitoes as it makes room to plenty of mosquito breeding sites (Ombugadu et al., 2024).
Figure 1: Map of Lafia Metropolis in Nasarawa State showing the
CollectionPoint (Created using QGIS Version 3.38.1)
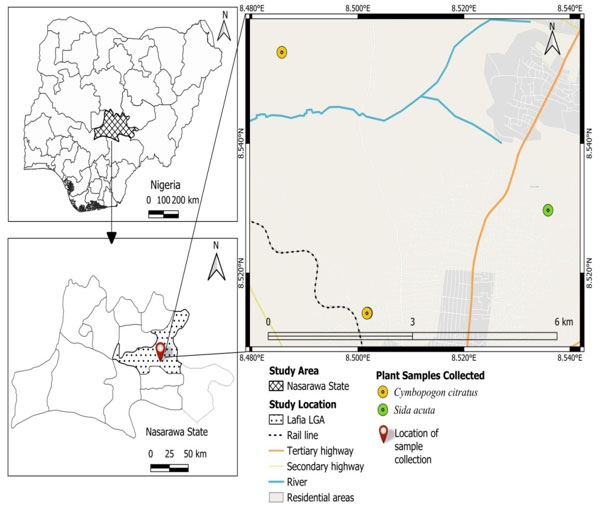
Sample Collection: The leaves of Sida acuta (Wireweed) were sought for and collected from houses and schools around Lafia metropolis of Nasarawa state, Nigeria, in the morning (0600 – 0700 hours) and evening (1800-1900 hours) period of the day. The plant collected was identified and authenticated botanically in the Department of Plant Science and Biotechnology Laboratory, Federal University of Lafia and assigned the voucher number FUL/SC/PSB/H.LAB/0082. Firstly, the leaves were cleaned with running tap water to remove dust and debris from their surface. Thereafter, they were allowed to air dry at room temperature without exposure to sunlight using methods described by Nortjie et al. (2022) in the Department of Zoological Laboratory, Federal University of Lafia, Nasarawa State, Nigeria.
Figure 2: Sida acutas Plant (Field Photo)

Preparation of Plant Extracts: The leaves of Sida acuta, which were dried at room temperature, became brittle and easy to crush, then ground with a mortar and pestle to produce a fine powder (Osuagwu et al., 2021). Plant extracts were made in a 1:2w/v ratio, meaning that 91.1 g (Sida acuta) powder was prepared for every 125 ml of solvent. For the n-Hexane, 911.2 g of the powder from Sida acuta was weighed using a digital electronic laboratory scale (500G x 0.01G – SF-400C) and added to two different 2.5 L amber bottles that each held 1250 ml of n-Hexane respectively. The bottles were then left to stand for three days (seventy-two hours) with continuous stirring (Osuagwu et al., 2021).
The goal of the procedure was to release the soluble phytochemicals by breaking and softening the plant’s cell wall (Nortjie et al., 2022). Using non-absorbent cotton wool and Whatman number (No.) 1 filter paper, the mixture solutions were filtered into conical flasks. The filtrates were concentrated by evaporating in a water bath at 60 °C, weighed and recorded (Osuagwu et al., 2021). After being scraped off the conical flasks, the dried crude oil of the solvent was quantitatively transferred into sample containers and kept fresh in the refrigerator. Following extraction, determination of percentage yield and phytochemical analysis was performed on the crude oil leaf extracts to determine their active components. Determination of the Percentage Yield of Oil Extracts.
The oil extract was weighed using a M-Metlar digital weighing balance (0.1 g – 3000 g) and the percentage yield of the oil extracts was calculated by the methods outlined by Mgbechidinma et al. (2023) as presented in equation 1.
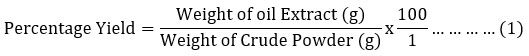
Percentage Yield of Sida acuta Oil Extracts

Qualitative Phytochemical Analysis of the Plant Oil Extract: The leaves of Sida acuta solvent oil extract were screened qualitatively for phytochemical constituents such as alkaloids, flavonoids, anthraquinones, steroids, saponins, phenols, terpenoids, tannins, and glycosides, using standard methods of analysis (Sofowora, 1993; Trease& Evans, 2002).
Test for Alkaloids: Few quantity of the each portion was stirred with 5 ml of 1% aqueous HCl on water bath and then filtered. Of the filtrate, 1 ml was taken individually into 2 test tubes. To the first portion, few drops of Dragendorff’s reagent were added; occurrence of orange-red precipitate was taken as positive. To the second portion, Mayer’s reagent was added and buff-coloured precipitate appearance was an indication for the presence of alkaloids (Sofowora, 1993).
Shinoda Test for Flavonoids: Few quantity of the each portion was dissolved in water and filtered; to this 2 ml of the 10% aqueous sodium hydroxide was later added to produce a yellow colouration. A change in colour from yellow to colourless on addition of dilute hydrochloric acid was an indication for the presence of flavonoids (Trease & Evans, 2002).
Borntrager’s Test for Anthraquinones: About 0.2 g of each portion to be tested was shaken with 10 ml of benzene and then filtered. Five millilitres of the 10% ammonia solution was then added to the filtrate and thereafter shaken. Appearance of a pink, red or violet colour in the ammoniacal (lower) phase was taken as the presence of free anthraquinones (Sofowora, 1993).
Test for Steroids: To 0.2 g of each portion, 2 ml of acetic acid was added, the solution was cooled well in ice followed by the addition of concentrated hydrogen tetraoxosulphate (VI) (H2SO4) carefully. Colour development from violet to blue or bluish-green indicated the presence of a steroidal ring i.e. aglycone portion of cardiac glycoside (Sofowora, 1993).
Ferric Chloride Test for Phenols: About 0.5 of each portion was boiled with distilled water and then filtered. To 2 ml of the filtrate, few drops of 10% ferric chloride solution were then added. A green-blue or violet colouration indicated the presence of a phenolic hydroxyl group (Trease & Evans, 2002).
Knollar’s Test for Terpenoids: A little of each portion was dissolved in ethanol. To it 1 ml of acetic anhydride was added followed by the addition of conc. H2SO4. A change in colour from pink to violet showed the presence of terpenoids (Sofowora, 1993).
Test for Tannins:The test extract was taken in water, warmed and filtered. 5 ml of filtrate was allowed to react with 1ml of 5% ferric chloride solution. If dark green or deep blue color is obtained, tannin is present (Dubale et al., 2023).
Test for Saponins: One (1) ml solution of extract was diluted with distilled water to 20 ml and shaken in a graduated cylinder for 15 minutes. Development of stable foam suggests the presence of saponins. Also, 1ml extract was treated with 1% lead acetate solution. Formation of white precipitates indicates the presence of saponins (Dubale et al., 2023).
Test for Glycosides: Two (2) ml of extract, 3 ml of chloroform and 10% ammonia solution were added. Formation of pink color indicates presence of glycosides.
Quantitative Phytochemical Analysis of the Plants Oil Extract: The quantitative phytochemical screening was conducted using the gravimetric analytical method of Khaled and Irani (2021) for the analysis of flavonoids, phenols, tannins, alkaloids, saponins, terpenoids, cyanide, oxalate and phytate. The methodological procedures employed for the gravimetric quantitative phytochemical analysis are shown below.
Determination of Flavonoids: One (1) g of the sample was weighed and repeatedly extracted with 100 cm3 of 80% methanol at room temperature. The mixture was then filtered through filter paper into a 250 cm3 beaker and the filtrate was transferred into a water bath and allowed to evaporate to dryness and weighed. The % of flavonoid was calculated (Krishnaiah et al., 2009) as expressed in equation 2.
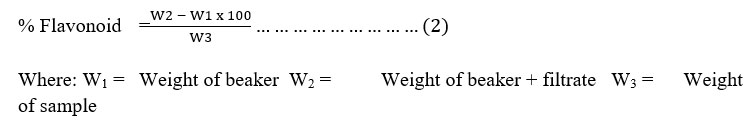
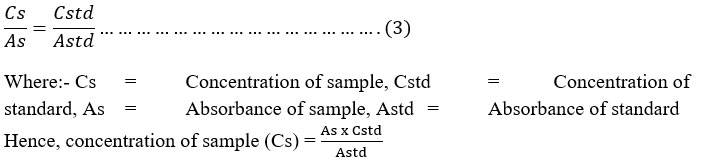
Estimation of Total Phenol: The fat free sample was boiled with 50 ml of ether for the extraction of the phenolic component for 15 min. Five ml of the extract was pipetted out into a 50 ml flask, then 10 ml of distilled water was added. Two ml of NH4OH solution and 5 ml of concentrated amyl alcohol were also added. The samples were made up to mark and left to react for 30 minutes for colour development. This was read at 505nm. A standard was prepared from phenol stock standard (supplied by Sigma Chemical Company Limited England), this was slightly modified (Santhi & Sengottuvel, 2016).The concentration of the sample was determined by using phenol standard as shown in equation 3.
Determination of Tannin: Tannin was determined using methods described by Horwitz and Albert (1996). This method was however slightly modified. About 2 g of sample was boiled with 300 ml of distilled water, diluted to 500 ml in standard volumetric flask and filtered through non- absorbent cotton wool. A volume of 25 ml of the infusion was measured into 250 ml conical flask and titrated with 0.1 N potassium permanganate (0.1 N) until pinkish colouration observed for at least 5 seconds. Potassium permanganate was standardized against sodium oxalate). The difference between the two titrates was multiplied by 3.0 to obtain the amount of tannin in the sample using equation 4.
0.1 ml potassium permanganate = 3.0 mg tannin ………………………….. (4)
Determination Alkaloids: Weigh 5.0 g of a given weight of each sample and disperse into 50 nl of 10% acetic acid solution in ethanol. Shake the mixture well and allow to stand for 4 hrs before filtering. Evaporate the filter to one quarter (1/4) of its original volume. Add drops wisely of concentrated ammonium hydroxide (NH4OH) to precipitate the alkaloids. Filter off the precipitate with a weighed filter paper and wash with 1% NH4OH solution. The filtering was done with a weighed filter paper. Dry the precipitate in filter paper in the oven at 60℃ for 30 min and reweigh. The weight difference, the weight of alkaloid is determined and expressed as a percentage of the sample weight analyzed (Khaled & Irani, 2021) as shown in equation 5.
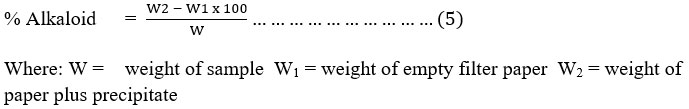
Test for Saponin: Saponin content: Total saponin content (% yield) was determined by gravimetric method as described by Kaur et al. (2015). The methanolic extracts from each plant (1 g in 10 ml) were macerated for 24 hours and then partitioned in water and n-butanol (1:1 ratio) solution. This solution was poured into the separator funnel and kept for 2 hours. The upper n-butanol layer was separated and the solvent was evaporated to obtain crude saponin extract as stated in equation 6.
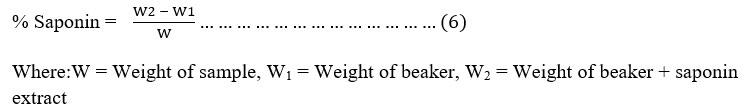
Quantitative Determination of Terpenoid: Dried plant extract 100 mg (wi) was taken and soaked in 9ml of ethanol for 24 hours (Malik, 2017). The extract after filtration was extracted with 10ml of petroleum ether using a separating funnel. The ether extract was separated in pre-weighed glass vials and waited for its complete drying (wf). Ether was evaporated and the yield (%) of total terpenoid content was measured by the formula in equation 7.
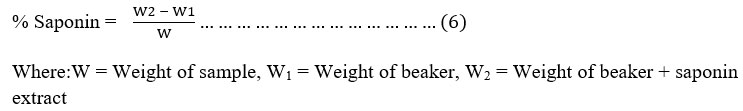
Cyanogenetic Glycosides (Cyanide) Alkaline Titration: A portion of 10-20 g was placed in number (No.) 20 sieve in 80 ml of Kjedahl flask, add approximately 200 ml of water and let it stand for 2 to 4 hours (Autolysis should be conducted with apparatus completely connected for distillation). Steam distill, collect 150 to 160 ml distillate in sodium hydroxide (NaOH) Solution (0.5 g in 20 ml water), and dilute to definite volume (250 ml). To 100 ml distillate (it is preferable to dilute to 250 ml and titrate 100 ml aliquot) add 8 ml 6 m ammonium hydroxide (NH4OH) and 2 ml 5% potassium iodide (KI) Solution and titrate with 0.02 m silver nitrate (AgNO3), using microburrete. End point is faint but permanent turbidity was easily recognized, especially against black background (Horwitz & Albert, 1996) as shown in equation 8.
1 ml 0.02 m AgNO3 = 1.08 g HCN (1 Silver equivalent to 2 Cyanide) …………… (8)
Determination of Oxalate Content by Titration: Oxalate ions are extracted from the plant parts by boiling them with dilute H2SO4 (0.5 N). Then oxalate concentration estimated volumetrically by titrating the extract with standard potassium per-manganate (KMnO4) solution MnO4– + 5C2O4 2- + 8H+ → Mn2+ + 10CO2 + 4H2O. This method was slightly modified.One gram (1 g) of plant material was weighed in electric weighing balance and transferred to 30 ml of 0.5 N sulfuric acid (H2SO4) and was boiled in a water bath for 15 minutes. Then the extract was filtered with Whatman’s No. 1 filter paper. Equal volume of deionised water was added. Then 10 ml of filtered extract was taken and 40 ml 0.5 N H2SO4 was added. Final 50 ml of mixture was heated to 60° C and was titrated against 0.05 N KMnO4. The end point was determined by permanent appearance of light pink colour (Mishra et al., 2017) as expressed in equation 9.
1 ml of KMnO4 = 2.24% Oxalate …………………..…………… (9)
Test for Phytate: Phytic acid was determined using a method reported by Yahaya et al. (2013). Four grams (4 g) of ground sample was soaked in 100 ml of 2% hydrochloric acid (HCl) for 3 hours and then filtered through two layers of filter paper, 25 ml of the filtrate was placed in a 250 ml conical flask and 5 ml of 0.3% ammonium thiocyanate (NH4SCN) solution was added as an indicator, 53.5 ml of distilled water was then added to reach the proper acidity. This mixture was titrated against ferric chloride (FeCl3) solution, which contains about 0.00195 g of iron per ml of FeCl3 solution until a brownish yellow colour that persisted for 5 minutes was obtained. The result was multiplied by factor1.95 to obtain phytate P. Phytate P result was multiplied by factor 3.55 to convert to phytate.
Preparation of Stock and Working Solution of Sida acuta Oil Extract: The stock solution was prepared using methods as suggested by WHO (2018), where 10 g of each oil extract (n-hexane) of both plants was weighed using an ADAM PW 124 (Max 120 g d = 0.0001 g) Electric Weighing Balance. The weighed extracts were added to 20 ml beaker then distilled water was added and allowed to stand for 1 hour, with occasional agitation. The suspension was subsequently transferred quantitatively into a 50 ml volumetric flask by filtering using Whatman No. 1 filter paper and the volume was adjusted to 50 ml by adding distilled water to make the stock solution of 100 mg/ml. From this stock solution, working solutions were prepared to obtain concentrations in parts per billion of 12.5 ppb, 25 ppb, 37.5 ppb, 50 ppb, 62.5 ppb and 125 ppb, respectively, a modification used from WHO (2020). The working solutions (diagnostic doses) were prepared using equation 10 by WHO (2018): C1V1 = C2V2 ………………………………………………… (10)
Where: C1 = Stock concentration (beginning concentration), V1 = Volume of stock required to prepare new solution, C2 = Concentration of new or working solution (desired concentration),V2 = Volume of new solution desired
Culturing of Aedes Eggs for Larvae: Prior to the hatching of Ae. Aegypti eggs gotten from National Arbovirus and Vectors Research Centre (NAVRC), Enugu State, Nigeria, nutrient broth was prepared by extracting nutrient broth from Agar powder, by adding, mixing and completely dissolving 13 g of nutrient agar powder (CM0001B) in 1L of distilled water and allowing the agar residue to settle to get our pure nutrient broth which was then poured into a conical flask and sterilized by autoclaving at 121°C for 15 minutes. Thereafter, the egg stripes were soaked into nutrient broth and allowed to stand for 12-24 hours in order for the eggs to hatch to first instar larvae. The larvae hatched were later fed by putting a pinch of finely powdered biscuit and yeast unto the surface of the water.
Preparation of Bioassay: The susceptibility of first-instar mosquito larvae were introduced and exposed to six concentrations (12.5 ppb, 25 ppb, 37.5 ppb, 50 ppb, 62.5 ppb and 125 ppb) of the n-hexane oil extract Sida acuta and was tested using the bioassay method in accordance with the recommended and advanced protocol of the World Health Organization [WHO] (2020) for testing of mosquito larvicides. For every concentration, four replicates were maintained, along with a positive and negative controls for every experiment. In each replicate, twenty-five larvae were put into each of four disposable transparent rubber bowls (150 mL capacity) that held 100 mL of distilled water.
Using a syringe, 1 milliliter of the solution for each diagnostic dose was dispensed into each rubber bowls for the n-hexane oil extracts. The positive control consisted of 1 ml of ethyl ether with 1 ml of n-hexane/100 ml of distilled water, while the negative control consisted of 100 ml of distilled water alone. The rate at which larvae were knocked down were noted at 10, 15, 20, 30, 40, 50, and 60 minutes and larval mortality was measured after exposure times of 24, 48, and 72 hours. A tiny needle puncture to the larvae’s belly to verified their mortality (WHO, 2018). A Taylor 1523 digital indoor/outdoor thermo-hygrometer was used to measure the temperature and relative humidity conditions in the lab.
Test Analysis :The interpretation of the mortality rate of mosquito larvae
based on World Health Organization (2018) is as follows.
- Mortality rate between 98 – 100 % within the diagnostic time connotes susceptible.
- Mortality rate between 80 – 97 % suggests possible resistance.
- Mortality rate < 80 % indicates resistance.
Determination of Percentage Mortality: Mortality was calculated using Abbott’s formula. Non-mobile and moribund larvae were recorded as dead as presented in equation 11.
![]()
Statistical Analysis: R Console software (Version 4.4.2) was used to analyze the observed data that was obtained. Pearson’s Chi-square test was used to compare larval percentage mortality rate in relation to treatments, plants and time of day, respectively. Level of significance was set at P < 0.05.
RESULTS
Phytochemicals Qualitatively Detected in Sida acuta Oil Extract: From the qualitative analysis done for Sida acuta oil extract gotten from that leaves showed a very high concentration (+++) of alkaloids, steroids and phenol were detected in both morning and evening periods collections followed by flavonoids, tannin, and glycosides which were present in low concentration (+) at both time of day (Table 1). Terpenoids was only detected in the morning period at a low concentration (+) whereas anthraquinones and saponin were not detected (–) at all in Sida acuta.
Table 1. Qualitatively Detected Metabolites in Sida acuta
Plants Oil Extracts in Relation to Time of Day
| Time of Day | ||
| Metabolites | Morning | Evening |
| Alkaloid | +++ | +++ |
| Flavonoids | + | + |
| Anthraquinone | – | – |
| Steroids | +++ | +++ |
| Saponins | – | – |
| Phenol | +++ | +++ |
| Terpenoids | + | – |
| Tannin | + | + |
| Glycosides | + | + |
Key: +++ = Present in high concentration, + = Present in low concentration= Not Detected
Quantitative Output of Phytochemicals in Sida acuta Oil Extracts: Table 2 shows the quantitative results using gravimetric method of analysis. With the exception of Tannin (120.28 mg/L), phenol (3.01%) and phytate (1.36%) that was higher in the evening hours, all other metabolites were more in the morning period. However, comparative analysis for each of the metabolite measured in relation to time of day showed no significant difference (P > 0.05).
Table 2. Quantitative Screening of Sida acuta Oil Extracts in Relation to Time of Day
| Time of Day | |||||
| Metabolite | Morning | Evening | χ2 | df | P– value |
| Flavonoids (%) | 6.73 | 1.65 | 3.0795 | 1 | 0.07928 |
| Phenols (%) | 1.86 | 3.01 | 0.2716 | 1 | 0.6023 |
| Tannin (mg/L) | 105.16 | 120.28 | 1.0141 | 1 | 0.3139 |
| Alkaloid (%) | 8.53 | 7.38 | 0.0831 | 1 | 0.7731 |
| Saponins (%) | 0.16 | 0.07 | 0.0352 | 1 | 0.8511 |
| Terpenoids (%) | 25.59 | 21.16 | 0.4198 | 1 | 0.517 |
| Cyanide (mg/100g) | 2.51 | 1.72 | 0.1475 | 1 | 0.7009 |
| Oxalate (%) | 1.60 | 0.55 | 0.5128 | 1 | 0.4739 |
| Phytate (%) | 1.07 | 1.36 | 0.0346 | 1 | 0.8524 |
Susceptibility Profile of Aedes aegypti Larvae in Relation to Sidaacuta Oil Extract Treatments: No mortality was observed among Ae. aegypti exposed to the morning period treatments (Table 3). A 1% mortality rate was recorded in the evening period at 12.5 ppb at 24 hours exposure time and differences in mortality rate in relation to treatment and time of day, respectively was not significant (P > 0.05). Also, at 48 hours exposures period, a mortality rate of 1% was obtained from the evening period treatments for 12.5 ppb, 62.5 ppb and 125 ppb, respectively. Although at 72 hours exposure period, 1% mortality rate was recorded in the morning period treatments at 50 ppb concentration. A high mortality rate of 3% was obtained in the evening at 62.5 ppb treatment followed by 2% mortality rate each at 12.5 ppb and 125 ppb. However, differences in mortality rate across all treatments as well as time of day showed no significant difference (P > 0.05).
Table 3. Mortality Rate of Aedes aegypti Larvae in Relation to Oil Extract Treatments
| Exposure Period (Hours) | Time of Day | % Mortality in Relation to Treatment (ppb) | χ2 | df | P-value | ||||||
| 0 | 12.5 | 25 | 37.5 | 50 | 62.5 | 125 | |||||
| 24 | Morning | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Evening | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6.00 | 6 | 0.4232 | |
| χ2 | 1 | ||||||||||
| df | 1 | ||||||||||
| P-value | 0.3173 | ||||||||||
| 48 | Morning | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Evening | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 4.00 | 6 | 0.6767 | |
| χ2 | 1 | 1 | 1 | ||||||||
| df | 1 | 1 | 1 | ||||||||
| P-value | 0.3173 | 0.3173 | 0.3173 | ||||||||
| 72 | Morning | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 6.00 | 6 | 0.4232 |
| Evening | 0 | 2 | 0 | 0 | 0 | 3 | 2 | 10.00 | 6 | 0.1247 | |
| χ2 | 2 | 1 | 3 | 2 | |||||||
| df | 1 | 1 | 1 | 1 | |||||||
| P-value | 0.1573 | 0.3173 | 0.08326 | 0.1573 | |||||||
DISCUSSION
This study in overall, based on qualitative processing, found the presence of eight metabolites in Sida acuta oil extract at varying concentrations, in which steroids, phenol alkaloid, glycosides, tannin, and flavonoids were recorded in both morning and evening period (Table 1). Studies by Musa et al. (2020) have shown that S. acuta is one of the plants with the largest group of phyto-chemicals, with amazing effects in humans, leading to the development of a powerful pain killer medication. This work agrees with previous study by Muneeswari et al. (2019) who reported that Sida acuta oil extract had the presence of alkaloids, steroids, tannins, glycosides, volatile oils, phenols and flavonoids as active phytochemical constituents. Our finding slightly agrees with other studies that revealed that tannins, alkaloids, saponin, flavonoids, steroids, phenols, terpenoids, and hydrogen cyanide were present in Sida acuta crude extract (Senthilkumar et al., 2018; Usman & Abdulkarim, 2023; Edward et al., 2023).
This study disagrees with that of Idowu (2023), which revealed a high presence of saponin content and also the moderate presence of anthraquinoun. These components could be responsible for the wide usage of the plant preparations for the treatment of various ailments, including asthma, fever, migraine, cough, cold, ulcer, snake bites, urinary diseases, and female disorders, and exploration as antidiuretic, antifertility and sedative agent (Akilandeswari et al., 2010).
In this research, terpenoid was absent in the evening collection of Sida acuta which reflects that the metabolite is only present in the early hours of the day which agrees with the findings of Hassan et al. (2022). The absence of anthraquinone and saponin in this study is in agreement with the findings of Hassan et al. (2022) who reported that anthraquinone and saponin were absent in Sida acuta collected from Kawo, Kaduna North Local Government area of Kaduna State.
The quantitative phytochemical screening of Sida acuta is presented in Table 2. The result revealed high amounts of tannins, alkaloids and terpenoids with moderate level of phenol, flavonoids, steroids, phytate, oxalate, cyanide and saponin metabolites which corroborate with the study by Nwankwo et al. (2023). The rich composition of the plant leaf fraction in phenolic compounds, steroids, terpenoids, alkaloids, and saponins suggests a potent pharmacological profile. Studies by Farooq et al. (2022) have proposed a correlation between the phytochemical composition of plant materials and their pharmacological activities. Steroids are well known to modulate inflammatory responses via their interactions with specific intracellular receptors which translates into either trans-repression (negative) or trans-activation (positive) of inflammation-related genes (Timmermans et al., 2019).
Flavonoids elicit anti-inflammatory and analgesic potentials by reducing intracellular Ca2+, thereby repressing and preventing the activation of phospholipase A2, whose activity produces the precursor molecules for the synthesis of a variety of inflammatory mediators (Yang et al., 2020; Waghole et al., 2022; Yang et al., 2022). Tannins are potent cyclooxygenase (COX) inhibitors with a notable propensity to suppress edemogenesis induced by phlogistic agents (Attiqet al., 2018). The rich phenolic composition of the leaf fraction also implies excellentbioactivities, as phenolics are known to exhibit COX-1-sparing activities (Tungmunnithum et al., 2018).
According to this research, the oil extracts of S. acuta exhibited very low larvicidal activity against Ae. aegypti larvae, possibly due to very low start of dosage at part per billions (ppb). The low mortality rate of larvae in all exposure periods for Sida acuta treatment across concentrations for both morning and evening sessions is consistent with the findings of Ombugadu et al. (2020) and Pam et al.
(2021), who reported a substantial variation in the mortality rate of Anopheles species larvae across aqueous extract doses in Lafia Local Government Area, Nasarawa State, Nigeria. Various facets of insect physiology can be affected by essential oils: in addition to interfering with the respiratory enzymes of the mitochondrial membrane, the neurological system of the insect itself, it may have deleterious effects on growth, development, and reproduction (Feroz, 2020; Nwokocha, 2024).
Whereas, the low mortality rate of larvae in all exposure periods for Sida acuta treatment across concentrations for both morning and evening sessions disagrees with that of Saharayaj, (2022) who recorded a high mortality with start of low dosage of fern plant oil extracts, also ferns such as Pteridium aquilinum, Polypodium vulgare, Schizaea dichotoma, and others frequently produce ecdysteroids, which can have a deterring, antifeedant (repellent) effect, or cause toxicity, interference, or alteration of oviposition.
It displayed miticidal (Oligonychus coffeae) and insecticidal (Helicoverpa armigera, Spodopteralitura, and Helopeltistheivora) properties. When ecdysteroids are consumed in greater quantities, monophagous insects that feed on them experience developmental abnormalities but would sooner starve to death than oligophagous insects (Saharayaj, 2022).
Sida acuta leaf hydrosol and volatile oils both exhibited variable degrees of larvicidal activity at various doses and exposure times according to reports from other authors who have shown that S. acuta contains phyto-chemicals that are effective in controlling insect pests, corroborating its insecticidal activity. Additionally, its ethanol extracts performed in a competitive manner against the target insect when used in conjunction with the synthetic insecticide, cypermethrin (Gadewad & Paedeshi, 2018; Babatunde et al., 2021).
The various properties and uses of Sida acuta plant extract in traditional medicine can be attributed to its bioactive constituents, which include alkaloids like vasicine, ephedrine, and cryptolepine (the main alkaloid in the plant), saponosides, coumarins, steroids (ecdysterone, β-sistosterol, stigmaterol, ampesterol), tannins, phenolic compounds (evofolin-A and B, scopoletin, loliolid, and 4-ketopinoresinol), polyphenol, sesquiterpene, and flavonoid (Nalini et al., 2021).
A variety of phytochemicals were found in the plant extracts utilized in this investigation and numerous substances that bear resemblance to these phytochemicals have been detected in different plant extracts and have demonstrated efficacy against a range of insects that cause disease (Torawane et al., 2021). Against Anopheles stephensi, the volatile oil and hydrosol oil of S. acuta shown significant in vitro cytotoxicity, with LC50 values of 106.40 mg/L – 80.00% killing and 101.22 mg/L – 70% killing at 500.00 mg/L, respectively.
Sida acuta contains chemicals known as insect pheromones, namely tritetracontane and hemeicosane. The former is efficient against Aedes aegypti, the mosquito vector, and the latter is a major component of Coleus aromaticus essential oil, which shown considerable larvicidal activity against Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Amarasinghe et al., 2020; Njoku et al., 2021).
CONCLUSION
The phytochemicals present in the qualitative and quantitative measurements at caring concentrations were responsibile for the cause of Ae. aegypti death at the maximum rate of 3%.. This deaths could be explained by the ability of the n-hexane to solubilize a greater variety of bioactive compounds found in plant materials, as well as its relatively high polarity, this facilitates the dissolution of both polar and non-polar compounds, faster extraction rates, and greater stability of extracted compounds. Moreover, its lower boiling point makes evaporation easier and leads to concentrated extracts with higher potency, emphasising the importance of carefully selecting the right solvent for each extraction. Higher concentration of this plant should be explored.
Conflict of interest: The Authors have no conflict of interest to declare
Data Availability: All data are available from the corresponding author on a reasonable request.
Funding : Nil
REFERENCES
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. J. econ. Entomol, 18(2), 265-267.
Abhishek, K., Charan, C. S., Nagendra, R. K., Hanumanthachar, Joshi, Prajwal, R., & Varalakshmi, H. B. (2023). Review of Sidaacuta – A Potent Drug. European Journal of Pharmaceutical and Medical Research, 10(4), 126-131.
Abiodun, T. I. (2022). Use of Climate Smart Agricultural Practices Among Smallholder Arable Crop Farmers of Nasarawa State, Nigeria (Master’s thesis, Kwara State University, Nigeria).
Akilandeswari, S., Senthamarai, R., Prema, S., &Valarmathi, R. (2010). Antimicrobial activity of leaf extracts of Sida acuta Burm. Int. J. Pharm. Sci. Res, 1(5), 248-250.
Amarasinghe, L. D., Wickramarachchi, P. A. S. R., Aberathna, A. A. A. U., Sithara, W. S., & De Silva, C. R. (2020). Comparative study on larvicidal activity of green synthesized silver nanoparticles and Annonaglabra (Annonaceae) aqueous extract to control Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Heliyon, 6(6).
Aminah, N. S., Laili, E. R., Rafi, M., Rochman, A., Insanu, M., & Tun, K. N. W. (2021). Secondary metabolite compounds from Sida genus and their bioactivity. Heliyon, 7(4).
Amitha, T. V., & Joseph, S. (2019). Morphological, anatomical and phytochemical screening of Sida acuta Burm. f., Kannur district, Kerala. International Journal of Botany Studies, 4(3), 01-03.
Attiq, A., Jalil, J., Husain, K., & Ahmad, W. (2018). Raging the war against inflammation with natural products. Frontiers in pharmacology, 9, 976.
Babatunde, S. F., Musa, A. K., Nneji, E. T., & Gambari, L. I. (2021). Evaluation of Ethanol Leaf Extracts of Sidaacuta and Chromolaenaodorata on Insect Pests of Celosia argentea L. and Amaranthus cruentus L. FUDMA Journal of Sciences, 5(1), 333-338.
Balboné, M., Soma, D. D., Namountougou, M., Drabo, S. F., Konaté, H., Toe, O., Bayili, K., Meda, G. B., Dabiré R. K., & Gnankine, O. (2022). Essential oils from five local plants: an alternative larvicide for anopheles gambiaesl (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae) control in Western Burkina faso. Frontiers in Tropical Diseases, 3, 853405.
Bassey, M. E., Johnny, I. I., Umoh, O. T., & George, U. I. M. (2021). Comparative Phytochemical Analysis of the Leaves and Stem of Five Species of Sida L. Journal of Complementary and Alternative Medical Research, 14(3), 26-31.
Dubale, S., Kebebe, D., Zeynudin, A., Abdissa, N., & Suleman, S. (2023). Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. Journal of Experimental Pharmacology, 51-62.
Edward, K. C., Chijioke, D. U., & Friday, C. G. (2023). Phytochemical Screening and Antibacterial Activities of Ethanolic and Aqueous Leaf Extracts of Alchornea cordifolia and Sida acuta on Organisms Isolated from Meat. Nigerian Journal of Microbiology.
Farooq, S., Shaheen, G., Asif, H. M., Aslam, M. R., Zahid, R., Rajpoot, S. R., Jabbar, S. & Zafar, F. (2022). Preliminary phytochemical analysis: In-Vitro comparative evaluation of anti-arthritic and anti-inflammatory potential of some traditionally used medicinal plants. Dose-Response, 20(1), 15593258211069720.
Feroz, A. (2020). Efficacy and cytotoxic potential of deltamethrin, essential oils of Cymbopogon citratus and Cinnamonum camphora and their synergistic combinations against stored product pest, Trogoderma granarium (Everts). Journal of Stored Products Research, 87, 101614.
Gadewad, M. G., & Pardeshi, A. (2018). Bioinsecticidal effect of Sidaacuta plant extract against red cotton bug, Dysdercus cingulatus Fab. International Journal of Zoology Studies, 3(1), 177-181.
Hassan, M., Musa, F. M., Adamu, A., & Baba, G. (2022). Phytochemical analysis and antibacterial activity of fractions of Sida acuta against some reference isolates of bacteria. Science World Journal, 17(1), 26-30.
Horwitz, W., & Albert, R. (1996). Reliability of the determinations of polychlorinated contaminants (biphenyls, dioxins, furans). Journal of AOAC International, 79(3), 589-621.
Huong, L. T., Hung, N. H., Dai, D. N., Tai, T. A., Hien, V. T., Satyal, P., & Setzer, W. N. (2019). Chemical compositions and mosquito larvicidal activities of essential oils from Piper species growing wild in Central Vietnam. Molecules, 24(21), 3871.
Idowu, A. A. (2023). Reactions of Clarias gariepinus Juveniles to the Toxicity of Sida acuta Leaf Ethanol Extract.
Kaur Rajinder, R. K., Saroj Arora, S. A., & Thukral, A. K. (2015). Quantitative and qualitative analysis of saponins in different plant parts of Chlorophytum borivilianum.
Khaled, K. L., & Irani, R. (2021). Antioxidant Activity and Anti-Nutritional Factors in Acacia Nilotica Gum. Int. J. Life.
Krishnaiah, D., Devi, T., Bono, A., & Sarbatly, R. (2009). Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res, 3(2):67-72.
Krishnaveni, A., Ezhilarasan, B., Iyappan, A., & Sathali, A. A. H. (2018).Preliminary phytochemical screening and in vitro antioxidant activity of Sida acuta Burm. Int J Res Pharmacol Pharmacother, 7(2), 157-65.
Lwande, O. W., Obanda, V., Lindström, A., Ahlm, C., Evander, M., Näslund, J., & Bucht, G. (2020). Globe-trotting Aedes aegypti and Aedes albopictus: risk factors for arbovirus pandemics. Vector-Borne and Zoonotic Diseases, 20(2), 71-81.
Malik, S. K. (2017). Qualitative and quantitative estimation of terpenoid contents in some important plants of Punjab, Pakistan. Pakistan Journal of Science, 69(2).
Mgbechidinma, C. L., Zheng, G., Baguya, E. B., Zhou, H., Okon, S. U., & Zhang, C. (2023). Fatty acid composition and nutritional analysis of waste crude fish oil obtained by optimized milder extraction methods. Environmental Engineering Research, 28(2).
Michael, C. E., Chinwokwu, C. E., & Inyang, M. N. (2016). Resident’s vulnerability to criminal victimization in Lafia, Nasarawa State, Nigeria. Online Journal of Arts, Management and Social Science, 2(1), 127-139.
Mishra, D. P., Mishra, N., Musale, H. B., Samal, P., Mishra, S. P., & Swain, D. P. (2017). Determination of seasonal and developmental variation in oxalate content of Anagallis arvensis plant by titration and spectrophotometric method. The Pharma Innovation, 6(6, Part B), 105.
Muneeswari, P., Bhaskaran, S. K., & Poornima, K. (2019). Identification of Active Pharmaceuticals of Sida acuta Burm. F Leaves using GC-MS and HPTLC Fingerprinting. International Journal of Pharmaceutical Science and Research, 10(3), 1194-07.
Musa, F. M., Muhmmad-Idris, Z. K., Abdulfatai, K., Wartu, J. R., & Shuaibu, S. B. (2020). Antibacterial activities of ethanol leaf and bark extracts of Terminalia avicennioides against methicillin resistant Staphyloccocus aureus. Science World Journal, 15(3), 119-123.
Mwingira, V., Mboera, L. E., Dicke, M., &Takken, W. (2020). Exploiting the chemical ecology of mosquito oviposition behavior in mosquito surveillance and control: a review. Journal of Vector Ecology, 45(2), 155-179.
Nalini, T. J., Keshamma, E., Ramesh, H. N., Rajeshwari, N., & Sridhar, B. T. (2021). GC MS identification of bioactive components of leaf extract of Sida acuta (BURM. F). International Journal of Herbal Medicine; 9(4), 19-24.
National Population Commission. (2006). Population census of the Federal Republic of Nigeria. Census Report. National Population Commission, Abuja.
Ngwamah, J. S., & Naphtali, R. S. (2019). Phytochemical Screening and Larvicidal Activities of Some Ethnobotanicals from North Eastern Nigeria against Culicine (Dipera: Culicidae) Mosquito.
Ninditya, V. I., Purwati, E., Utami, A. T., Marwaningtyaz, A. S., Fairuz, N. K., Widayanti, R., & Hamid, P. H. (2020). Artemisia vulgaris efficacies against various stages of Aedes aegypti. Veterinary World, 13(7), 1423.
Njoku, I. S., Ichide, M. U., Rahman, N. U., Khan, M. A., Chibuko, N. A., Asekun, O. T., & Familoni, O. B. (2021). Extraction, Characterization and Larvicidal Activity of Essential Oil and Hydrosol from Sida acuta Burm. f. Leaves Grown in Nigeria: doi. org/10.26538/tjnpr/v5i1. 29. Tropical Journal of Natural Product Research (TJNPR), 5(1), 211-216.
Torawane, S., Andhale, R., Pandit, R., Mokat, D., & Phuge, S. (2021). Screening of some weed extracts for ovicidal and larvicidal activities against dengue vector Aedes aegypti. The Journal of Basic and Applied Zoology, 82, 1-9.
Nortjie, E., Basitere, M., Moyo, D., &Nyamukamba, P. (2022). Extraction methods, quantitative and qualitative phytochemical screening of medicinal plants for antimicrobial textiles: a review. Plants, 11(15), 2011.
Nwankwo, N. E., Ezeako, E. C., Nworah, F. N., Ogara, A. L., Oka, S. A., Aham, E. C., Joshua, P. E., Nwiloh, B. I., Ezike, T. C., Ashiakpa, N. P., Ngozi, H. C. & Batiha, G. E. S. (2023). Bioactive compounds, anti-inflammatory, anti-nociceptive and antioxidant potentials of ethanolic leaf fraction of Sida linifolia L. (Malvaceae). Arabian Journal of Chemistry, 16(1), 104398.
Nwokocha, C. E. (2024). Larvicidal activity of three plants extracts against Aedes aegypti mosquito. Master’s Dissertation Submitted to the College of Postgraduate Studies, Federal University of Lafia, Nasarawa State. 143.
Ombugadu, A., Micah, E. M., Adejoh, V. A., Odey, S. A., Pam, V. A., Ahmed, H. O., Aimankhu, O. S., Uzoigwe, N. R., Samuel, M. D., Dogo, K. S., &Mafuyai, M. J. (2020). Capsicum chinensis (Hot Pepper) Powder Larvicidal Activity against Mosquitoes Larvae in Lafia Local Government Area, Nasarawa State, Nigeria. Biomedical Journal of Scientific & Technical Research, 31(5), 24507-24511.
Ombugadu, A., Muhammed, S. U., Maikenti, J. I., Ali, A. A., Ashigar, M. A., Ahmed, H. O., Igboanugo, S., Yina, G. I. & Pam, V. A. (2024). Mosquito Species Composition in Nasarawa Local Government Area, Nasarawa State, Nigeria. UMYU Scientifica, 3(4), 46-56.
Osuagwu, S. O., Ichor, T. S., Oluma, H. O. A., & Adibe, O. (2021). Antibacterial Activity and Phytochemical Properties of Selected Medicinal Plants against Salmonella typhi, Salmonella paratyphi A, B & C, Clinical Isolates. American Academic Scientific Research Journal for Engineering, Technology and Sciences, 81(1), 2313-4410.
Pam, D. D., Kopdorah, S. W., Lapang, P. M., Jwanse, I. R., Joseph, S. T., Agwom, F., Istifanus, G., Ombugadu, A., Amajoh, C.N. & Dakum, Y. D. (2021). Larvicidal efficacy and gc-ms analysis of Hyptis suaveolens leaf extracts against anopheles species. International Journal of Biochemistry Research & Review, 30(1), 8-19.
Rodrigues, A. M., Martins, V. E. P., & Morais, S. M. (2020). Larvicidal efficacy of plant extracts and isolated compounds from Annonaceae and Piperaceae against Aedes aegypti and Aedes albopictus. Asian Pacific Journal of Tropical Medicine, 13(9), 384-396.
Sahayaraj, K. (2022). Ferns, a source of phytoecdysones, and their applications in pestiferous insect management. In Ferns: Biotechnology, Propagation, Medicinal Uses and Environmental Regulation (pp. 181-198). Singapore: Springer Nature Singapore.
Santhi, K., & Sengottuvel, R. (2016). Qualitative and quantitative phytochemical analysis of Moringa concanensis Nimmo. International Journal of Current Microbiology and Applied Sciences, 5(1), 633-640.
Scalvenzi, L., Radice, M., Toma, L., Severini, F., Boccolini, D., Bella, A., Guerrini, A., Tacchini, M., Sacchetti, G., Chiurato, M., Romi, R., & Di Luca, M. (2019). Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite, 26.
Selvakumaran, J., Ragavendran, K., Muthukanagavel, M., Ignacimuthu, S., Vasanth, N., Krishnamoorthy, R., Ahmed, M.Z., Alqahtani, A.S., Stalin, A., Ganesan, P., & Mutheeswaran, S. (2024). Evaluation of mosquitocidal, histopathological and non-target effect of botanical pesticides from Stemodia viscosa and their mixtures against immature stages of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. Biologia, 79(5), 1425-1437.
Senthilkumar, R. P., Bhuvaneshwari, V., Malayaman, V., Ranjithkumar, R., & Sathiyavimal, S. (2018). Phytochemical screening of aqueous leaf extract of Sida acuta burm. F. And its antibacterial activity. Journal of Emerging Technologies and Innovative Research, 5(8), 474-478.
Shittu, M. D., & Alagbe, J. O. (2020). Phyto-nutritional profiles of broom weed (Sida acuta) leaf extract. Annals of Clinical Toxicology, 3(2).
Silverio, M. R., Espindola, L. S., Lopes, N. P., & Vieira, P. C. (2020). Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules, 25(3484),1-9. doi:10.3390/molecules25153484.
Sofowora A. (1993). Medicinal Plants and Traditional Medicinal in Africa. (2nd Ed.) Sunshine House, Ibadan, Nigeria: Spectrum Books Ltd; Screening Plants for Bioactive Agents; pp. 134-156.
Souheila, N., Lamia, E., Rania, E., & Rebai, B. (2020). Evidence based efficacy of three medicinal plant extracts against Culex quinquefasciatus (Say) larvae.
Tcheghebe, O. T., Seukep, A. J., & Tatong, F. N. (2017). Ethnomedicinal uses, phytochemical and pharmacological profiles, and toxicity of Sida acuta Burm. F.: a review article. The Pharma Innovation, 6(6, Part A), 1.
Timmermans, S., Souffriau, J., & Libert, C. (2019). A general introduction to glucocorticoid biology. Frontiers in immunology, 10, 1545.
Trease, G. E., & Evans, W. C. (2002). Pharmacognosy. 15th Ed. London: Saunders Publishers; pp. 42–44. 221–229, 246–249, 304–306, 331–332, 391–393 Tropical Plants Database, Ken Fern. Tropical.theferns.info. 2023-10-16 <tropical.theferns.info/viewtropical.php?id=Hyptis%20spicigera>
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., & Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines, 5(3), 93.
Ullah, Z., Ijaz, A., Mughal, T. K., & Zia, K. (2018). Larvicidal activity of medicinal plant extracts against Culex quinquefasciatus Say. (Culicidae, Diptera). Int. J. Mosq. Res, 5(2), 47-51.
Usman, H., & Abdulkarim, I. (2023). Phytochemical and Antihelmintic Studies of the Leaves Extract of Sida acuta Burm. F against Taenia saginata. FUDMA Journal of Sciences, 7(2), 223-226.
Waghole, R. J., Misar, A. V., Kulkarni, N. S., Khan, F., Naik, D. G., & Jadhav, S. H. (2022). In vitro and in vivo anti-inflammatory activity of Tetrastigma sulcatum leaf extract, pure compound and its derivatives. Inflammopharmacology, 30(1), 291-311.
World Health Organization (WHO). (2018). World malaria report 2018 (World Health Organization). Geneva, Switzerland.
World Health Organization. (2020). Ethics and vector-borne diseases: WHO guidance.
Yahaya, I. A., Nok, A. J., & Bonire, J. J. (2013). Chemical studies of the peel of Xanthosoma sagittifolium (Tannia Cocoyam).Pakistan Journal of Nutrition, 12(1), 40–44.
Yang, N. N., Zhang, Y. F., Zhang, H. T., Ma, X. H., Shen, J. H., Li, P., Zhong, T. H. & Zhang, Y. H. (2020). The in vitro and in vivo anti-inflammatory activities of triterpene saponins from Clematis florida. Natural Product Research, 35(24), 6180-6183.
Yang, T., Hu, Y., Yan, Y., Zhou, W., Chen, G., Zeng, X., & Cao, Y. (2022). Characterization and evaluation of antioxidant and anti-inflammatory activities of flavonoids from the fruits of Lycium barbarum. Foods, 11(3), 306.


