1Department of Agriculture, Uttaranchal College of Science and Technology, Dehradun, Uttarakhand, India
2School of Environment and Sustainable Development, Central University of Gujarat, Gandhinagar, Gujarat, India
3School of Chemical Sciences, Central University of Gujarat, Gandhinagar, Gujarat, India
4Center of Research for Development, Parul University, Vadodara, Gujarat, India
Corresponding author email: smartramanravi@gmail.com
Article Publishing History
Received: 13/10/2020
Accepted After Revision: 08/12/2020
Pesticides are chemical substances applied to mitigate the agricultural and domestic pests, but indiscriminate and unsafe use leads to their accumulation in the environment. Bioaccumulation of pesticides is of great public health concern due to their toxicity. The enhanced production and formulation of pesticides has posed serious problem through contaminating the nearby surroundings, which ultimately affect the biological diversity. Therefore, the present study is focussed on physico-chemical and microbial characterization of pesticides contaminated industrial soil nearby pesticides industry. For this, the soil sample was collected from nearby pesticides industries from GIDC Naroda, Ahmedabad district of Gujarat state. The soil sample was analysed for various physico-chemical characteristics such as temperature, pH, electrical conductivity, moisture contents, water holding capacity, bulk density, hardness, chloride, alkalinity, sulphate, available phosphorus, total phosphorus, nitrate, nitrite, ammonium, total organic carbon and total organic matter.
For microbial characterization, isolation of bacteria was carried out using serial dilution pour plate technique and characterized morphologically, biochemically. The molecular characterization of the bacteria isolates was done by 16S rRNA sequencing. For this genomic DNA was extracted using CTAB method and amplified. The amplified DNA was sequenced for identification of bacteria. The isolated bacteria were identified as Bacillus licheniformis, Bacillus subtilis, Bacillus sp., Bacillus thuringiensis, Bacillus pumilus, Bacillus amyloliquefaciens, Bacillus velezensis, Kocuria flava, Pseudomonas sp. and Bacillus cereus. The obtain results infers that pesticides contaminated soil contains diversified bacterial species. As these bacterial species are growing in the pesticides contaminated soil and can be resistant to the toxicity of the pesticides, therefore, they may be the potential candidate for the removal of that compounds for environmental clean-up.
Pesticides, Industrial Soil, Microbial Characterisation, 16S rRNA Sequencing
Ravi R. K, Fulekar M. H, Hiranmai R. Y, Singh M. Physico-Chemical and Microbial Characterization of Soil Collected from Pesticides Infused Industrial Area, Gujarat Industrial Development Corporation (GIDC) Naroda, Ahmedabad, Gujarat. Biosc.Biotech.Res.Comm. 2020;13(4).
Ravi R. K, Fulekar M. H, Hiranmai R. Y, Singh M. Physico-Chemical and Microbial Characterization of Soil Collected from Pesticides Infused Industrial Area, Gujarat Industrial Development Corporation (GIDC) Naroda, Ahmedabad, Gujarat. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: <a href=”https://bit.ly/2JOOc4H”>https://bit.ly/2JOOc4H</a>
Copyright © Ravi et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Pesticides are natural or synthetic compounds that are poisonous and can kill pests including insects, nematodes and rodents etc. Over the past few decades, pesticides and other agrochemicals have become a vital component of modern agricultural system, leading to a substantial improvement in crop productivity by controlling insects and other diseases (Carvalho 2017; Ali et al. 2019). The state like Gujarat, agriculture is one of the most important sector as it is the primary sources of livelihood for more than half (~ 60%) of its workforce (Planning Commission 2004; UNDP 2004). Currently, huge amount of pesticides are being used in agricultural field for protection of crops from the pests. Due to increasing demand of pesticides, a lots of manufacturing and formulation industries has been established in Gujarat to fulfil the requirements (Morillo and Villaverde 2017; Varjani et al. 2018).
On the other hand, enhanced Industrial and agricultural activities in recent years, in India especially in Gujarat has led to considerable contamination of air, soil and groundwater resources due to release of large amounts of pesticides and other chemicals in the environment (Morillo and Villaverde 2017; Varjani et al. 2018). Indiscriminate use of pesticides for pest infestation and vector control has drawn special attention of scientific community globally due to the multifaceted toxicity, mobility, persistence and recalcitrant properties (Fantke and Jolliet 2016; Liu et al. 2016; Varjani et al. 2018).
The rapid increase in pesticides industries also has polluted the nearby environment severely. Nevertheless, these pesticides possesses several negative impacts ranging from ecological pollution to damage of biodiversity (Pico et al. 2018; Barbieri et al. 2019; Köck-Schulmeyer et al. 2019). This can influences the physico-chemical properties and microbial diversity of the soil and possibly can cause a threat to both the environment and human health (Samant et al. 2018). Although, some studies show physico-chemical and microbial characterization of pesticides contaminated industrial soil from different industrial area of Gujarat (Doolotkeldieva et al. 2018; Ravi et al. 2019), but pesticides infused industrial soil of GIDC Naroda, Ahmedabad, Gujarat is yet to be studied. Therefore, the present study was carried out to characterize the pesticide infused soil from Gujarat for their physico-chemical analysis and microbial diversity.
MATERIAL AND METHODS
The soil sample was collected randomly from five sites from nearby pesticides industries from Gujarat Industrial Development Corporation (GIDC), Naroda, Ahmedabad district (23°06’02″N – 72°42’14″E) of Central Gujarat, in sterilized polyethene zipper bag using auger up to a depth of 1- 15 cm and stored at 4 °C for further analysis (Jayashree and Vasudevan 2006). The important physico-chemical properties of the soil, viz. temperature, pH, electrical conductivity, moisture contents, water holding capacity, bulk density, hardness, chloride, alkalinity, total organic carbon, total organic matter, sulphate, nitrate, nitrite, ammonium, available phosphorus and total phosphorus were analysed using standard methods (APHA1998). All the physico-chemical properties of the soil were analyzed in triplicates and their mean and standard deviation (SD) was calculated. The obtained values were described as Mean ± SD.
The Isolation of indigenous bacteria from pesticide contaminated soil was performed by serial dilution pour plate technique using nutrient agar medium. Well grown bacterial colonies were picked and further purified by streaking. The colonies were characterized morphologically, biochemically and identified by 16S rRNA techniques. The colonies were counted and the average number of colonies per three plates was determined for CFU (colony forming unit) count. Morphological Characterization of isolated bacterial species: The isolated pure bacterial species were grown on nutrient agar medium and examined morphologically for their shape, size, margin, constancy, elevation, opacity, and pigmentation. The gram staining test was carried out by Gram’s Method using a Gram staining kit and observed under a microscope for colour and shape. Biochemical Characterization of isolated bacterial species: The isolated bacterial species were analyzed for various biochemical properties such as motility, starch hydrolysis, catalase, oxidase, urease, indole production test, nitrate reduction test, citrate utilization test, xylose, maltose, fructose, dextrose, trehalose, inositol, sucrose, L-arabinose, glycerol, melezitose, lactose, esculin hydrolysis, amylase, gelatinase, methyl red (MR) test, vogus-proskauer (VP) test, etc. using KB001 Biochemical Test Kit (HI Media, India).
Identification of bacterial species using 16S rRNA sequencing techniques: The identification of bacterial species was carried out by 16S rRNA gene sequencing. For this genomic DNA was extracted done using the CTAB method. 1 ml of well grown broth culture was centrifuged at 8000 rpm for 10 min. The pellet was resuspended in 200 µl of DW and 200 µl of buffer saturated phenol was added and incubated at 60ºC for 1 hour and centrifuged at 8000 rpm for 5 min. Again 400 µl of chilled ethanol was added to the aqueous stage of DNA precipitation. The precipitated DNA pellet was washed with 70% ethanol and resuspended in nuclease-free water (Das et al. 2019). The extracted DNA was used for amplification of the 16S rRNA gene sequence with primer A109 (F) AC (G/T) GCTCAGTAACACGT and 1510 (R) GGTTACCTTGTTACGACTT (Birbir et al. 2007, Mani et al. 2012). The PCR reaction combination contained 10X Taq buffer, 2 mM MgCl2, 10 mM of dNTPs, 10 µM of each primer, 1 µl of DNA, 2U Taq Polymerase. The reaction was initiated by denaturation at 94ºC for 5 min followed by 35 cycles of denaturation, annealing, and elongation. The reaction was terminated after a final elongation. The amplified product was exposed to electrophoresis on a 1.5 % agarose gel. The refined product was sequenced bidirectionally using an automated DNA sequences.
Construction of the molecular phylogenetic tree: The phylogenetic tree was constructed using 16S rRNA sequences of isolated bacteria in FASTA format. The sequences closely related to the bacteria and fungus of the present study were recovered from the NCBI and aligned using ClustalW. The phylogenetic tree was prepared using the MEGA software version 7.0 and the Maximum Likelihood method (Tamura and Nei 1993, Kumar et al. 2016). All the sequences were deposited to the National Center for Biotechnology Information (NCBI).
RESULTS AND DISCUSSION
Physico-chemical characteristics of soil collected from GIDC Naroda, Ahmedabad, Gujarat: The physico – chemical properties such as temperature, pH, electrical conductivity, moisture contents, water holding capacity, bulk density, chloride, alkalinity, hardness, sulphate, available phosphorus, total phosphorus, all types of nitrogen (nitrate, nitrite, ammonium), total organic carbon and total organic matter of the soil samples collected from GIDC Naroda of Ahmedabad district, Gujarat was carried out as shown in Table 1, which infers temperature (°C), pH, electrical conductivity (µs cm -1) value of 26.12 ± 0.13, 8.24 ± 0.13, 121 ± 27.65 respectively. The moisture content (%) and water holding capacity (%) was observed 15.85 ± 1.13 and 37.45 ± 0.82 respectively. The present results are supported by earlier studies in the case of rice field contaminated with pesticides (Raman Kumar Ravi et al. 2015) and pesticides contaminated industrial soil (Ravi et al. 2019).
The total organic carbon and total organic matter are the significant property of soil and play important role in its fertility (Yennawar et al. 2013; King et al. 2020). The result obtained of total organic carbon (%) is 0.9 ± 0.17, whereas total organic matter (%) is 1.67 ± 0.03. The recorded results for bulk density (g/ml), chloride (mg/kg), alkalinity (mg/kg), hardness (mg CaCO3/kg), sulphate (mg/kg), available phosphorus (mg/kg) and total phosphorus (mg/kg) was 1.73 ± 0.02, 236.5 ± 36.04, 203 ± 15.65, 60 ± 5.10, 3.91 ± 1.22, 0.87 ± 0.18 and 8.30 ± 0.60 respectively. The different nitrogen contents are 10.53 ± 0.71 for nitrate, 0.09 ± 0.01 for nitrite and 1.94 ± 0.38 for ammonium nitrogen. The result obtained is supported by earlier research performed by Baishya and Sarma 2014, where slight greater value of nitrate was found, and slight difference in the nitrite and ammonical nitrogen value as reported earlier by Jia and Conrad 2009. These findings are also supported by previous studies for pesticides contaminated industrial soil (Ravi et al. 2019; Meena et al. 2020).
Table 1. Physico-chemical Analysis of soil samples collected from GIDC Naroda, Ahmedabad
| Parameters | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Average ± SD |
| Temperature (°C) | 26.1 | 26 | 26.5 | 26.2 | 26.3 | 26.12 ± 0.13 |
| pH | 8.28 | 8 | 8.31 | 8.3 | 8.3 | 8.24 ± 0.13 |
| Electrical Conductivity (µs cm -1) | 99 | 104 | 101 | 159 | 142 | 121 ± 27.65 |
| Moisture contents (%) | 14.75 | 14.76 | 16.15 | 17.44 | 16.13 | 15.85 ± 1.13 |
| Water Holding Capacity (%) | 37.07 | 37.46 | 36.86 | 36.86 | 37.02 | 37.45 ± 0.82 |
| Bulk Density (g/ml) | 1.75 | 1.75 | 1.74 | 1.71 | 1.70 | 1.73 ± 0.02 |
| Chloride (mg/kg) | 283 | 216.5 | 200 | 266.5 | 216.5 | 236.5 ± 36.04 |
| Alkalinity (mg/kg) | 200 | 190 | 230 | 195 | 200 | 203 ± 15.65 |
| Hardness(mg CaCO3/kg) | 64 | 60 | 66 | 56 | 54 | 60 ± 5.10 |
| Sulphate (mg/kg) | 4.43 | 5.39 | 4.21 | 2.15 | 3.38 | 3.91 ± 1.22 |
| Available Phosphate (mg/kg) | 0.84 | 0.58 | 0.92 | 1.06 | 0.93 | 0.87 ± 0.18 |
| Total Phosphorus (mg/kg) | 9.08 | 8.61 | 7.73 | 7.66 | 8.40 | 8.30 ± 0.60 |
| Nitrate (mg/kg) | 11.21 | 9.55 | 10.05 | 11.11 | 10.73 | 10.53 ± 0.71 |
| Nitrite (mg/kg) | 0.11 | 0.09 | 0.08 | 0.09 | 0.10 | 0.09 ± 0.01 |
| Ammonium (mg/kg) | 2.09 | 1.30 | 2.23 | 2.17 | 1.91 | 1.94 ± 0.38 |
| Total Organic Carbon (%) | 1.2 | 1.05 | 1.2 | 1.35 | 0.9 | 0.9 ± 0.17 |
| Total Organic Matter (%) | 1.64 | 1.70 | 1.65 | 1.69 | 1.68 | 1.67 ± 0.03 |
Note: SD – Standard Deviation
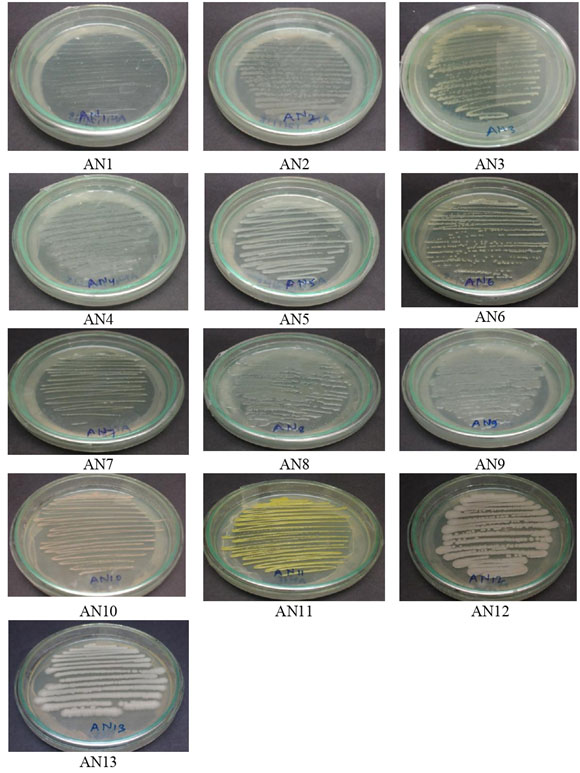
Microbiological Characterization of soil collected from GIDC Naroda, Ahmedabad (Central Gujarat): The bacterial species were isolated from contaminated soil using serial dilution pour plate technique on nutrient agar medium. A total of 13 bacterial species were isolated and pure cultured from collected soil sample of GIDC Naroda, Ahmedabad, Central Gujarat (Fig. 1).
Figure 1: Bacteria isolated from GIDC Naroda soil, Ahmedabad
Morphological characterization of isolated bacterial species: The isolated pure culture of bacteria was characterized by several morphological properties like shape, size, margin, elevation, pigmentation, optical character, surface, and consistency by growing them on nutrient agar plates (Table 2).
Table 2. Morphological characterization of bacteria isolated from GIDC Naroda soil, Ahmedabad
| Isolates | Shape | Size | Margin | Elevation | Pigment | Optical character | Surface | Consistency |
| AN1 | Irregular | Large | Undulate | Flat | Whitish | Opaque | Smooth | Moist |
| AN2 | Elliptical | Intermediate | Undulate | Flat | Whitish | Opaque | Smooth | Moist |
| AN3 | Round | Large | Undulate | Convex | Whitish | Opaque | Smooth | Moist |
| AN4 | Round | Intermediate | Entire | Convex | Creamy | Sebaceous | Smooth | moist |
| AN5 | Elliptical | Intermediate | Curled | Unbonate | Creamy | opaque | Smooth | moist |
| AN6 | Round | Small | Entire | Convex | White | sebaceous | Smooth | moist |
| AN7 | Round | Small | Entire | Convex | Yellowish | sebaceous | Smooth | moist |
| AN8 | Round | Intermediate | Entire | Convex | Yellow | opaque | Smooth | moist |
| AN9 | Round | Small | Entire | Convex | Orange | opaque | Smooth | moist |
| AN10 | Round | Intermediate | Entire | Convex | Creamy | Sebaceous | Smooth | moist |
| AN11 | Round | Small | Entire | Convex | Yellowish | sebaceous | Smooth | moist |
| AN12 | Round | Intermediate | Entire | Convex | Pinkish | opaque | Smooth | moist |
| AN13 | Round | Small | Entire | Convex | Orange | opaque | Smooth | moist |
Biochemical characterization of bacterial species: Several biochemical analyses were carried out to describe the isolated bacterial species. All the bacterial species were found gram-positive except isolate AN12 (Pseudomonas sp. strain RKRAN12) (Table 3). All the bacterial species were rod-shaped except isolate AN11 (Kocuria flava strain RKRAN11). All the bacterial species were negative for urease, starch hydrolysis, lactose, xylose, galactose, raffinose, sodium gluconate, inositol, dulcitol, arabitol, erythritol, α-methyl -D glucoside, rhamnose, α-methyl- D mannoside, xylitol, esculin hydrolysis, malonate utilization and sorbose test, while positive for catalase and dextrose test. The isolate AN2 (Bacillus subtilis strain CAKDS3), isolate AN4 (Bacillus sp. strain AN4), isolate AN5 (Bacillus thuringiensis strain RKRAN5), isolates AN9 (Bacillus velezensis strain RKRAN9), isolate AN11 (Kocuria flava strain RKRAN11) and isolate AN12 (Pseudomonas sp. strain RKRAN12) were able to reduce nitrates.
The isolate AN2 (Bacillus subtilis strain CAKDS3), isolate AN3 (Bacillus sp strain Y174007), isolate AN4 (Bacillus sp. strain AN4), isolate AN7 (Bacillus pumilus strain RKRAN7), isolate AN9 (Bacillus velezensis strain RKRAN9), isolate AN10 (Bacillus sp. strain RKRAN10) and isolate AN11 (Kocuria flava strain RKRAN11) were found positive for oxidase test. The isolate AN3 (Bacillus sp strain Y174007) and isolate AN10 (Bacillus sp. strain RKRAN10) were able to utilize citrate and adinitol, therefore positive for that test. The isolate AN1 (Bacillus licheniformis strain A28), isolate AN6 (Bacillus subtilis strain RKRAN6), isolate AN8 (Bacillus amyloliquefaciens strain RKRAN8) and isolate AN13 (Bacillus cereus strain RKRAN13) were positive for maltose, methyl red, sucrose, trehalose and melibiose test.
Table 3. Biochemical characterization of isolated bacteria from GIDC Naroda soil, Ahmedabad
| Tests | AN1 | AN2 | AN3 | AN4 | AN5 | AN6 | AN7 | AN8 | AN9 | AN10 | AN11 | AN12 | AN13 |
| Gram stain | +ve | +ve | +ve | +ve | +ve | +ve | +ve | +ve | +ve | +ve | +ve | -ve | +ve |
| Shape | Rods | Rods | Rods | Rods | Rods | Rods | Rods | Rods | Rods | Rods | Cocci | Rods | Rods |
| CFU counts
( N x 105 cfu/g) |
1.90 |
1.41 |
2.15 |
1.65 |
2.42 |
1.12 |
1.12 |
2.01 |
1.48 |
2.30 |
1.69 |
2.72 |
1.56 |
| Motility test | – | – | + | + | – | + | – | – | – | + | + | – | + |
| Catalase test | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Oxidase test | – | + | + | + | – | – | + | – | + | + | + | – | – |
| Urease test | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Indoletest | – | – | + | – | – | + | – | – | – | + | – | – | + |
| Starch hydrolysis | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Nitrate reduction | – | + | – | + | + | – | – | – | + | – | + | + | – |
| Methyl Red test | + | + | + | + | + | + | – | + | + | + | + | + | + |
| V-P test | – | + | + | + | – | + | + | – | + | + | + | – | + |
| O-F test | O-/F- | O+/F- | O-/F- | O-/F- | O+/F+ | O-/F- | O-/F- | O-/F- | O+/F- | O-/F- | O-/F- | O+/F+ | O-/F- |
| Triple Sugar Iron | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A | K/A |
| Glucose | + | + | + | + | + | + | V | + | + | + | + | + | + |
| Sorbinol | V | – | – | – | – | – | – | V | – | – | – | – | – |
| Lactose | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Xylose | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Maltose | + | – | + | + | + | + | + | + | – | + | + | + | + |
| Fructose | + | + | – | + | + | – | – | + | + | – | + | + | – |
| Dextrose | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Galactose | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Raffinose | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Trehalose | + | – | + | + | + | + | + | + | – | + | + | + | + |
| Melibiose | + | – | – | – | – | + | – | + | – | – | – | – | + |
| Sucrose | + | + | – | + | + | + | – | + | + | – | + | + | + |
| L-Arabinose | – | – | – | – | + | – | – | – | – | – | – | + | – |
| Mannose | – | + | – | + | + | + | – | – | + | – | + | + | + |
| Inulin | – | – | – | – | + | – | – | – | – | – | – | + | – |
| Sodium gluconate | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Glycerol | – | + | + | – | – | + | – | – | + | + | – | – | + |
| Salicin | – | – | + | – | + | + | – | – | – | + | – | + | + |
| Dulcitol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Inositol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sorbitol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Mannitol | – | + | – | – | + | – | + | – | + | – | – | + | – |
| Adonitol | – | – | + | – | – | – | – | – | – | + | – | – | – |
| Arabitol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Erythritol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| α-methyl D glucoside | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Rhamnose | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Cellobiose | – | – | + | – | + | + | – | – | – | + | – | + | + |
| Melezitose | + | – | – | – | + | – | – | + | – | – | – | + | – |
| α-methyl- D-mannoside | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Xylitol | – | – | – | – | – | – | – | – | – | – | – | – | – |
| ONPG | + | – | – | – | – | – | – | + | – | – | – | – | – |
| Esculin hydrolysis | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D- Arabinose | – | – | – | – | + | – | – | – | – | – | – | + | – |
| Citrate utilization | – | – | + | – | – | – | – | – | – | + | – | – | – |
| Malonate utilization | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Sorbose | – | – | – | – | – | – | – | – | – | – | – | – | – |
Note: (+ = Positive, – = Negative, V= 11-89% positive, O+/F–= only oxidative; O+/F+ = Oxidative and fermentative; O–/F– = glucose not metabolised; A/A= Glucose, lactose & sucrose fermentation; K/A = Glucose fermentation; K/ K = Non fermentative)
Identification of bacterial species by 16S rRNA gene sequencing technique: Identification of bacterial species was done by 16S rRNA gene sequencing technique. The pesticides contaminated soil show diversity of bacterial species such as Bacillus licheniformis strain A28, Bacillus subtilis strain CAKDS3, Bacillus sp strain Y174007, Bacillus sp. strain AN4, Bacillus thuringiensis strain RKRAN5, Bacillus subtilis strain RKRAN6, Bacillus pumilus strain RKRAN7, Bacillus amyloliquefaciens strain RKRAN8, Bacillus velezensis strain RKRAN9, Bacillus sp. strain RKRAN10, Kocuria flava strain RKRAN11, Pseudomonas sp. strain RKRAN12 and Bacillus cereus strain RKRAN13 (Fig.1). All the sequences of bacterial species (AN1 to AN13) were submitted in NCBI under the accession number MH806397, MH806398, MH806399, MK182800, MK215844, MK215849, MK215850, MK215851, MK229344, MK229345, MK229346, MK229347 and MK229348 respectively (Table 4).
Table 4. Identification of isolated bacteria from soil
| Isolates | Identification | Accession Number |
| AN1 | Bacillus licheniformis strain A28 | MH806397 |
| AN2 | Bacillus subtilis strain CAKDS3 | MH806398 |
| AN3 | Bacillus sp strain Y174007 | MH806399 |
| AN4 | Bacillus sp. strain AN4 | MK182800 |
| AN5 | Bacillus thuringiensis strain RKRAN5 | MK215844 |
| AN6 | Bacillus subtilis strain RKRAN6 | MK215849 |
| AN7 | Bacillus pumilus strain RKRAN7 | MK215850 |
| AN8 | Bacillus amyloliquefaciens strain RKRAN8 | MK215851 |
| AN9 | Bacillus velezensis strain RKRAN9 | MK229344 |
| AN10 | Bacillus sp. strain RKRAN10 | MK229345 |
| AN11 | Kocuria flava strain RKRAN11 | MK229346 |
| AN12 | Pseudomonas sp. strain RKRAN12 | MK229347 |
| AN13 | Bacillus cereus strain RKRAN13 | MK229348 |
Molecular Phylogenetic tree: The 16S rRNA sequences of bacterial species were studied to define the relationship between development and nomenclature using a phylogenetic tree. The partial sequences of the 16S rRNA gene from the isolated bacterial species (AN1 to AN13) were associated to find the closest match using the Basic Alignment Search Tool (BLAST). The BLAST analysis revealed that the genes showed 100% similarity to Bacillus licheniformis (MH806397:AN1), 100 % homology to Bacillus subtilis (MH806398:AN2), 100 % similarity to Bacillus sp (MH806399:AN3), 100 % homology to Bacillus sp. (MK182800:AN4), 100 % similarity to Bacillus thuringiensis (MK215844:AN5), 100 % homology to Bacillus subtilis (MK215849: AN6), 100 % similarity to Bacillus pumilus (MK215850:AN7), 100 % homology to Bacillus amyloliquefaciens (MK215851:AN8), 100 % similarity to Bacillus velezensis (MK229344:AN9), 100 % homology to Bacillus sp. (MK229345:AN10), 100 % similarity to Kocuria flava (MK229346:AN11), 100 % homology to Pseudomonas sp. (MK229347:AN12) and 99.88 % similarity to Bacillus cereus (MK229348: AN13). The construction of a molecular phylogenetic tree was done by aligning the sequences of bacterial species.
Figure 2: Phylogenetic tree based on 16S rRNA gene sequences showing the relationship among 13 strains and some of their closest phylogenetic relatives
The present study show that slight variation in the physico-chemical properties of soil around the pesticides industry are occurred, which provide better environmental condition for the bacterial diversity to grow. The study show that the soil around the pesticides industry of GIDC, Naroda, Ahmedabad district contains diversity of bacterial species. The soil is mainly dominated by different species of genus Bacillus. This finding was also supported was earlier studies for the soil contaminated with pesticides (Doolotkeldieva et al. 2018; Ravi et al. 2019).
CONCLUSION
The pesticides industries are adding different types of pesticides to environment which bring changes in natural properties of the soil. Pesticides are observed to influence the physico-chemical and biological properties of soil. The present study has indicated slight variations in observed parameters. The soil samples from five different sight exhibited similar pattern in selected parameters which indicates the changes brought about by deposition from industries. The microbial analysis show diversified bacterial species, mainly dominated by different species of Bacillus. As these bacterial species are growing in the pesticides contaminated soil, therefore, this study will help to determine the variations and possibilities for remediation.
ACKNOWLEDGMENTS
The authors acknowledge the Central University of Gujarat, Gandhinagar to fulfil the required facilities for the research work. RKR was supported by fellowships from University Grant Commission, New Delhi, India.
Conflict of Interest: The authors declare that there is no conflict of interest.
REFERENCES
Ali, N., Khan, S., Khan, M.A., Waqas, M., and Yao, H. (2019). Endocrine disrupting pesticides in soil and their health risk through ingestion of vegetables grown in Pakistan. Environ. Sci. Pollut. Res. 1-13.
APHA (1998). Standard Methods for the Examination of Water and Wastewater, twentieth ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC
Baishya, K., and Sarma H P (2014). Effect of agrochemicals application on accumulation of heavy metals on soil of different land uses with respect to its nutrient status. IOSR Journal of Environmental Science, Toxicology and Food Technology, 8(7), 46-54.
Barbieri, M.V., Postigo, C., Guillem-Argiles, N., Monllor-Alcaraz, L.S., Simionato, J.I., Stella, E., and de Alda, M.L. (2019). Analysis of 52 pesticides in fresh fish muscle by QuEChERS extraction followed by LC-MS/MS determination. Sci. Total Environ. 653, 958-967.
Birbir, M., Calli, B., Mertoglu, B., Bardavid, R.E., Oren, A., Ogmen, M.N., and Ogan, A. (2007). Extremely halophilic Archaea from Tuz Lake, Turkey, and the adjacent Kaldirim and Kayacik salterns. World Microbiol Biotechnol 23,309-316. http://doi.org/10.1007/s11274-006-9223-4
Carvalho, F.P. (2017). Pesticides, environment, and food safety. Food and Energy Security 6 (2), 48–60.
Das, D, Kalra, I, Mani, K, Salgaonkar, B.B., and Braganca, J.M. (2019). Characterization of extremely halophilic archaeal isolates from Indian salt pans and their screening for production of hydrolytic enzymes. Environ Sustain 227-239. http://doi.org/10.1007/s42398-019-00077-x
Doolotkeldieva, T., Konurbaeva, M., and Bobusheva, S. (2018) Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ Sci Pollut Res 25, 31848-31862. https://doi.org/10.1007/s11356-017-0048-5
Fantke, P., and Jolliet, O. (2016). Life cycle human health impacts of 875 pesticides. International Journal of Life Cycle Assessment. 21, 1-12.
Jayashree, R., and Vasudevan, N (2006). Residues of organochlorine pesticide in agricultural soils of Thiruvallur District. J. Food Agric. Environ. 4 (1), 313–316.
Jia, Z., and Conrad, R. (2009). Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil, Environmental Microbiology.11 (7), 1658–1671.
Köck-Schulmeyer, M., Postigo, C., Farré, M., Barceló, D., and de Alda, M.L. (2019). Medium to highly polar pesticides in seawater: analysis and fate in coastal areas of Catalonia (NE Spain). Chemosphere 215, 515-523.
King, A. E., Ali, G. A., Gillespie, A. W., and Wagner-Riddle, C. (2020). Soil organic matter as catalyst of crop resource capture. Front. Environ. Sci. 8, 50. doi: 10.3389/fenvs.2020.00050
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33, 1870-1874. http://doi.org/10.1093/molbev/msw054
Liu, Y., Li, S., Ni, Z., Qu, M., Zhong, D., and Ye, C. (2016). Fubin Tang pesticides in persimmons, jujubes and soil from China: Residue levels, risk assessment and relationship between fruits and soils. Science of the Total Environment. 542,620-628.
Mani, K., Salgaokar, B.B., and Braganca, J.M. (2012). Culturable halophilic archaea at the initial and crystallization stages of salt production in a natural solar saltern of Goa, India. Aquat Biosyst 8, 15. http://doi.org/10.1186/2046-9063-8-15
Meena, R. S., Kumar, S., Datta, R., Lal, R., Vijayakumar, V., Brtnicky, M., Sharma, M. P., Yadav, G. S., Jhariya, M.K, Jangir, C.K., Pathan, S. I., Dokulilova, T, Pecina, V., and Marfo, T. D. (2020). Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land. 9, 34. doi: 10.3390/land9020034
Morillo, E., and Villaverde, J. (2017) Advanced technologies for the remediation of pesticide contaminated soils. Sci. Total Environ 586, 576-597.
Pico, Y., El-Sheikh, M.A., Alfarhan, A.H., and Barceló, D. (2018). Target vs non-target analysis to determine pesticide residues in fruits from Saudi Arabia and influence in potential risk associated with exposure. Food Chem. Toxicol. 111, 53-63
Planning Commission Gujarat Human Development Report. (2004).
Ravi, R K., Pathak, B., and Fulekar, M H. (2015). Bioremediation of Persistent Pesticide Pollutants in Rice field Soil Environment using Surface Soil Treatment Reactor, International Journal of Current Microbiology and Applied Sciences, 4(2), 359-369.
Ravi, R. K., Singh, M., and Fulekar, M. H. (2019) Isolation and characterization of bacterial species from pesticides contaminated industrial soil, Ankleshwer, Bharuch, Gujarat, International Journal of Research and Analytical Reviews, 6 (2), 210-214
Samant, M., Pandey, S.C., and Pandey, A. (2018) Impact of hazardous waste material on environment and their management strategies. In: Microbial biotechnology in environmental monitoring and clean up. pp 175-192
Tamura, K., and Nei, M. (1993) Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Molecular Biology and Evolution. 10,512-526.
UNDP. Gujarat Human Development Report (2004). United Nations Development Programme.
Varjani, S.J., Joshi. R.R., Kumar, P.S., Srivastava, V.K., Kumar, V., and Banerjee, C., Kumar, R.P. (2018). Polycyclic aromatic hydrocarbons from petroleum oil industry activities: effect on human health and their biodegradation. In: Varjani SJ, Gnansounou E, Gurunathan B, Pant D, Zakaria ZA (Eds.), Waste Bioremediation. Springer Nature, Singapore, pp. 185-199.
Yennawar, V. B., Bhosle, A. B., and Khadke, P. A. (2013). Soil Analysis and Its Environmental Impact on Nanded City, Maharastra. Research Front. 1(1), 65-70.