1Department of Biochemistry, K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, India.
2Department of Pharmacology, Nandha College of Pharmacy, Erode, India.
3Department of Biochemistry, PGP College of Arts and Science, Namakkal, India.
Corresponding author email: sarabioc@gmail.com
Article Publishing History
Received: 05/03/2021
Accepted After Revision: 18/05/2021
Cardiovascular diseases (CVDs) are a significant health burden with an ever-increasing prevalence. They remain the leading causes of morbidity and mortality worldwide. Contemporary medicine has been used to take care of myocardial infarction (MI), a subset of CVDs, and have been relatively successful but not without adverse effects. As a result, this question has stimulated interest in the use of natural products, which may be equally effective and better tolerated. Therefore, this study aims to analyze the cardioprotective effect of partially purified phenolic fraction derived from Kedrostis foetidissima leaves (PFK) on isoproterenol (ISO) induced MI in male Wistar rats. Animals pretreated with different doses of PFK (50 and 100 mg/kg body weight) and α-tocopherol for 45 days were subjected to subcutaneous ISO injection (20 mg/kg body weight) for two consecutive days to induce MI. Heart tissue and serum of the sacrificed rats were used for assay of hs-CRP, homocysteine, NF-kB, IL-6, TNF-α levels, and lysosomal enzymatic activities (β-glucuronidase, β-N-acetylglucosaminidase, cathepsin-B & D, and β-galactosidase) to evaluate cardiac damage. The results obtained from this study confirms the severe ISO-induced cardiac cell damage in terms of rapidly increased lysosomal enzyme concentration (β-glucuronidase, β-N-acetylglucosaminidase, β-galactosidase), raised hsCRP, homocysteine, NF-kB, IL-6, and TNF-α levels, and reduced activity of the β-glucuronidase and cathepsin-D lysosomal enzymes versus the normal rats. Contrarily, the experimental rats pretreated with PFK had a significantly altered lysosomal enzyme activity, reduced hsCRP, homocysteine, NF-kB, IL-6, and TNF-α level when compared to MI control rats. In conclusion, this study revealed that the PFK play a cardio protective role by regenerating the cardiac defence system against ISO induced cardiac damage.
Lysosomal Enzymes, Medicinal Plants, Myocardial Infarction, Phenols
Pavithra, Uddandrao V. V. S, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Sethumathi P. P, Vadivukkarasi S, Saravanan G. Phenolic-fractions of Kedrostis foetidissima leaves ameliorate lysosomal damage and inflammation of isoproterenol-induced myocardial infarction in rats. Biosc.Biotech.Res.Comm. 2021;14(2).
Pavithra, Uddandrao V. V. S, Chandrasekaran P, Sengottuvelu S, Tamilmani P, Sethumathi P. P, Vadivukkarasi S, Saravanan G. Phenolic-fractions of Kedrostis foetidissima Leaves Ameliorate Lysosomal Damage and Inflammation of Isoproterenol-Induced Myocardial Infarction in Rats. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3fQYhew“>https://bit.ly/3fQYhew</a>
Copyright © Pavithra et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cardiovascular disease (CVD) is an important cause of mortality and morbidity both in developed and developing countries. A major form of CVD is myocardial infarction (MI), which is commonly encountered as a silent infarct owing to its detection well late in the disease progression. When the homeostasis between the blood supply to the cardiac vessels and the requirement of the cardiac tissue is disturbed, the cardiomyocytes are subjected to a prolonged ischemic insult resulting in necrosis, commonly referred to as acute MI (Boarescu et al. 2019).
With a recent change in lifestyle and predisposition to co-morbidities, the incidence of MI has been reported in the young and increasing with age in the middle and older age groups, affecting both men and women (Sangeethadevi et al. 2020). The reduced blood flow to the heart results in a shortage of oxygen to the cardiac muscles, which if left untreated results in irreversible necrotic damage to the myocardium. In most cases, the heart attack is silent, no obvious symptoms of pain and usually has a sudden acute onset (Hoffman and Buckberg 2014; Virani et al. 2020).
Oxidative stress is the most important of all the causative factors in inducing MI by generating highly toxic free-radicals that cause a wide array of metabolic changes and myocardium dysfunction (Sammeturi et al. 2020). Additionally, it destroys the bio molecules (carbohydrates, lipid, proteins, and nucleic acids) and interrupts their interaction with each other leading to cell death (Jansy et al. 2021). Conventional synthetic drugs have been quite successful in the treatment of CVD, but the side effects of prolonged use pose a challenge, necessitating the need for alternative sources like drugs of natural origin (Shaito et al. 2020). Isoproterenol (ISO) is an artificially synthesized catecholamine and a β-adrenergic agonist, if used in large doses can induce cellular damage to the myocardial membrane (Procaccini et al. 2019; Shaito et al. 2020).
Experimental rats when injected with ISO underwent hypoxia, hypertension, increased formation of free radicals and calcium overload (Pavithra et al. 2020; Sangeethadevi et al. 2020).A probable reported mechanism of this ISO-induced cardiac damage is the production of highly toxic free-radicals by auto-oxidation of catecholamines.This excess oxidative stress damages the structural integrity of the cardiac tissue, besides a progressive decline in the function of several antioxidants and an increase in the lipid peroxidation products. Henceforth, irreversible damage of the heart tissues sets in (Alam et al. 2018; Sangeethadevi et al. 2021).
Medicinal plants are comparatively cost effective and are since considered as a better alternative to synthetic drugs owing to their cardioprotective properties. They provide nutritional substances, mainly phytochemicals, that are potentially restorative and help maintain a balanced body system. Antioxidant compounds derived from plant sources act as free radical scavengers, protecting the body by delaying or inhibiting the free radical induced cellular damage (Swapna et al. 2020).A variety of free radical neutralizing antioxidants are found in various dietary sources like fruits, vegetables, and fresh leaf vegetables, etc (Uddandrao et al. 2019; Kalaivani et al. 2020).
Many researchers have also reported the biological role of phytoconstituents in ameliorating cardiotoxicity (Alam et al. 2018; Parim et al. 2019). Besides, plant derived phenolic compounds are reported to inhibit the auto oxidation of lipid molecules which limits the formation of low density oxidized lipoproteins, thereby impeding further cardiovascular damage (Cosme et al. 2020). Recently, a group of phenolic compounds, known as polyphenols, have come to light because of their biological activity, especially the antioxidant and free radical scavenging properties (Khan et al. 2019). Kedrostis foetidissima, an annual herb of the Cucurbitaceae family, is traditionally used by inhabitants of the Asian and African countries to prevent and cure diseases, besides serving as a dietary component.
Previously, reported that K. foetidissima has a high amount of antioxidant properties and cardioprotective efficacy (Pavithra et al. 2020). However, to the best of our knowledge, the effect of K. foetidissima as a cardioprotective agent on hsCRP, membrane damage, and inflammatory mechanism in MI is yet to be studied. Hence, the present research aims to study this effect of the partially-purified phenolic fraction of K. foetidissima leaves (PFK) on cellular and serum indicators of cardiac tissue damage in male Wistar rats with ISO-induced MI.
MATERIAL AND METHODS
For the preparation of PFK, the ethyl acetate fraction of methanolic leaf extract of K. foetidissima leaves were subjected to fractionation in silica gel column chromatography using ethyl-acetate and methanol as solvent (the procedure has been described in an earlier report by Pavithra et al. 2020). Male albino Wistar rats (weight – 150-180g) were used in the study obtained from the Nandha College of Pharmacy, Erode, Tamilnadu, India. All experimental animals were kept under standard laboratory conditions and fed with permitted commercial food and water adlibitum. The experiments of this study was carried out as per the procedures of animal ethical committee constituted by the Nandha College of Pharmacy, Erode, Tamilnadu, India and the study approval number is NCP/IAEC/2018-19/14. For the experimental design, the procured 30 male Wistar rats were divided into five groups of six rats each.
Group I: Normal control Group II: MI untreated control Group III: Albino Wistar rats treated with oral dose-I of PFK (50mg/kg BW) (Pavithra et al. 2020) for 45 days and isoproterenol (20 mg/100g BW) (Saravanan et al. 2013) administered subcutaneously twice at an interval of 24hours.Group IV: Albino Wistar rats treated with oral dose-II of PFK (100mg/kg BW) (Pavithra et al. 2020) for 45 days and isoproterenol (20 mg/100g BW) administered subcutaneously twice at an interval of 24 hours.Group V: α-tocopherol as standard for 45 days (60 mg/kg b.w) (Saravanan et al. 2013) and isoproterenol (20 mg/100g BW) administered subcutaneously twice at an interval of 24 hours.
After 45 days of treatment, all the animals were sacrificed under light ether anaesthesia by cervical decapitation. Heart tissues were dissected, and blood was collected for plasma separation. For the estimation of hsCRP and homocysteine, the plasma was used to assay the hsCRP. The standard chemiluminescence immunoassay kit (Roche Diagnostics, Switzerland) was used for assaying plasma hsCRP levels. While the Microtiter Plate Assay package (Diazyme Laboratories) was employed for estimating the plasma homocysteine concentration.
For the evaluation of serum inflammatory cytokines, the serum levels of inflammatory cytokines such as nuclear factor kappa B (NF-kB), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) were measured as per standard protocols (Signosis Inc., Santa Clara, CA, USA). For the assay of lysosomal enzyme, the lysosomal enzyme activity, namely β-glucuronidase, β-N-acetyl glucosaminidase, and β-galactosidase, were estimated using the methods described by Kawai and Anno (1971), Moore and Morris (1982) and Conchie et al (1959) respectively. One contrary, as per the procedure by Barret (1980) and Sapolsky et al. (1973) the Cathepsin-B and D lysosomal enzymes were assessed. For the statistical analysis, all data were analyzed using SPSS version 10.0. Besides the descriptive analysis, the five groups were compared for hypothesis testing using a one-way analysis of variance (ANOVA), followed by the least significance test (LSD) in case of significant results. p <0.05 was considered significant for all data (α= 5%).
RESULTS AND DISCUSSION
In this study, we made an attempt to observe the effect of the medicinal plant extract, PFK, as a cardio protective agent against ISO-induced cardio toxicity leading to MI. CRP, known as hsCRP, is an acute-phase protein and an important inflammatory marker, often increased in case of tissue damage and infection contributing to the major process of coagulation. The association between the size of the infarct in an acute MI and the level of circulating hsCRP is a well-established fact (Saravanan and Ponmurugan 2012; Zhong et al. 2013), suggesting hsCRP to be a reliable indicator for diagnosing underlying coronary artery damage in addition to the myocardial necrosis (Saravanan and Ponmurugan 2012; Carrero et al. 2019). In this study, we found that a significant (p<0.05) increase in the plasma hsCRP levels in the rats injected with ISO to induce MI when compared to normal control rats (Fig 1).
Figure 1: Effect of PFK on hs-CRP levels of control and ISO treated rats.
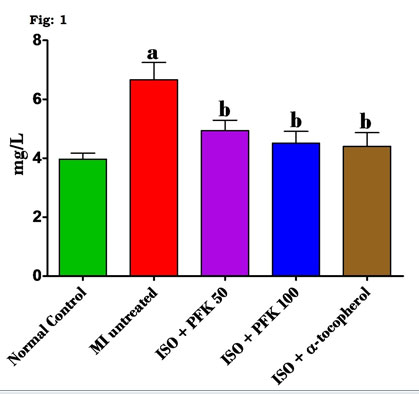
Values are mean ± SD, n = 6, aSignificantly (p < 0.05) different from normal control, bSignificantly (p<0.05) different from MI untreated.
Whereas, on comparing groups with the ISO-induced MI, near normal levels of plasma hsCRP were obtained in rats pretreated with PFK than the rats receiving ISO injections only.These findings are further reinforced by studies that point to a direct correlation between hsCRP and acute MI in humans (Badiger et al. 2014; Carrero et al. 2019). Furthermore, serum hsCRP level is not merely an indicator of the extent of tissue damage following an MI, but also responsible for the development of the irreversible necrosis of the myocardium (Reindl et al. 2020). This also suggests that elevated serum levels may be influenced by the size of the myocardial infarct (Zhong et al. 2013; Lucci et al. 2020).
The induction of MI by subcutaneous ISO injection used in this study caused injury to the cardiomyocytes, initiating an inflammatory response. This tissue damage produced intramyocardial haemorrhage and pericarditis because of the gradual leakage of blood into the pericardial space. The local inflammation in the heart increases the production of proinflammatory mediators leading to a continuous marked rise in CRP levels in the blood (Sammeturi et al. 2020).
However, the results of the present study show that the orally administered PFK significantly reduced these plasma hsCRP levels in the rats with ISO-induced cardiotoxicity. A possible explanation is the inhibition of the platelet mediated inflammation leading to a reduction in the release of proinflammatory mediators (Chen et al. 2020). Hyper-homocysteinemia, a phenomenon associated with the early development of heart and blood vessel disease (Babu et al. 2015). The key features include the production of free radicals, lipid peroxidation of the polyunsaturated fatty acids bound to the cell membrane, monocytic production of interleukins, and up-regulation of the vascular cell adhesion molecules, which encourage the development of atherosclerosis (Shahzad et al. 2019; Jansy et al. 2021). The changes in homocysteine levels due to PFK administration has been described in figure 2.
Figure 2: Effect of PFK on homocysteine levels of control and ISO treated rats.
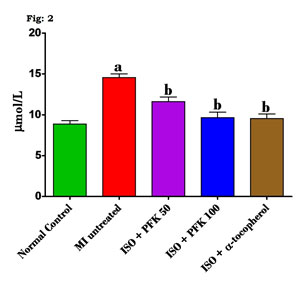
Values are mean ± SD, n = 6, aSignificantly (p<0.05) different from normal control, bSignificantly (p < 0.05) different from MI untreated.
In the experimental rats, the introduction of ISO caused a significant increase in plasma levels of homocysteine compared with control rats (p<0.05). Alternately, these changes in ISO-injected rats were reversed as a result of the prior administration of PFK and α-tocopherol. In the present study, the elevated homocysteine is due to an imbalance in the dietary methionine that contributed to the development of atherosclerosis. The available literature supports the use of highly lipotropic molecules (cerivastatin and fluvastatin) to reduce the risk of CVD and the likelihood of formation of atherosclerotic plaque, the proposed mechanism for which are non-lipid pathways like a reduced expression of interleukins (Boren et al. 2020; Jansy et al. 2021).
PFK contained more phenolic compounds with high lipotropic properties, which can easily pass through the smooth muscle cell membrane of the vessels, thus limiting the production of both homocysteine and interleukin. The recent evidence supports the association between lipid metabolism in the body and the inflammatory process (Uddandrao et al. 2020). Strikingly, hyperlipidemia which is the major pathogenic process underlying the development of atherosclerotic plaque in the blood vessels has been found to inhibit the acute inflammatory response. Additionally, adipose cells produce inflammatory cytokines which are known contributors to a host of metabolic disorders including MI. These findings led to the use of circulating inflammatory proteins CRP, TNF-α and IL-6 as markers to predict an impending cardiovascular adverse event (Carrero et al. 2019; Sammeturi et al. 2020; Hamad et al. 2020).
The effect of PFK administration on the inflammatory markers (NF-kB, IL-6, and TNF-α) has been illustrated in figure 3A-C. A statistically significant increase (p<0.05) in the NF-kB (Fig. 3A), IL-6 (Fig. 3B), and TNF-α (Fig. 3C) was seen in the rats injected with ISO versus the control rats. However, these levels were significantly low (p<0.05) in the PFK pre-treated rats when compared to those who were administered only ISO (Sammeturi et al. 2020; Hamad et al. 2020).
Figure 3: Effect of PFK on the protein levels of (A) NF-kB, (B) IL-6 and (C) TNF-α levels in serum of control and ISO treated rats.
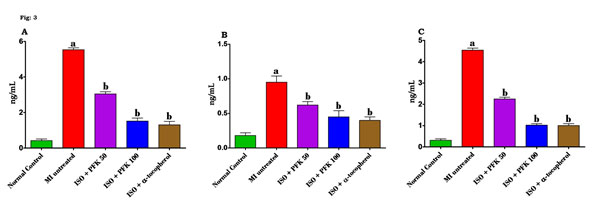
Values are mean ± SD, n = 6, aSignificantly (p<0.05) different from normal control, bSignificantly (p<0.05) different from MI untreated.
IL-6 is a fundamental inflammatory mediator that induces the production of hsCRP in the hepatic tissue. Many metabolic disorders with an inflammatory component, including acute MI, are often precipitated by hyperlipidemia leading to greater expression of pro-inflammatory genes, such as IL-6 and TNF-α (Sammeturi et al. 2020). The results of the present study concur with these findings, evident from the changes in transcription factors such as NF-kB and a boost in the production of the pro-inflammatory factors IL-6 and TNF- α, which is closely linked with the steatotic and inflammatory responses found in coronary heart disease. Usually, in a dormant cell, the cytoplasmic protein, inhibitory kappa B (IkB), checks the expression of the NF-kB factor (Chen et al. 2014; Albensi 2019).
Whereas, upon activation, NF-kB translocates into the nucleus up regulate the gene expression of various pro-inflammatory cytokines (Uddandrao et al. 2019; Jansy et al. 2021). Our results add to this knowledge as the serum IL-6 and TNF levels increased in the ISO-intoxicated rats, signifying an acute inflammatory process; this is in line with results reported by Sangeethadevi et al. (2021). Whereas, the rats treated with the experimental drugs, PFK had lower levels of IL-6 TNF-α and NF-kB, reinforcing the role of PFK in limiting the inflammatory process (Sangeethadevi et al. 2021).
A great emphasis has been laid on the lysosomal alterations occurring concurrently with ischemic or hypoxic damage to muscle cells. Lysosomes are the cell organelles in the animal cell responsible for intracellular digestion of cell components by the processes of autophagy, heterophagy, and endocytosis. The typical sequences of vacuole formation, lysosomal disruption, and spread of the lysosomal enzymes in the cell have reportedly been observed in the case of cardiac ischemia (Nirmala and Pandian 2015; Chi et al. 2020).
Besides this, lysosomes also aid in the cellular secretion and transport process (Bonam et al. 2019). On the other hand, the activity of various lysosomal enzymes was significantly increased (p<0.05) as evident from the serum and cardiac tissue assays of rats treated with ISO versus the normal control rats. Conversely, the experimental drugs (PFK and α-tocopherol) when orally given, significantly reduced (p<0.05) the serum (Table 1) and cardiac tissue (Table 2) activity of these enzymes in ISO-injected rats pretreated with PFK versus only ISO-injected rats (Bonam et al. 2019).
Table 1. Effect of PFK on the activities of lysosomal hydrolases in serum of experimentally induced myocardial infarcted rats
| Groups | β-glucuronidase (μmol of p-nitrophenol liberated/h/mg protein) | β-N-acetyl glucosaminidase (μmol of p-nitrophenol liberated/h/mg protein) | β-galactosidase (μmol of p-nitrophenol liberated/h/mg protein) | Cathepsin-B (μmol of p-nitrophenol liberated/h/100 mg protein) |
Cathepsin-D (μmol of tyrosine liberated/h/100 mg protein) |
| Normal control | 12.60±0.78 | 8.567±0.6 | 10.6±0.81 | 15.64±0.47 | 15.98±0.77 |
| MI untreated | 19.65±±2.38 | 20.88±1.48a | 17.87±0.81a | 17.81±1.41a | 27.89±1.69 |
| ISO + PFK 50 | 16.65±0.96b | 12.45±0.73b | 13.58±0.88b | 16.23±0.52b | 19.67±0.7 |
| ISO + PFK 100 | 15.66±1.1b | 11.65±0.45b | 13.52±1.02 b | 16.19±0.39b | 19.31±1.08 |
| ISO+ α-tocopherol |
15.6±0.73b | 11.42±0.55b | 13.28±0.41b | 16.11±0.32b | 17.68±1.13 |
Values are mean ± SD, n = 6.
aSignificantly (P<0.05) different from normal control
bSignificantly (P<0.05) different from MI untreated
Table 2. Effect of PFK on the activities of lysosomal hydrolases in heart of experimentally induced myocardial infarcted rats
| Groups | β-glucuronidase (μmol of p-nitrophenol liberated/h/mg protein) | β-N-acetyl glucosaminidase (μmol of p-nitrophenol liberated/h/mg protein) | β-galactosidase (μmol of p-nitrophenol liberated/h/mg protein) | Cathepsin-B
(μmol of p-nitrophenol liberated/h/100 mg protein) |
Cathepsin-D
(μmol of tyrosine liberated/h/100 mg protein) |
| Normal control | 16.24±1.57 | 14.37±0.81 | 46.75±4.63 | 11.37±0.51 | 10.61±1.15 |
| MI untreated | 28.73±3.3a | 26.55±2.24a | 81.29±3.19a | 20.23±0.69a | 19.38±1.31a |
| ISO + PFK 50 | 20.31±1.42b | 16.17±0.69 b | 59.74±7.39b | 14.63±0.67b | 12.90±1.15b |
| ISO + PFK 100 | 18.96±3.07b | 15.71±1.06b | 58.46±5.69b | 14.14±0.61b | 12.16±0.85 b |
| ISO+ α-tocopherol |
19.80±0.82b | 15.51±0.78b | 56.79±6.18b | 14.03±0.7b | 11.54±0.66b |
Values are mean ± SD, n = 6.
aSignificantly (P<0.05) different from Normal control
bSignificantly (P<0.05) different from MI untreated
Acid hydrolases located in the myocardial cells, along with the lysosomal and cytosolic enzymes are responsible for the myocardial injury and cell death in the state of ischemia (Prince and Hemalatha 2018; Mishra et al. 2019). Similarly, in this study, enhanced activity of the lysosomal enzymes, both in serum and heart tissue was observed in the ISO-intoxicated rats. The lysosomal membrane is a potential site of an attack by free radicals because of its lipid moiety. This phospholipid rich membrane loses its stability leading to the release of lysosomal enzymes within the cell, that are capable of progressively damaging the cardiac cells, progressing from a reversible ischemic injury to irreversible cardiomyocyte necrosis (Akila et al. 2017; Chi et al. 2020).
On the other hand, β-glucuronidase and cathepsin-D activity significantly reduced (p<0.05) in the lysosomal fraction of the heart of rats with ISO-induced cardiotoxicity when compared to normal control rats. Also, the cytosolic fraction of these enzymes increased considerably (p<0.05) in rats with ISO-induced cardiotoxicity versus normal control rats. Conversely, pretreatment with PFK and α-tocopherol to the rats subjected to ISO-induced MI showed a significant increase in the lysosomal fraction (p<0.05) and a decrease in the cytosolic fraction (p<0.05) of the heart (Table 3) (Akila et al. 2017; Chi et al. 2020).
Table 3. Effect of PFK on the activity of β-glucuronidase and Cathepsin-D in lysosomal and cytosolic fractions of the heart in normal and experimentally induced myocardial infarcted rats
| Groups | β-glucuronidase
(μmol of p-nitrophenol liberated/h/mg protein) |
Cathepsin-D
(μmol of tyrosine liberated/h/100 mg protein) |
|
| Normal control | Lysosomal | 15.23±2.7 | 27.87±2.56 |
| Cytosol | 20.18±1.58 | 21.93±1.71 | |
| MI untreated | Lysosomal | 9.05±0.46a | 10.45±1.51a |
| Cytosol | 30.43±3.3a | 32.50±3.26a | |
| ISO + PFK 50
|
Lysosomal | 11.30±0.87b | 19.03±2.86b |
| Cytosol | 23.75±1.33b | 20.14±1.14b | |
| ISO + PFK 100
|
Lysosomal | 12.82±1.54b | 20.80±1.71b |
| Cytosol | 23.57±1.82b | 19.79±2.71b | |
| ISO + α-tocopherol |
Lysosomal | 13.20±1.57b | 23.42±2.59b |
| Cytosol | 22.35±1.45b | 19.44±0.79b | |
Values are mean ± SD, n = 6.
aSignificantly (P<0.05) different from normal control
bSignificantly (P<0.05) different from MI untreated
We observed significantly lower levels of β-glucuronidase and cathepsin-D in the lysosomal fractions of the ISO-intoxicated rats as compared to normal control rats. This reduced activity confirms the aforementioned mechanism of abnormal release of lysosomal enzymes into the cell leading to cell death in the myocardium (Akila et al. 2017; Trivedi et al. 2020). Administration of PFK significantly altered the concentration of these enzymes in the cardiac tissues of the ISO-intoxicated rats. A probable explanation could be the inhibition of cellular peroxidation or preventing the iron catalyzed oxidative reactions, which lead to peroxidation of cellular membranes. This leads to a stabilization of the lysosomal membrane, thus avoiding the leakage of lysosomal contents (Prince and Hemalatha 2018; Lopez et al. 2020).
CONCLUSION
The findings of the present study reveal that pretreatment of rats with PFK had a significant protective role against the ISO-induced MI by maintaining the levels of hsCRP, TNF-α, IL-6, NF-kB and lysosomal enzymes activities in both serum and heart tissues. This effect may be attributed to the anti-lipoperoxidative and antioxidant properties of phenolic compounds in the partially purified fraction. Based on these results, it can be concluded that PFK may be used as potential source in the management of CVD.
ACKNOWLEDGEMENTS
This study was financially supported by the Indian Council of Medical Research (ICMR), Government of India as a Senior Research Fellowship (Project Ref No: 45/24/2018-BIO/BMS – Dt.16/04/2018). We also express our sincere thanks to the K.S. Rangasamy College of Arts and Science (Autonomous), Tiruchengode, India for their constant support throughout the study.
Conflict Of Interests: The authors declare no conflicts of interests to disclose.
REFERENCES
Akila, P., Asaikumar, L., and Vennila, L. (2017) Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomedicine & Pharmacotherapy, 85: 582-591.
Alam, M.N., Hossain, M.M., Rahman, M.M., Subhan, N., Mamun, M.A. Al, Ulla, A., Reza, H.M., and Alam, M.A. (2018) Astaxanthin prevented oxidative stress in heart and kidneys of isoproterenol-administered aged rats. Journal of Dietary Supplements, 15: 42-54.
Albensi, B.C. (2019) What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion?. Frontiers in Cell and Developmental Biology, 7: 154.
Badiger, R., Dinesha, V., Hosalli, A., and Ashwin, S.P. (2014). hs-C-reactive protein as an indicator for prognosis in acute myocardial infarction. Journal of the Scientific Society, 41: 118.
Barrett, A.J. (1980) Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. The Biochemical Journal, 187: 909-912.
Boarescu, P.M., Chirila, I., Bulboaca, A.E., Bocsan, I.C., Pop, R.M., Gheban, D., and Bolboaca, S.D. (2019) Effects of Curcumin Nanoparticles in Isoproterenol-Induced Myocardial Infarction. Oxidative Medicine and Cellular Longevity, 19: 7847-142.
Bonam, S.R., Wang, F., and Muller, S. (2019) Lysosomes as a therapeutic target. Nature Reviews Drug Discovery, 18: 923-948.
Boren, J., Chapman, M.J., Krauss, R.M., Packard, C.J., Bentzon, J.F., Binder, C.J., Daemen, M.J., Demer, L.L., and Hegele, R.A. (2020) Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal, 41(24): 2313-2330.
Carrero, J.J., Andersson Franko, M., Obergfell, A., Gabrielsen, A., and Jernberg, T. (2019) hsCRP Level and the Risk of Death or Recurrent Cardiovascular Events in Patients With Myocardial Infarction: a Healthcare-Based Study. Journal of the American Heart Association, 8: 012638.
Carrero, J.J., Andersson, F.M., Obergfell, A., Gabrielsen, A., and Jernberg, T. (2019) hsCRP Level and the Risk of Death or Recurrent Cardiovascular Events in Patients With Myocardial Infarction: a Healthcare-Based Study. Journal of the American Heart Association, 8(11): 012638.
Chen, S., Yin, Z.J., and Jiang, C. (2014) Asiaticoside attenuates memory impairment induced by transient cerebral ischemia-reperfusion in mice through anti-inflammatory mechanism. Pharmacology Biochemistry and Behavior, 122: 7-15.
Chen, Y., Zhong, H., Zhao, Y., Luo, X., and Gao, W. (2020) Role of platelet biomarkers in inflammatory response. Biomarker Research, 8: 28.
Chi, C., Riching, A.S., and Song, K. (2020) Lysosomal Abnormalities in Cardiovascular Disease. International Journal of Molecular Sciences, 21(3): 811.
Conchie, J., Findlay, J., and Levvy, G.A. (1959) Mammalian glycosidases. Distribution in the body. Biochemical Journal, 71: 318-325.
Cosme, P., Rodriguez, A.B., Espino, J., and Garrido, M. (2020) Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants, 9(12): 1263.
Hamad, A.H.A., Zenab, B.H.M., and Muhammad, B.I. (2020) Basic pathogenic mechanisms of atherosclerosis. Egyptian Journal of Basic and Applied Sciences, 7: 116-125
Hoffman, J.I., and Buckberg, G.D. (2014) The myocardial oxygen supply:demand index revisited. Journal of the American Heart Association, 3: 000285.
Jagadeeswari, M., and Hemashenpagam, N. (2019) In Vitro antioxidant and anti-inflammatory potential of Kedrostis foetidissima (Jacq) Cogn leaf extracts. Journal of Pharmacy Research, 8: 360-365
Jansy, A.I.R., Uddandrao, V.V.S., Ganapathy, S., Chandrasekaran, P., Sengottavelu, S., Tamilmani, P., Sethumathi, P.P., and Vadivukkarasi, S. (2021) Biochanin A attenuates obesity cardiomyopathy in rats by inhibiting oxidative stress and inflammation through the Nrf-2 pathway. Archives of Physiology and Biochemistry, DOI: 10.1080/13813455.2021.1874017.
Kalaivani, A., Vadivukkarasi, S., Sathibabu Uddandrao, V.V., and Saravanan G. (2020) Attenuation of Obesity-Associated Oxidative Stress by Cucurbita maxima Seed Oil in High Fat Diet-Induced Obese Rats. In: Tappia P., Ramjiawan B., Dhalla N. (eds) Pathophysiology of Obesity-Induced Health Complications. Advances in Biochemistry in Health and Disease, 19, Springer, Cham. https://doi.org/10.1007/978-3-030-35358-2_18
Kawai, Y., and Anno, K. (1971) Mucopolysaccharides degrading enzymes from the liver of the squid Ommastrephes solani paciicus. I. Hyaluronidase. Biochim Biochimica et Biophysica Acta, 242: 428-436.
Khan, M.K., Paniwnyk, L., and Hassan S. (2019) Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. In: Li Y., Chemat F. (eds) Plant Based “Green Chemistry 2.0”. Green Chemistry and Sustainable Technology. Springer, Singapore. https://doi.org/10.1007/978-981-13-3810-6_8
Lopez, D.M., Kahlau, L., Jungnickel, K.E.J., Low, C., and Damme, M. (2020) Characterization of the complex of the lysosomal membrane transporter MFSD1 and its accessory subunit GLMP. FASEB Journal, 34(11): 14695-14709.
Lucci, C., Cosentino, N., Genovese, S., Campodonico, J., Milazzo, V., De Metrio, M., Rondinelli, M., Riggio, D., Biondi, M.L., Rubino, M., Celentano, K., Bonomi, A., Capra, N., Veglia, F., Agostoni, P., Bartorelli, A.L., and Marenzi, G. (2020) Prognostic impact of admission high-sensitivity C-reactive protein in acute myocardial infarction patients with and without diabetes mellitus. Cardiovascular Diabetology, 19(1): 183.
Mishra, P.K., Adameova, A., Hill, J.A., Baines, C.P., Kang, P.M., Downey, J.M., Narula, J., Takahashi, M., Abbate, A., Piristine, H.C., Kar, S., Su, S., Higa, J.K., Kawasaki, N.K., and Matsui, T. (2019) Guidelines for evaluating myocardial cell death. American Journal of Physiology-Heart and Circulatory Physiology, 317(5): 891-922.
Moore, J.C., and Morris, J.E. (1982) A simple automated colorimetric method for determination of N-acetyl- beta-D-glucosaminidase. Annals of Clinical Biochemistry, 19: 157-9.
Nirmala, J., and Pandian, R. (2015) Study of anti-diabetic activity of Kedrostis feoditissima (Jacq.) Cogn., International Journal of Current Research and Academic Review, 3: 50-55.
Parim, B., Sathibabu Uddandrao, V.V., and Saravanan, G. (2019) Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Failure Reviews, 24: 279-299.
Pavithra, K., Sathibabu Uddandrao, V.V., Chandrasekaran, P., Brahmanaidu, P., Sengottuvelu, S., Vadivukkarasi, S., and Saravanan, G. (2020) Phenolic fraction extracted from Kedrostis foetidissima leaves ameliorated isoproterenol-induced cardiotoxicity in rats through restoration of cardiac antioxidant status. Journal Food Biochemistry, 44: 13450.
Prince, S.M.P., and Hemalatha, K.L.A. (2018) molecular mechanism on the antiapoptotic effects of zingerone in isoproterenol induced myocardial infarcted rats. European Journal of Pharmacology, 821: 105-111.
Procaccini, D.E., Sawyer, J.E., and Watt, K.M. (2019) Pharmacology of cardiovascular drugs. In: Critical Heart Disease in Infants and Children. third ed. Elsevier Inc.
Ravi Babu, B., Jagadish Naik, M., and Janardhan, M. (2015) Cardio protective activity of Passiflora foetida extract and silver nanoparticles in doxorubicin induced cardiac disease in rats. Indian Journal of Research in Pharmacy and Biotechnology, 3: 329-34.
Reindl, M., Tiller, C., Holzknecht, M., Lechner, I., Henninger, B., Mayr, A., Brenner, C., Klug, G., Bauer, A., Metzler, B., and Reinstadler, S.J. (2020) Association of Myocardial Injury With Serum Procalcitonin Levels in Patients With ST-Elevation Myocardial Infarction. JAMA network open, 3(6): 207030.
Sammeturi, M., Hussain Shaik, A., and Prasad, M.E. (2020) Cardioprotective molecular mechanism of syringic acid against isoproterenol induced post- myocardial toxicity in male albino wistar rats. Journal of King Saud University Science, 32: 1375-1381.
Sangeethadevi G, Sathibabu, U.V.V., Jansy Isabella, R.A.R., Saravanan, G., Ponmurugan, P., Chandrasekaran, P., Sengottuvelu, S., and Vadivukkarasi, S. (2021) Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug and Chemical Toxicology, DOI: 10.1080/01480545.2021.1894707.
Sangeethadevi, G., Sathibabu Uddandrao, V.V., and Vadivukkarasi, S. (2020). Therapeutic Potential of Biochanin-A Against Isoproterenol-induced Myocardial Infarction in Rats. Cardiovascular & Hematological Agents in Medicinal Chemistry, 18: 1-6.
Sapolsky, A.I., Altman, R.D., and Howell, D.S. (1973) Cathepsin D activity in normal and osteoarthritic human cartilage. Federation Proceedings, 32(4): 1489-93.
Saravanan, G., and Ponmurugan, P. (2012) Amaranthus viridis Linn., a common spinach, modulates C-reactive protein, protein profile, ceruloplasmin and glycoprotein in experimental induced myocardial infarcted rats. Journal of the Science of Food and Agriculture, 92: 2459-2464.
Saravanan, G., Ponmurugan, P., Sathiyavathi, M., Vadivukkarasi, S., and Sengottuvelu, S. (2013). Cardioprotective activity of Amaranthus viridis Linn: Effect on serum marker enzymes, cardiac troponin and antioxidant system in experimental myocardial infarcted rats. International Journal of Cardiology, 165: 494-498.
Sathibabu Uddandrao, V.V., Brahmanaidu, P., Ravindarnaik, R., Suresh, P., Vadivukkarasi, S., and Saravanan, G. (2019) Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. European Journal of Nutrition. 58(6): 2425-2437.
Shahzad, S., Mateen, S., Naeem, S.S., Akhtar, K., Rizvi, W., Moin, S. (2019) Syringic acid protects from isoproterenol induced cardiotoxicity in rats. European Journal of Pharmacology, 849: 135-145.
Shaito, A., Thuan, D., Phu, H.T., Nguyen, T., Hasan, H., Halabi, S., Abdelhady, S., Nasrallah, G.K., Eid, A.H., and Pintus, G. (2020) Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Frontiers in Pharmacology, 11: 422.
Swapna, K., Uddandrao, V.V.S., Vadivukkarasi, S., and Saravanan, G. (2020) Asiatic Acid Attenuate Type 2 Diabetes Mellitus Induced Alterations in Acetylcholinesterase and Antioxidant System of Brain in Rats. Bioscience Biotechnology Research Communications, 13(4): 2193-2199.
Trivedi, P.C., Bartlett, J.J., and Pulinilkunnil, T. (2020) Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells, 9(5): 1131.
Uddandrao, V.V.S., Rameshreddy, P., Brahmanaidu, P., Ponnusamy, P., Balakrishnan, S., Ramavat, R.N., Swapna, K., Pothani, S., Nemani, H., Meriga, B., Vadivukkarasi, S, P R, N., and Ganapathy, S. (2020) Antiobesity efficacy of asiatic acid: down-regulation of adipogenic and inflammatory processes in high fat diet induced obese rats. Archives of Physiology and Biochemistry, 126(5): 453-462.
Virani, S.S., Alonso, A., Benjamin, E.J., Bittencourt, M.S., Callaway, C.W., Carson, A.P., Chamberlain, A.M., Chang, A.R., Cheng, S., and Delling, F.N. (2020) Djousse LHeart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation, 141(9): 139-596.
Zhong, J., Wang, Y., Wang, X., Li, F., Hou, Y., Luo, H., and Chen, H. (2013). Significance of CAVI, hs-CRP and homocysteine in subclinical arteriosclerosis among a healthy population in China. Clinical and Investigative Medicine. Medecine Clinique et Experimentale, 36: 81-86.


