1Department of Pharmaceutical Sciences, Sree Vidyanikethan College of Pharmacy, A. Rangampet, Tirupati -517502, Chittoor Dist., Andhra Pradesh, India.
2Department of Pharmaceutical Sciences, Jawaharlal Nehru Technological University Ananthapur (JNTUA), Ananthapuramu- 515002, A. P., India.
3Department of Chemistry, Krishna University, Machilipatnam-521001, Krishna Dist., A. P., India.
Corresponding author email: radhikapharma11@gmail.com
Article Publishing History
Received: 12/03/2021
Accepted After Revision: 15/06/2021
Amaranthus roxburghianus is a small-sized tree mustly used for iron tonic and inflammatory bowel disease.The aim of present investigation was to evaluate pharmacological screening for anti arthritic activity of total flavonoids of Amaranthus roxburghianus nevski in freund’s complete adjuvant-induced arthritic rats model.The extraction of A. roxburghianus dried aerial parts was done using ethyl alcohol: water (70: 30) by the hot soxhlet method. Total flavonoids were separated from the extract and two doses 20 and 40 mg/kg of TFAR, were used against Freund’s complete adjuvant-induced chronic immunological arthritis in Wistar rats. Arthritis study was carried out using morphological parameters, haematological studies, proinflammatory cytokines (TNF-alpha,IL-6) and histopathological findings to explore the mechanism of Antiarthritic potential.
The results showed significant paw oedema inhibition for TFAR at a dose of 40mg/kg which was assisted by the results of paw volume and diameter. The TFAR also strongly reduced proinflammatory cytokines levels and depicts the histopathological alterations induced by Freund’s complete adjuvant model.Finally it is concluded that TFAR protects synovial membrane by improving the health status exhibits promising anti-arthritic activity. This finding thus supports the traditional use of A. roxburghianus for arthritis.
A. roxburghianus, Haematological, Histopathological, Interleukin-6, Organ weight, Tumor Ncrosis Factor-Alpha.
Chikatipalli R, Saravanakumar K, Bannoth C. S. K. Pharmacological Screening of Amaranthus roxburghianus Nevski Total Flavonoids for Anti-Arthritic Activity in Freund’s Complete Adjuvant-Induced Arthritis Rat Model. Against Human Pathogens. Biosc.Biotech.Res.Comm. 2021;14(2).
Chikatipalli R, Saravanakumar K, Bannoth C. S. K. Pharmacological Screening of Amaranthus roxburghianus Nevski Total Flavonoids for Anti-Arthritic Activity in Freund’s Complete Adjuvant-Induced Arthritis Rat Model. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3eMRjpi“>https://bit.ly/3eMRjpi</a>
Copyright © Chikatipalli et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic, progressive autoimmune inflammatory disease that occurs in several joints as symmetrical polyarthritis associated with swelling and discomfort. RA is a complex multi-system disorder whose primary site of damage to inflammatory tissue is in the finger and foot joints. Destructive cartilage and bone changes and bone outgrowths limit joint mobility(Bihani et al., 2014). RA is a chronic inflammatory condition characterised by pain, inflammation of the synovial membrane, inflammation of the peripheral joints, morning stiffness, articular tissue destruction and reduced joint mobility (Choudhary et al., 2015; Uttra et al., 2017). The anatomy and aetiology of RA is complex and unclear, can cause serious impairment, and eventually affects the capacity of a person to perform daily activities, reduces the quality of life, and causes premature death.
RA is the most common inflammatory condition that affects about 1% of the global adult population, compared to males females are three times more prone to RA (Patil et al., 2012). Conventional treatment of RA with NSAIDS, corticosteroids, immunosuppressants and anti-rheumatic agents (TNF-alpha & monoclonal antibodies) has impediments (Yende et al., 2010; Kola et al., 2018). Chronic treatment with the aforementioned agents has significant adverse effects, such as haematological, cardiovascular, GIT and renal toxicity (Saleem et al., 2020).
Patients suffering from chronic autoimmune disorders are urged to take alternative symptomatic relief strategies(Kauthale et al., 2017; Zhang et al., 2018 ). Amaranthus roxburghianus Nevski (Family: Amaranthaceae) is a wild plant with tender leaves and edible shoots, widely used as a leafy plant(Mary et al., 2011). In Telugu, it is widely known as Chirikoora. It is used as an iron tonic(Nirmal et al., 2013) and its herbal formulations are rich in alkaloids mostly used in traditional Ayurvedic medicine(Oyeleke et al., 2018). Amaranthus roxburghianus is used as an abortifacient by tribes of Chittoor district, Andra Pradesh state of India(Alves de Almeida et al., 2017).
In conjunction with piperine, its root extract is used in the successful treatment of inflammatory bowel disease (Pamila et al., 2017) and its leaves have been reported for use in the treatment of sunstroke and urinary disorders(Bansod et al., 2010). Based on the above evidences for treatment of various diseases we made an attempt to analyse the anti-arthritis efficacy of Amaranthus roxburghianus. So we separeted the total flavonoids fraction using hydro-alcoholic solvent system and analysed its efficacy for treatment of RA using in chronic models of albino Wistar rats.
MATERIAL AND METHODS
A.roxburghianus leafy vegetables were acquired from near by Tirupati areas. The plant authentication was performed at Botany Department, Sree Venkateswara University, Andhra Pradesh, India with a plant voucher specimen (Ref. No. 1656). The aerial parts of the plant were washed with tap water and dried in the shade. The dried leaves were powdered in the grinder and defatted with petroleum ether and successive extraction was carried out with ethyl alcohol: water with (70: 30) ratio, by the hot soxhlet process.
The hydro-alcoholic extract was concentrated under reduced pressure in a rotary evaporator (Heidolph Instrument,Laborota 4000, Germany). The dried crude hydro-alcoholic A.roxburghianus extract was collected and preserved in an airtight glass container at 4-8 °C until final use(Ajayi et al., 2018). The total fraction of flavonoids (TFAR) (60g) was derived from an eluted fraction of H2O: ethanol (3:7) by standard Association of analytical chemists(AOAC) methods.
Animal:Male Wistar rats weighing between 180-200 g were used for experimental studies. The Institutional Animal Ethics Committee of the Sree Vidyanikethan College of Pharmacy, Tirupati, Chittoor Dist., A.P., India (Approval No.: SVCP/IAEC/I-001/2018-19 dated 01/04/2019) approved all animal experiment protocols in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Animal Experiments (CPCSEA).
The animals were housed in Poly propylene cages and held in the light/dark cycle at 24 C ± 2 °C under 12 h and were fed with a regular pellet diet and had free access to water.A total of 30 male Wistar albino rats, 180-200 g in weight, were selected and assigned to 5 groups of 6 rats in each group (n=6). As a standard control, Group I was using. Group 2 was used as an Arthritic control group, Group 3 was used as a 10 mg/kg diclofenac, Group 4 was used as a 20 mg/kg TFAR group, and Group 5 was used as a 40 mg/kg TFAR group.
Acute Toxicity Studies: The toxicity effect of extract was recorded in previous studies(Oyeleke, et al., 2018) and found as safety of up to 200 mg/kg. Based on earlier studies, which recorded a better response, the desired doses of 20 and 40 mg/kg were selected (Hosseini, 2018).
Freund’s Complete Adjuvant (FCA): All rats were injected intradermally with 0.1 mL of FCA (1mg/ml) into the left hind paw on day ‘0’, with the exception of those in the normal control group. For arthritis to grow, an interval of 7 days was given. During this time, all the animals developed signs of arthritis, such as swelling, redness and restricted movement. The treatment finished on the 28th day (Alamgeer et al., 2015).Morphological studies including Paw volume and diameter, body weight, haematological studies, histopathologicaland the experimental animal blood was obtained and the serum was isolated by the process of centrifugation .
ELISA kits were used to test the protein concentration of serum proinflammatory cytokines such as TNF-alpha and IL-6, and the procedure was performed in accordance with standard instructions.The values were expressed as Mean ± SEM (n = 3).
Statistical Analysis: Using one-way variance analysis (ANOVA) followed by the Dunnet test, the statistical significance was tested and P<0.05, P<0.01, and P<0.001 were considered statistically significant.
RESULTS AND DISCUSSION
Morphological studies:Paw volume and diameter s:The effect of TFAR on paw volume and diameter in arthritic rats induced by FCA was reflected in (Table 1). Challenge with CFA (0.1 mL) suggests paw edoema production that reached peak edoema on the 21st day of injection. The group treated with diclofenac demonstrated substantial inhibition of paw edoema on day 7th (P<0.05), day 14th (P<0.01), day 21st (P<0.001) and day 28 (P<0.001). TFAR (20mg/kg) demonstrates substantial paw edoema inhibition on day 21 and day 28 with (P<0.01). Important paw edoema inhibition was also shown in rats treated with TFAR (40 mg/kg) on days 7th (P<0.05), 14th (P<0.05), 21st (P<0.01) and 28th (P<0.01).
The paw diameter was raised and subsequently decreased marginally until the 21st day of adjuvant induction. The group treated with diclofenac demonstrated substantial paw diameter inhibition on day 14 th(P<0.01), day 21th (P<0.001) and day 28th (P<0.001). TFAR (20 mg/kg) demonstrates strong paw diameter inhibition on day 21th and day 28th with (P<0.01).Also rats treated with TFAR (40 mg/kg) shows significant inhibition of paw diameter on day 21st and day 28th (P<0.01).
Table 1. Effect of TFAR on Paw Volume in FCA-induced arthritic rats
| Groups | Paw volume (mL) | ||||
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Normal control | 0.31±0.06 | 0.0.29±0.09 | 0.29±0.09 | 0.28±0.08 | 0.29±0.06 |
| Arthritis control | 0.83 ± 0.09 | 1.25 ± 0.19 | 2.12 ± 0.16 | 2.46 ± 0.13 | 2.59 ± 0.12 |
| Diclofenac 10 mg/kg | 0.47 ± 0.03 | 0.68± 0.21** | 0.97±0.19** | 1.13±0.21*** | 0.59±0.15*** |
| TFAR 20 mg/kg | 0.61 ± 0.02 | 0.88 ± 0.26 | 1.77 ± 0.16* | 1.52 ± 0.12** | 0.88 ± 0.12*** |
| TFAR 40 mg/kg | 0.59 ± 0.05 | 0.81± 0.17* | 1.56 ± 0.20** | 1.39 ± 0.21** | 0.78 ± 0.23*** |
Values are expressed as mean ± SEM (n=6). *P<0.05,**P<0.01,***P<0.001 as compared with Arthritis control. (One-way ANOVA followed by Dunnet’s test).
Body weight studies: Effect of TFAR on body wt. in FCA-induced arthritic rats was tabulated in (Table 2) indicates the increased body wt. during treatment of standard drug and TFAR.
Table 2. Effect of TFAR on body wt. in FCA-inducd arthritic rats
| Groups | Mean Body wt. (gm) | Mean Difference in Body wt | |
| Before Induction | After Induction | ||
| Normal control | 179±1.23 | 179±1.23 | — |
| Arthritis control | 165 ± 3.13 | 186 ± 2.4 | 21 ± 1.26 |
| Diclofenac 10 mg/kg | 175 ± 2.24 | 206 ± 1.38 | 31 ± 1.11** |
| TFAR20 mg/kg | 173 ± 1.12 | 202 ± 3.21 | 29 ± 2.03* |
| TFAR40 mg/kg | 176 ± 3.65 | 207 ± 5.01 | 31 ± 1.67* |
Values are expressed as mean ± SEM (n=6); *P<0.05, **P<0.01 as compared with control followed by Dunnet’s test.
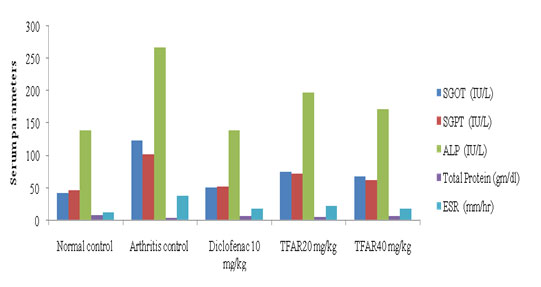
Hematological studies:The effect of TFAR on different serum and blood parameters in arthritic rats induced by FCA was tabulated in (Figure 1). The CFA (0.1 mL) study showed increase in the levels of SGOT, SGPT, ALP and decrease in the total protein levels in the control group. The group treated with Diclofenac reported a decrease in SGOT (P<0.01), SGPT (P<0.01), ALP (P<0.001) and Total Protein (P<0.01) levels. TFAR (20 mg/kg) suggested a substantial decrease in SGPT (P<0.05), ALP (P<0.05) and total protein (P<0.05) levels respectively. The treated TFAR (40 mg/kg) community showed a decrease in SGOT (P<0.05), SGPT (P<0.05), ALP (P<0.01) and total protein (P<0.05) levels.
Figure 1: Effect of TFAR on hematological parameters in FCA-induced arthritic rats
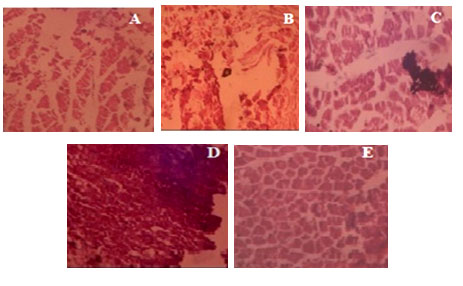
Histopathological Studies: The histopathology of wistar rat ankle joint tissue are shown in Figure 2 and suggested damaging connective tissue lesions, joint space vascularity, and the development of granuloma in the arthritis control group. The normal control group showed the absence of necrosis in the ankle joint with normal connective tissue function. Treatment with standard showed normal ankle joint connective tissue with less oedema and no necrosis, compared to arthritis control group rats. TFAR (20mg/kg) treated rats showed oedema and necrosis with few inflammatory cells and granuloma formation. Rats treated with TFAR (40mg/kg) showed mild oedema necrosis, but there was no granuloma in the ankle joint.
Figure 2: Histopathological observation of the rat ankle tissues (A) Normal control (B) Arthritis control(C) Diclofenac (10 mg/kg) (D) TFAR (20 mg/kg) (E) TFAR (40 mg/kg) treated rats. Magnification: x100; thickness: 5 µm.
Organs weight studies: A decrease was observed in thymus wt. in comparison with the control group, the mean spleen wt was increased in the FCA treated rats (Table 3). In rats treated with TFAR (20 and 40 mg/kg) and diclofenac (10 mg/kg) compared to FCA treated rats, the increase in spleen wt (P <0.01) was significantly inhibited. TFAR (40 mg/kg) and diclofenac (10mg/kg) therapy attenuated the decrease in wt. Significantly, of thymus
(P <0.01).
Table 3. Effect of TFAR on thymus and spleen with FCA-induced arthritic rats.
| Groups | Spleen wt.
(mg/100 g b.wt.) |
Thymus wt.
(mg/100 g b.wt.) |
| Normal control | 189.53±3.12 | 100.5±1.01 |
| Arthritis control | 259.34±3.61 | 71.18±2.34 |
| Standard(10 mg/kg) | 199.83±4.20** | 91.00±1.46** |
| TFAR 20 mg/kg | 224.50±2.36* | 83.50±1.43 |
| TFAR 40 mg/kg | 210.00±2.34* | 85.75±1.53* |
Values are expressed as the mean ± SEM (n= 6); *P<0.05, **P<0.01 as compared with control (One-way ANOVA followed by Dunnet’s test
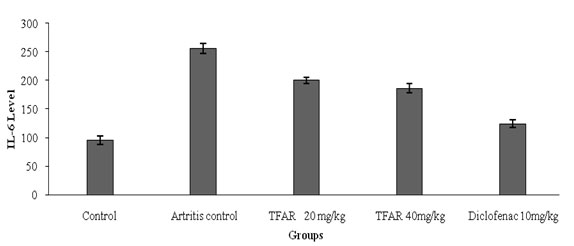
Proinflammatory cytokines (TNF-alpha and IL-6):The proinflammatory cytokine analysis was performed and TFAR(40mg/kg) showed a substantial effect (P<0.5) relative to the regulation of arthritis. The results showed that proinflammatory cytokine inhibition was dose based. Compared to TFAR(40mg/kg) and athritis regulation, the regular diclofenac showed substantial (P<0.01) decreases in Proinflammatory cytokines. The TNF-Alpha and IL6 level results are shown in Figure 3 and 4.
TNF-alpha and IL6 play a key role in inflammatory responses due to generation and propagation of inflammation. Various studies have been performed the effect of extract on inhibition of proinflamatory cytokines and reported dose-dependently reduced IL-6 in macrophages at both the gene and protein expression levels. It was also found that flavonoids inhibited the production of cytokines, TNF-α, macrophage inflammatory protein-1, and IL-6 via the activated monocytes (Farzaei et al. 2019).
Figure 3: Effect of TFAR on TNF-Alpha level in FCA-induced arthritis rats
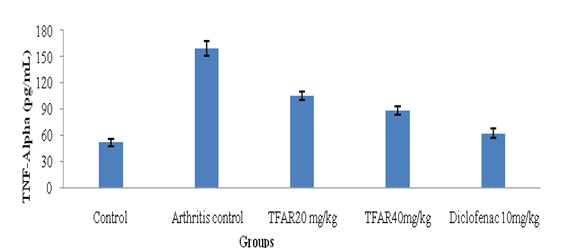
Figure 4: Effect of TFAR on IL6 level in FCA-induced arthritis rats
CONCLUSION
The antiathritics activity of A.roxburghianus extract (TFAR) was performed by FCA induced athritis in rat model and it can be concluded from the findings that the total flavonoid fraction showed promising anti-arthritis activity by reducing the amount of proinflammatory cytokines and preserving the weight of the spleen and thymus. The TFAR treated rats showed oedema and necrosis with few inflammatory cells and granuloma formation were observed by histopathological study. Thus, A.roxburghianus plant could be a promising candidate for treatment of various antiinflammatory diseases.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Anna Balaji, Principal of Sree Vidyanikethan College of Pharmacy, Tirupati for providing necessary infrastructure and facilities to conduct this research work.
Conflicts of Interests:The Authors declare that there is no conflict of interest.
REFERENCES
Ajayi, A. M., Umukoro, S., Aderibigbe, A., and Ademowo, O. G. (2018). Anti-inflammatory activity of Theobroma cacao L. stem bark ethanol extract and its fractions in experimental models. J Ethnopharmacol, 222, 239–248.
Alamgeer, H.U.H., Uttra, A.M., and Rasool, S. (2015). Evaluation of in vitro and in vivo anti-arthritic potential of Berberis calliobotrys. Bangladesh J Pharmacol, 10, 807–819.
Alves de Almeida, A. C., de-Faria, F. M., Dunder, R. J., Manzo, L. P. B., Souza-Brito, A. R. M., and Luiz-Ferreira, A. (2017). Recent trends in pharmacological activity of alkaloids in animal colitis: Potential use for inflammatory bowel disease. Evid-Based Compl Alt Med, 2017, 1-24.
Horwitz, W., and Latimer, G.W. (2006). Official methods of analysis of AOAC International
18th edition, In:Horwitiz W, (eds) Gaithersburg, Maryland : AOAC International.
Bansod, M. S., and Kagathara, V. G. (2010). Evaluation of analgesics and anti- inflammatory activity of a polyherbal formulation. Int J Pharm Tech Res, 2, 1520–1527.
Bihani, G. V., Rojatkar, S. R., and Bodhankar, S. L. (2014). Anti-arthritic activity of methanol extract of Cyathocline purpurea (whole plant) in Freund’s complete adjuvant-induced arthritis in rats. Biomed Aging Pathol, 4(3), 197–206.
Choudhary, M., Kumar, V., Malhotra, H., and Singh, S. (2015). Medicinal plants with potential anti-arthritic activity. J Intercul Ethnopharmacol, 4(2), 147–179.
Farzaei, M.H., Singh, A.K., Kumar, R., Croley, C.R., et al. (2019). Targeting Inflammation by Flavonoids:Novel Therapeutic Strategy for Metabolic Disorders. Int J Mol Sci, 20, 4957.
Habibur Rahman, M., Eswaraiah, C., and Dutta, A. M. (2015). In-vitro anti-inflammatory and anti-arthritic activity of Oryza sativa var. Joha rice (an aromatic indigenous rice of Assam). Am Eurasian J Agric Environ Sci, 15, 115–121.
Hosseini, A., Shorofi, S.A. and Davoodi, A. (2018). Starting dose calculation for medicinal plants in animal studies; recommendation of a simple and reliable method. Res J Pharmacogn, 5(2), 1-7.
Kauthale, V., Kulkarni, D., Chavan, L., Patil, S., and Nalawade, A. (2017). Diversity of wild edible plants in Dhadgaon block of Nandurbar District in Maharashtra, India. Int J Curr Res Biosci Plant Biol, 4(6), 62–73.
Kola,V., Mondal, P., Thimmaraju, M. K., Mondal, S., and Rao, N.V.(2018). Antiarthritic potential of aqueous and ethanolic fruit extracts of Momordica charantia using different screening models. Pharmacogn Res, 10(3), 258.
Manan, M., Saleem, U., Akash, M.S.H., Qasim, M., Hayat, M., Raza, Z., and Ahmad, B. (2020). Antiarthritic Potential of Comprehensively Standardized Extract of Alternanthera bettzickiana: In vitro and in vivo Studies. ACS Omega, 5(31), 19478–19496.
Mary, D. A., Franco, F. M., and Babu, V. (2011). Assessing the contribution of local and traded biodiversity in community health care: A case study from Keelakodankulam village, South India. Ethnobot Res Appl, 9, 275–286.
Nirmal, S.A., Ingale, J.M., Pattan, S.R., and Bhawar, S.B. (2013). Amaranthus roxburghianus root extract in combination with piperine as a potential treatment of ulcerative colitis in mice. J Integrat Med, 11(3), 206–212.
Pamila, U.A.,and Karpagam,S.(2017). Antimicrobial activity of Alternanthera bettzickiana (regel) Nicholson and its phytochemical contents. Int J Pharmaceuti Sci Res, 8, 2594–2599.
Patil, M.V.K., Kandhare, A.D., and Bhise, S.D.(2012). Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomed Aging Pathol, 2(1), 6–15.
Perumal, S.S., Ekambaram, S.P., and Dhanam, T. (2017). In vivo antiarthritic activity of the ethanol extracts of stem bark and seeds of Calophyllum inophyllum in Freund’s complete adjuvant induced arthritis. Pharmaceuti Biol, 55(1), 1330–1336.
Prasad, S. B., and Yashwant, Aeri V. (2013). In vitro anti-inflammatory activity of Raupya (silver) Bhasma. J Chem Pharmaceuti Res, 5, 194–197.
Ruiz, A., Hermosín-Gutiérrez, I., Mardones, C., Vergara, C., Herlitz, E., Vega, M., Dorau, C., Winterhelter, P., and Von Baer, D. (2010). Polyphenols and anti-oxidant activity of Calafate (Berberis microphylla) fruits and other native berries from southern Chile. J Agricul Food Chem, 58(10), 6081–6089.
Saleem A., Saleem, M., Akhtar, M. F., Shahzad, M., and Jahan, S. (2020). Moringa rivae leaf extracts attenuate Complete Freund’s adjuvant-induced arthritis in Wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacol, 28(1), 139–151.
Tripathy, S., Pradhan, D., and Anjana, M. (2010). Anti inflammatory and antiarthritic potential of Ammania baccifera linn. Int J Pharm Biologic Sci, 1(3), 1–7.
Uttra, A.M., and Hasan, U. H.(2017). Anti-arthritic activity of aqueous-methanolic extract and various fractions of Berberis orthobotrys Bien ex Aitch. BMC Complement and Alternati Med, 17(1), 1–16.
Yang,Y., Qi, J., Wang, Q., Du, L., Zhou, Y., Yu, H., Kijlstra, A., and Yang, P. (2013). Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci, 54(4), 2516–2522.
Yende, S.R., Sannapuri, V.D., Vyawahare, N.S., and Harle, U. N. (2010). Antirheumatoid activity of aqueous extract of Piper nigrum on freund’s adjuvant-induced arthritis in rats. Int J Pharmaceutic Sci Res, 1, 129–133.
Zhang, Q., Yu, Y., Li, J., Guan, Y., Huang, J., Wang, Z., Zhang, Z., Zhang, W., Guo, J., Li, J., Chen, J., and Zhou, Q. (2018). Anti-arthritic activities of ethanol extracts of Circaea mollis Sieb. & Zucc.(whole plant) in rodents. J Ethnopharmacol, 225, 359–366.


