¹Department of Botany, Bishop Heber College (Autonomous), Tiruchirappalli – 17.
²The Rapinat Herbarium and Centre for Molecular Systematics, St. Joseph’s College (Autonomous), Tiruchirappalli-02.India
Article Publishing History
Received: 19/10/2019
Accepted After Revision: 12/12/2019
The present study evaluates the preliminary phytochemical profile of the leaf and stem of Hydnocarpus macrocarpus with reference to their antifungal activity. Antifungal activity was evaluated using Agar-Well Diffusion Method and the preliminary phytochemical screening method was done using Pew’s tests, Alkaline test, Ninhydrin test, Xantho proteins test, Conc. H2SO4 test, Xanthoprotetic test, Biuret test, Mayer’s test, Hager’s test, Wagner’s test Salkowski’s Test, Keller killiani Test, Glycoside Test, Keller kiliani Test Foam Test, Test for Coumarins, Braymer’s Test, Potassium dichromate test and Ferric chloride test. Antifungal activity of leaf and stem was tested with three fungal pathogens. It revealed that the leaf and stem extracts of acetone and methanol showed the highest activity against the pathogens Aspergillus niger and Mucor indicus. Phytochemical screening showed the presence of alkaloids, carbohydrates, flavonoids, steroids, glycosides, phenols, proteins, tannis, saponins, terpenoids, and fixed oil in the leaf and stem of H. macrocarpus. The result obtained in the in vitro methods suggest that H. macrocarpus stem and leaves may be administered for their phytochemical and antifungal activity.
Hydnocarpus macrocarpus, Phytochemical profile, Antifungal activity.
Mariyaraj J, Gideon V. A, Britto S. J, Francis S. Pharmaceutical Activities of Certain Phytochemicals from the Leaf and Stem of Hydnocarpus macrocarpus. Biosc.Biotech.Res.Comm. 2019;12(4).
Mariyaraj J, Gideon V. A, Britto S. J, Francis S. Pharmaceutical Activities of Certain Phytochemicals from the Leaf and Stem of Hydnocarpus macrocarpus. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2Oyu9WC
INTRODUCTION
Medicinal plant products possess unique chemical diversity because of diverse bioactive compounds in them. Constant uses of herbals have led to the effective drug discovery for the treatment of human diseases, (Galm and Shen, 2007, Afolayan 2013 Ganesh et al., 2019). H. macrocarpus is a huge tree, evergreen. The tree is probably harvested from the wild for the gifted seeds, known for medicinal uses. It is an endangered tree confined to southwest India. The prime habitat of the tree has been severely damaged because of anthropogenic pressure as example the Kodayar Hydroelectric Project the establishment of plantation crops in Tamilnadu, South India. Outlying tree populations exist further north towards the Anamalais. The fruits with medicinal properties are stimulants of respiration and enable digestion. In excess, however, they can cause respiratory failure and even death. The leaf and stem also showed high medicinal values inhibiting microbes, both Gram positive and negative, (Ganesh et al 2019).
Botanical descriptions: Trees up to 12 m tall. Bark greyish brown, lenticellate; blaze cream. Branches with architecture of “Aubreville model” branchlet sterete with fallen leaf scars, lenticellate, rusty or greyish stellatetomentose. Leaves simple, alternate, spiral, clustered at twig ends; petiole 2.5 cm; lamina 7.5-10 × 5-7.5 cm, elliptic, folded boat-shaped, apexacute to shortly acuminate, base acute, with very shallow serrations. Inflorescence in racemes, 5-10 cm long, 10 flowered; pedicels 2 cm long, tomentose; petals cream, laciniate; anthers awned Distribution: Southern Peninsula (Western Ghats). Corner, Gard. Bull. Straits Settlem. 10: 319, 325. 1939; Matthew, III. FI. Palni hills t. 81. 1996.
As said earlier leaves and fruits in H. macrocarpus are used as stimulants for respiration and as agents of improved digestion. It is also claimed to be of benefit in the treatment of cancer. Seed powder is used against constipation, irritation and other skin diseases. The oil of H. macrocarpus plays the greatest role in medicinal field, not only the seed but also the leaf and stem as an effective antibiotic, (Ganesh et al., 2019). The investigation of the paper confirms the reported validity of H. macrocarpus in the plant organs of leaf and stem as ant microbial in nature.
MATERIALS AND METHODS
Collection and Authentication
The plant was collected from the Western Ghats, Kerala, India, during April 2017. The plant was identified by Dr. S. John Britto, Director and Head, The Rapinat Herbarium and Center for Molecular Systematics St. Joseph’s College (Autonomous) Tiruchirappalli, India. The voucher specimen RHT: 68237 were deposited at The Rapinat Herbarium.
Extraction of plant material: Leaves were air dried under shade at room temperature, ground with electric grinder into fine powder and stored in air tight container for further use. 10 grams of powdered sample mixed in 150 ml of solvents (i.e. methanol, ethanol, acetone, Chloroform, Petroleum either and water) for extraction, was kept in rotary shaker for three days at room temperature. The extracts were filtered by using Whatmann filter paper then air dried and stored for further usage. The crude extracts were further re-suspended in 1 ml of respective solvents for the investigation of phytochemical and antibacterial activities.
Phytochemical screening and Antifungal activity: For alkaloids: Wagner’s Test: 2 ml of extract was treated with few drops Waner’s reagent. Formation of reddish brown precipitate indicated the presence of alkaloids.
Hager’s Test: 2 ml of extract was treated with few drops of Hager’s reagent (saturated solution of picric acid). Formation of yellow color precipitate signified positive result. Mayer’s Test: 2 ml of extract was treated with few drops of Mayer’s reagent. Formation of cream precipitate indicated the presence of alkaloids. Test for proteins Biuret Test: 2 ml of extract was treated with 2 ml 5%NaOH and 2 ml 1% CuSO4 solutions. Violet or purple coloration indicated presence of proteins and free amino acids. Xanthoprotetic Test: 2 ml of extract was treated with few drops of concentrated HNO3. Formation of yellow color indicated the presence of proteins. Conc. H2SO4 Test: 2 ml extract was treated with few drops of conc. H2SO4. Formation of white precipitate indicated the presence of proteins. Xantho proteins Test: 2 ml of extract was treated with few drops of conc. HNO3 and NH3 solution. Formation of reddish orange precipitate indicated the presence of xantho proteins.
Test for amino acids: Ninhydrin test: 2 ml of extract was treated with 1ml of freshly prepared 0.25% ninhydrin reagent and boiled for few minutes. Formation of blue color indicated the presence of amino acids. Test for flavonoids: Alkaline Test: 2-3 ml of extract was treated with few drops of NaOH solution. Formation of intense yellow color which turned colorless on addition of few drops of dilute HCl. Pew’s tests: 2-3 ml of extract was treated with zinc powder in a test tube, followed by drop wise addition of conc. HCl. Formation of purple, red or cherry color indicates the presence of flavonoids. Lead acetate test: 1 ml extract was treated with 1 ml 10% lead acetate (Pb(OAc)4) solution. Formation of yellow Color precipitate indicated the presence of flavonoid.
Conc.H2SO4 test: 5ml of dilute ammonia solution was added to the extract followed by conc.H2SO4. Yellow color indicated the presence of flavonoids.Test for fixed oils CuSO4 test: 2 ml of extract was treated with 1 ml of 1%CuSO4 solution and 10% NaOH solution. Blue coloration indicated the presence of fixed oils.Test for phenols and tannins Ferric chloride test: 2 ml of extract was treated 2-3 drops of 5% ferric chloride solution. Formation of bluish-black color showed presence of phenols and black color tannins.Potassium dichromate test: 2 ml of extract was treated with 5% potassium dichromate solution. Positive result was confirmed by a formation of brown precipitate (for phenol). Braymer’s Test: 2 ml of extract was treated with 2 mlH2O and followed with 2-3 drops of FeCl3 (5%). Green precipitate proved presence of tannins.Test for Coumarins: 2 ml of extract was treated with 3ml of 10% NaOH solution. Yellow coloration indicated the presence of coumarins. Test for saponins Foam Test: 2 ml extract was diluted with 10 ml of distilled water and warmed gently. It was shaken for 5 minutes. Persistent froth indicated the presence of saponins. The same extract was added with few drops of olive oil. Formation of a soluble emulsion, confirmed the presence of saponins.
Test for Glycosides: Keller kiliani Test (Test for cardiac glycoside): 2 ml extract was treated with 1 ml glacial acetic acid, one drop 5% FeCl3 and 1 ml conc. H2SO4. A brown ring of the interface indicated the presence of cardiac glycosides. Glycoside Test: Small amount of extract was treated with1 ml water and shaken well. Then aqueous NaOH was added. The appearance of yellow color indicated the presence of glycosides.Test for sterols Salkowski’s Test: 2 ml of extract was treated with 2 ml chloroform and 2 ml conc. H2SO4. Chloroform layer appeared red and acid layer showed greenish yellow fluorescence indicated the presence of sterols. Keller killiani Test: (Test for cardiac glycoside): 2 ml extract was treated with 1 ml glacial acetic acid, one drop. 5% FeCl3 and 1 ml conc. H2SO4. A brown ring of the interface indicated the presence of cardiac glycosides. Test for Terpenoids Salkowski’s Test: 2 ml of chloroform and 1 ml ofconc. H2SO4 was added to 1 ml of extract and observed for reddish brown color that indicated the presence of terpenoids.
Antifungal activity: The antifungal assay was carried out in the Tropical Institute of Ecological Sciences, Kottayam, affiliated to Mahatma Gandhi University, Kerala. Selection of Fungal organisms. The pathogenic fungal species were obtained from Tropical Institute of Ecological Sciences, Kottayam, Kerala. Aspergillus fumigate, Aspergillus niger and Mucor sp. Agar-Well Diffusion Method (Murray et al., 1995; Olurinola,1996). Protocol for the antifungal activity was adopted from Murray (1995) in which ethanolic extracts of twenty ethnomedicinal plants were investigated earlier. Olurinola later modified the Method in 1996.
Preparation of Fungal Inoculums: The inoculum was prepared from 5 – 6 days old culture grown on Potato Dextrose Agar Medium (PDA). Petri dishes were flooded with distilled water and conidia were scraped using a sterile spatula to release the spores. The spore density of each fungus adjusted by spectrophotometer (A595 nm) to get a final concentration of approximately 105 spores/ml (Mahesh & Satish, 2008).Culture Media Used> The Potato Dextrose Agar (PDA) medium was used for the antifungal studies. It consisted of following composition (for 1000 ml): –
Potato – 200g, Dextrose – 20g,Agar – 20g, Distilled water – 1000ml
pH – 7.0 The PDA was weighed as per the requirement and was dissolved in 1000 ml of distilled water. The pH was adjusted to 7.0 with a digital pH meter. The medium was kept for boiling until complete dissolution of the ingredients. Then, it was autoclaved for at 151bs pressure and 121°C.Procedure: 20ml PDA containing Petri plates were seeded with the matured culture of fungal strains. Wells were cut using a sterile Cork Borer and 100µl (200µg/well) of extracts were added into the well. For the negative control, distilled water was added to the wells. Then plates were kept for incubation for about a week at room temperature. The antifungal activity was examined by measuring the diameter of the inhibition zone formed around the well in millimeters(mm).
RESULTS AND DISCUSSION
Serving as a phytomedicine, generally plants have contributed to human health and well-being. Firsthand information recorded by ancient physicians were evaluated and detail about the properties and therapeutic values are being investigated in recent times (Shrestha and Dhillion, 2003, Afolayan 2013 and Ganesh et al 2019). The results of qualitative screening of phytochemicals of H. macrocarpus leaf and stem showed the presence of Alkaloids, Carbohydrates, Glycosides, Flavonoids, Phenols, Tannins, and Fixed oils, Sponins, Sterols and Terpenoids. High concentrations of phytochemicals were found in methanolic, ethanolic, acetone and aqueous extracts while a very low concentration in chloroform and petroleum ether extracts (Table 1).
Table 1: Phytochemicals of H. macrocarpus leaf and stem
| Extracts | |||||||||||||||||||||||||
| S.No. | Phytochemical | Acetone | Aqueous | Chloroform | Ethanol | Methanol | Petroleum | ||||||||||||||||||
| constituents | ether | ||||||||||||||||||||||||
| L | S | L | S | L | S | L | S | L | S | L | S | ||||||||||||||
| 1. | Test for Alkaloids | ||||||||||||||||||||||||
| Hager’s Test | ++ | + | ++ | ++ | – | – | ++ | – | ++ | ++ | + | – | |||||||||||||
| Mayer’s Test | ++ | ++ | +++ | ++ | – | – | ++ | – | ++ | ++ | – | – | |||||||||||||
| Wagner’s Test | ++ | ++ | – | ++ | – | – | ++ | + | ++ | ++ | – | + | |||||||||||||
| 2. | Test for Carbohydrates | ||||||||||||||||||||||||
| Molisch’s Test | + | ++ | ++ | + | – | – | + | ++ | + | ++ | – | – | |||||||||||||
| Fehling test | + | – | + | – | – | – | – | + | – | + | – | – | |||||||||||||
| Benedict’s Test | + | + | + | – | – | – | + | + | + | + | – | – | |||||||||||||
| 3. | Test for Flavanoids | ||||||||||||||||||||||||
| Alkaline Test | + | + | + | ++ | – | – | + | – | + | + | – | – | |||||||||||||
| Conc.H2SO4 Test | + | + | ++ | + | – | – | + | + | ++ | + | – | – | |||||||||||||
| Pew’s Test | ++ | ++ | ++ | – | – | – | + | ++ | ++ | ++ | – | – | |||||||||||||
| Lead acetate | ++ | + | ++ | + | – | – | ++ | + | ++ | + | – | – | |||||||||||||
| 4. | Test for fixed oils | ||||||||||||||||||||||||
| CuSO4 Test | ++ | +++ | ++ | ++ | + | + | ++ | ++ | + | ++ | + | + | |||||||||||||
| 5. | Test for Phenols | ||||||||||||||||||||||||
| Ferric chloride Test | +++ | + | ++ | – | – | – | ++ | ++ | ++ | ++ | – | – | |||||||||||||
| Potassium | – | + | – | – | – | – | – | ++ | – | ++ | – | – | |||||||||||||
| Dichromate Test | |||||||||||||||||||||||||
| 6. | Test for Tannins | ||||||||||||||||||||||||
| Ferric chloride Test | – | + | + | – | – | – | + | + | + | + | – | – | |||||||||||||
| Braymer’s Test | + | + | + | – | – | – | + | + | + | + | – | – | |||||||||||||
| 7. | Test for saponins | ||||||||||||||||||||||||
| Foam Test | ++ | + | ++ | + | – | – | ++ | + | + | ++ | + | – | |||||||||||||
| 8. | Test for Glycosides | ||||||||||||||||||||||||
| Keller kiliani Test | ++ | ++ | ++ | + | – | + | ++ | ++ | ++ | ++ | – | – | |||||||||||||
| Glycoside Test | + | + | + | + | – | + | + | + | + | + | – | – | |||||||||||||
| 9. | Test for Coumarins | ||||||||||||||||||||||||
| 10%NaOH Test | + | + | + | + | – | + | + | + | + | + | + | – | |||||||||||||
| 10. | Test for Sterols | ||||||||||||||||||||||||
| Salkowshi’s Test | + | ++ | + | – | + | + | + | ++ | + | ++ | – | – | |||||||||||||
| Keller killiani Test | ++ | ++ | ++ | + | – | + | ++ | ++ | ++ | ++ | – | – | |||||||||||||
| 11. | Test for Proteins | ||||||||||||||||||||||||
| Biuret Test | – | – | – | – | – | – | – | – | – | – | – | – | |||||||||||||
| Xanthoproteic Test | + | + | + | + | + | + | + | + | + | + | – | + | |||||||||||||
| Conc.H2SO4 Test | – | – | – | + | + | – | + | + | + | + | – | – | |||||||||||||
| 12. | Test for Amino acids | ||||||||||||||||||||||||
| Ninhydrin Test | – | – | – | – | – | – | – | – | – | – | – | – | |||||||||||||
| 13. | Test for Terpenoids | ||||||||||||||||||||||||
| Salkowshi’s Test | ++ | ++ | ++ | + | – | + | ++ | ++ | ++ | ++ | – | – | |||||||||||||
Antifungal Activity: Acetone extracts of leaf and stem were tested for in vitro the antifungal activity against Aspergillus fumigatus, Aspergillus niger and Mucor indicus were investigated. Acetone leaf extract showed highest activity for Aspergillus niger (12 ± 1.67). On the other hand the stem extract showed the highest activity for acetone against the pathogen Aspergillus niger (24.33± 0.81). it is seen in the Table 2, figure.Methanol extracts of leaf and stem were tested for in vitro antifungal activity against Aspergillus fumigatus, Aspergillus niger and Mucor indicus were investigated. methanolic leaf extract showed highest activity for Mucor (15.5 ± 0.54). On the other hand the stem extract showed the highest activity for methanol against the pathogen Aspergillus niger (26.33 ± 0.51) figure, Plate.
Table 2: Antifungal Activity
| S. No | Samples Fungal
Strains |
Acetone stem |
Methanol stem |
Acetone leaf |
Methanol leaf |
| 1 | Aspergillus fumigatus | 20.83 ±0.89 | 15.66 ± 0.51 | 7 ± 0 | 11.5 ± 0.54 |
| 2 | Aspergillus niger | 24.33± 0.81 | 26.33 ± 0.51 | 12 ± 1.67 | 13.33 ±2.10 |
| 3 | Mucor indicus | 19.16±1.16 | 11.16 ± 0.75 | 10.1 ± 0.75 | 15.5 ± 0.54 |
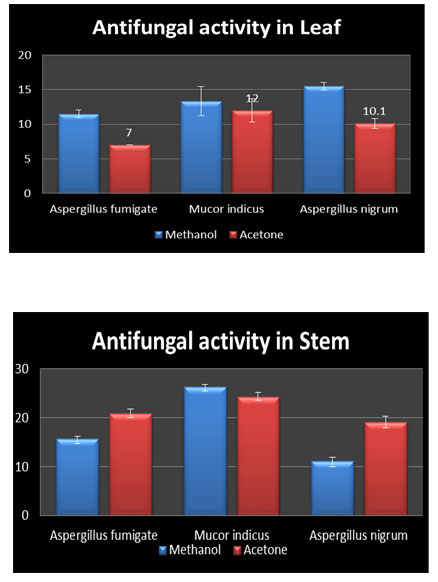 |
Figure 1 |
 |
Plate 1 |
CONCLUSION
The study on the leaf of H. macrocarpa for its phytochemical constituents has revealed the presence of secondary metabolites. Methanol, ethanol, acetone and aqueous are good extractive solvents and the antifungal studies have shown the highest inhibition activity against the pathogens Aspergillus nigrum and Mucor indicus.
ACKNOWLEDGMENT
The authors are thankful to Bishop Heber College (Autonomous) Tiruchirappali and to the Director and Head, The Rapinat Herbarium and Center for Molecular Systematic, St. Joseph’s College (Autonomous) for providing necessary facilities.
REFERENCES
Afolayan AJ, (2013) Extracts from the shoots of Arctotis artotoides inhibit the growth of bacteria and fungi. Pharm. Biol. 41: 22-25
Alam M, Khan H, Samiullah L, Siddique KM. (2012) A review on Phytochemical and Pharmacological Studies of Kundur (Boswellia serrate Roxb exColebr.) -A Unani drug. J App Pharma Sci., 2(3): 148-156.
Bose S, Pal P. (2011) Phytopharmacological and Phytochemical Review of Butea monosperma. Int J of Res in Pharma and Biomed Sci 2(3): 1374-1388.
Bharathi B, Siva Sankar S, Swamidoss Danial. (2010) Incidence of bacterial and fungal coinfections in some HIV infected Indian population. Indian Journal of Biotechnology, 3(2):199.
Bhagwat, M. K., & Datar, A. G. (2014). Antifungal activity of herbal extracts against plant pathogenic fungi. Archives of Phytopathology and Plant Protection, 47(8), 959-965.
Matthew, K.M. (1983). The Flora of Tamilnadu Carnatic.Vol 1.The Rapinat Herbarium, Tiruchirappalli.
Galm, U., & Shen, B. (2007). Natural product drug discovery: the times have never been better. Chemistry & biology, 14(10),1098-1104.
Ganesh M, Sung Gil, Lee Jayabalan Jayaprakas, Murugan Mohankumar, Hyun Tae Jang (2019) Hydnocarpus alpina Wt extract mediated green synthesis of ZnO nanoparticle and screening of its anti-microbial, free radical scavenging, and photocatalytic activity Biocatalyst and Agricultural Biotech Vol 19 https://doi.org/10.1016/j.bcab.2019.101129
Pallithanam, J.P. (2001). A pocket flora of the Sirumalai Hills, South India, The Rapinat Herbarium, Tiruchirappalli.
Janakiraman, M., & Jeyaprakash, K. (2015). Evaluation of Phytochemical Compounds in Leaf Extract of Vitex negundo L. Using TLC, UV-VIS and FTIR Analysis. International Journal of Health Sciences and Research (IJHSR), 5(8), 289-295.
Joseph, B., Kumbhare, P., & Kale, M. (2013). Preliminary phytochemical screening of selected Medicinal Plants. International Research Journal of Engineering and Technology, 1, 55-62.
Kamal, A., & Khan, M. M. R. (2014). Phytochemical evaluation of some medicinal plants. Indian Journal of Plant Sciences, 3(4), 5-8.
Ramesh, B.R. and J.P. Pascal, (1997). Atlas of endemic of the Western Ghats (India): Distribution of tree species in evergreen and semi-evergreen forest. Institute Francias, Pondicherry.
Palei K, Ananta, Nishteswar K, Shukla VJ. (2013). Phytochemical screening of Soymida febrifuga Roxb. (Meliaceae) root bark. Int J of Pharmacy & Life Sciences. 4 (2): 2371-2374.
Malarkodi V, Armstrong, Ravichandran, Jeyakum, Hemalatha,Vijayalakshmi, Srikanth J. (2009). Preliminary phytochemical studies on the stem bark of Soymida febrifuga (Roxb) Res J of Pharmacog and Phytochem. 1(3): 213-216.
Siva N, Ganesan N, Banumathy and Muthuchelian. (2008). Antifungal effect of leaf extract of some medicinal plants against Fusarium oxysporum causing wilt disease of Solanum melogena L .Ethnobotanical Leaflets. 12: 156-163.
Paliwal, S., R. Chauhan, A.A. Siddiqui, S. Paliwal and J. Sharma, (2007). Evaluation of antifungal activity of Salvadora persica Linn, leaves. Natural Product Radiance, 6: 372-374.
Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A. (2010). Antifungal properties of Salvadora persica and Juglansregia L. extracts against oral Candida strains. European Journal of Clinical Microbiology. 29(1): 81-88.
Shrestha, P. M., & Dhillion, S. S. (2003). Medicinal plant diversity and use in the highlands of Dolakha district, Nepal. Journal of Ethnopharmacology, 86(1), 81-96.
Sahoo MR, Dhanabal SP, Jadhav AN, Reddy V, Muguli G, Babu UV, Rangesh P. (2014). Hydnocarpus: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 154(1): 17


