1Department “Plant Protection and Horticulture”, Saratov State Agrarian University named after N.I. Vavilov, Saratov, Russia.
2Department “Plant Protection and Horticulture”, Saratov State Agrarian University named after N.I. Vavilov, Saratov, Russia.
3Department of Plant Protection and Horticulture, Saratov State Agrarian University named after N.I. Vavilov, Saratov, Russia.
4Department “Plant Protection and Horticulture”, Saratov State Agrarian University named after N.I. Vavilov, Saratov, Russia.
5Department of Plant Protection and Horticulture, Saratov State Agrarian University named after N.I. Vavilov, Saratov, Russia.
Corresponding author email: mladenzevv@mail.ru.
Article Publishing History
Received: 12/04/2021
Accepted After Revision: 29/05/2021
Overall, the study of the population dynamics of brown-tail moth (Euproctis chrysorrhoea L.) in the Penza region van help identify the main factors which affect the change in the number of the pest. This scientific approach is necessary for the development of effective methods for the protection of forest plantations. Hence, the chief purpose of this study is to investigate the peculiarities of the population dynamics of brown-tail moth in the plantations of the Penza region. In order to meet the purpose of the study, the examination of the population dynamics of brown-tail moth was carried out in 2018-2020 on permanent stationary test plots laid in various forest inventory and forest growing conditions in Penza region. The solution to this issue was on the basis of quantitative counts with a simultaneous assessment of the identified mortality factors at different age intervals. Arrangement and sampling of trial units was random and systematic according to the existing statistical methodology.
Furthermore, the authors applied generally accepted and specially developed techniques. As a result of the study, examining the population dynamics of brown-tail moth in the plantations of the Penza region led to recognizing 19 different factors of the biotic environment. The regulating role of each of them was established at a specific stage of development of brown-tail moth during ontogenesis. In conclusion, it can be inferred that these results and findings of the study van grant a considerable chance to consider and perform forest protection measures against phytophagous organisms. Some feasible and practical measures are recommended at the end of the article that can be applied in future researches.
Entomophages; Population Dynamics; Population; Survival Rate; The Brown-Tail Moth.
Mladentsev V, Dubrovin V, Eskov I, Ryabushkin Y, Kritskaya E. Peculiarities of the Population Dynamics of Brown-Tail Moth Euproctis chrysorrhoea, in the Plantations of Penza Region of Russia. Biosc.Biotech.Res.Comm. 2021;14(2).
Mladentsev V, Dubrovin V, Es’kov I, Ryabushkin Y, Kritskaya E. Peculiarities of the Population Dynamics of Brown-Tail Moth Euproctis chrysorrhoea, in the Plantations of Penza Region of Russia. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=https://bit.ly/339HA70“https://bit.ly/339HA70</a>
Copyright © Mladentsev et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The brown tail moth (BTM) is a forest and ornamental pest in Russia and the United States. Its ultimate polyphagia and documented phonological shift related to host use recommend the appearance of distinct host races ((Iliiinskii, 1965; Wu et al., 2020). Population genetic variety indices reveal a higher genetic diversity in Russia, predominantly in the samples from Penza region in Russia (Sheikhshoaie et al., 2018; Ali et al., 2020).
The region has all the conditions for the development of brown-tail moth, and in the event of its massive foci, it can cause significant damage to trees and shrubs. Based on the literary sources, brown-tail moth is a poorly studied species throughout the growing area of deciduous plantations (Dubrovin, 2011; Dubrovin, 2016; Sajjadi and Moosavi, 2019; Ramachandran and Rosen, 2020). In the Penza region, this species has practically not been studied (Tachi et al., 2021).
Given the necessity of this matter, the objective of this work is to study the factors of the population dynamics of brown-tail moth population that affect change in the insect population per generation to use the data obtained in the development of effective methods for protecting forest plantations.
MATERIAL AND METHODS
As mentioned earlier, to carry out this survey, the study of the population dynamics of brown-tail moth was conducted over the years 2018-2020 on permanent stationary test plots laid in various forest inventory and forest growing conditions in Penza region. Field survey data were supplemented by laboratory raising of insects. At the same time, a fixed number of the studied phase of ontogenesis was taken, and the percentage of transition of pests to the next phase of development was determined during the observation periods.
The transition to 10% of individuals was noted as the beginning of the next phase of ontogenesis, the transition to 50% of individuals – the mass appearance of the phase, the transition to 80% of individuals – the end of the development period of the phase under study. Thus, the egg mortality was determined by the ratio of hatched caterpillars to the total number of eggs taken from the natural population. Caterpillars were kept in 0.5-liter jars, 2 to 5 caterpillars of II – IV ages each before the emergence of adult insects. The number of caterpillars died from parasites, diseases, and unidentified causes was considered.
The number of caterpillars in the nest was determined using the first developed method, by measuring the circumference of the nest in its widest part and calculating its volume. Further, the actual number of the caterpillars was calculated. The result obtained was converted into a certain unit of the nest volume (Dubrovin & Mladentsev, 2019; Ali et al., 2020). The mortality of caterpillars in wintering nests from various factors, including damage from birds by characteristic pecks, was also determined in laboratory settings. Pupal mortality in natural conditions was registered once a week according to external signs. When a butterfly flew out of the pupa, it formed a large triangular opening on the shell along the borders of the antenna inmantle and chest segments (Dubrovin, 2016; Tachi et al., 2021).
After the emergence of ichneumons, large or small (depending on the type of parasite) rounded holes remained on the shell of the host pupae. The pupae, eaten by tahinas, had irregular holes. The pupae, eaten by the beauties larvae and other predators, had large irregular holes in their different parts. At the end of the survey, the total mortality from parasites, diseases and unidentified causes was determined. The mortality of insects from unspecified causes was determined by the difference in the total number of dead individuals and individuals that died from established causes.
Mortality from interspecies competition was determined in the natural population of pests by keeping caterpillars in isolated sleeves located on the branches of model trees. Feeding caterpillars of the investigated and accompanying insect species, as well as the investigated species alone, were studied in the sleeves. The dead caterpillars without signs of infection and disease, and the difference in mortality were counted to record the total mortality from interspecies competition (Dubrovin, 2016; Ramachandran and Rosen, 2020).
Analysis of the dynamics of the number converted the results of the counts to a unified accounting unit equal to 100 growth points. Material processing and compilation of survival tables were carried out according to the methods (Ali et al., 2020; Ramachandran and Rosen, 2020). Analysis of survival tables determined statistical relationships between population density and survival at different age intervals. The factors of the highest mortality per generation were identified. Mortality from one group of factors over an elementary
period of time: g=nd/nt [1]
where nd – the number of dead individuals,
nt – the total number of individuals counted.
The survival rate from one group of factors was calculated by formula:
W=1–q [2]
The survival rate from one group of factors for the entire observation period was:
Wf=W1+W2+W3+…+Wn [3]
Mortality over the entire observation period:
Q=1–Wf [4]
Survival tables assumed that all factors acting on the populations of the studied insects acted independently of each other.
To identify parasites, predators, and diseases of insects during the research the authors used “A review of braconid ichneumons (Hymenoptera, Braconidae) of the fauna of the USSR”, Iliinskii A.I. Supervision, accounting, and forecast of mass reproduction of needle- and leaf-eating insects in the forests of the USSR / A.I. Iliiinskii, I.V. Tropin. – M.: Forest industry, 1965. – 525 p. (Iliiinskii, 1965), as well as with the help of the staff of the Department of Plant Protection and Horticulture, N.I. Vavilov Saratov State Agrarian University.
RESULTS AND DISCUSSION
The research identified the factors of the natural environment influencing the size of the brown-tail moth population. Among them there are 19 species of entomophages and diseases that affect the decline in the number of brown-tail moth at all stages of development (Table 1).
Table 1. Identified factors of mortality affecting the population dynamics of brown-tail moth
| No. | Mortality factor | Phase when an insect dies | Mortality, % |
| Fam. Chalcidoidae | |||
| 1 | Telenomus laeviusculus Rtzb. | Egg | 18.1 |
| Undefined causes | 17.2 | ||
| Fam. Bracoпidae | |||
| 3 | Meteorus versicolor Wesm. | Caterpillar | 16.2 |
| 4 | Meteorus ictericus Nees. | 9.1 | |
| Fam. Chalcidoidae | |||
| 5 | Eupteromalus nidulans Toer | Caterpillar | 5.3 |
| Fam. Tachiпidae | |||
| 6 | Zenillia libathrix Рапz. | Caterpillar | 25.4 |
| 7 | Blondelia nigripes Fall | 18.4 | |
| 8 | Pteromalus puparium L. | 9.3 | |
| 9 | Pareudora praeceps Mg. | 6.8 | |
| Fam. Chalcidoidae | |||
| 10 | Brachymeria secundaria Rast. | Caterpillar | 7.3 |
| Fam. Carabidae | |||
| 11 | Calosoma inguisitor L. | Caterpillar | 3.4 |
| 12 | Calosoma sycophanta L. | 5.0 | |
| 13 | Birds | Caterpillar | 21.0 |
| Fam. Entomophthoraceae | |||
| 14 | Entomophthora aulicae Reich. род Род Beauveria | Caterpillar | 11.2 |
| 15 | Beauveria bassiana (Bals) Vuill. | 16 | |
| Fam. Braconidae | |||
| 16 | Microgaster calceatus Hal. | Pupa | 5 |
| Fam. Carabidae | |||
| 17 | Calosoma inguisitor L. | Pupa | 8.3 |
| 18 | Calosoma sycophanta L. | 12.4 | |
| Fam. Entomophthoraceae | |||
| 19 | Entomophthora aulicae Reic h. | Pupa | 13.6 |
For a comparative analysis of the factors of population dynamics affecting the brown-tail moth population, the role of each of them was studied. Data obtained were converted to unified accounting units, i.e., per 100 growth points, which facilitated the analysis of population dynamics (Table 2).
Table 2. The survival rate of brown-tail moth over the age intervals
| Unified unit of account
(100 growth points) |
||||
| 2018 | 2019 | 2020 | Mean | |
| Eggs | 178.00 | 154.97 | 132.97 | 155.31 |
| I-III-age caterpillars | 107.15 | 93.29 | 80.05 | 93.50 |
| IV-V-age caterpillars | 12.02 | 9.41 | 9.28 | 10.24 |
| Pupae ♀ + ♂ | 3.44 | 3.68 | 3.59 | 3.57 |
| Sex ratio (I) | 0.58 | 0.46 | 0.43 | 0.49 |
| Pupae ♀ | 1.98 | 1.71 | 1.55 | 1.75 |
| Butterflies ♀ | 1.14 | 0.98 | 0.89 | 1.01 |
In 2018-2020, there was a high number of pests in the studied plantations, the percentage of damage in some cases reached more than 80%. Currently, there has been a decline in the insect population (Dubrovin & Mladentsev, 2019; Ramachandran and Rosen, 2020; Wu et al., 2020). One of the main factors was the biotic factors of the environment, described in the article. Analysis of Table 2 shows the changes the brown-tail moth population undergoes in a generation. From the egg to adult phase, the population size decreases by about 76 times, but due to the high fertility of females the population is restored.
Figure 1: Changes in the abundance of brown-tail moth by development phases in a generation
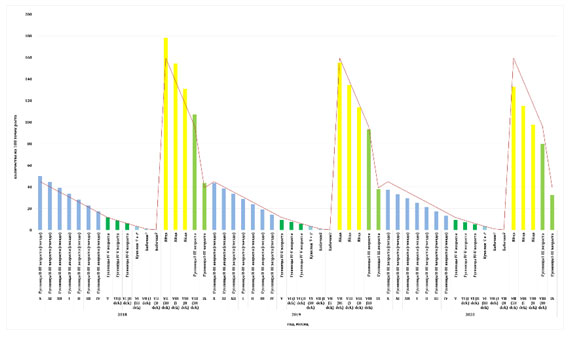
The greatest loss of abundance occurs during the overwintering period of caterpillars and the transition to active feeding, approximately 9 times. In this period of time, there is high mortality from inter- and intraspecific competition. The sex ratio decreased over the years of the study and averaged 0.49, which indicates a gradual transition of the gradation cycle to the phase of population decline. The presented analysis of the survival rate of brown-tail moth over the age interval made it possible to construct a curve of the number of the pest by years of study (Fig. 1). Based on the factors of population dynamics identified in the course of the study, a table of the survival rate of the regulation of the brown-tail moth population over the time interval was compiled (Table 3).
Table 3. The survival rate of brown-tail moth in the plantations of the Penza region
| Age interval x | Initial number of live individuals, lx | Factors causing population decline, dx F | Number of individuals died during the interval, dx | 100 qx (dx to lx ratio, %) |
| 1 | 2 | 3 | 4 | 5 |
| Egg | 155.31 | Parasites | 54.82 | 35.3 |
| Undefined causes | 6.99 | 4.5 | ||
| Total | 61.81 | 39.8 | ||
| I-III-age caterpillars | 93.50 | Parasites | 21.89 | 23.4 |
| Predators | 23.66 | 25.3 | ||
| Diseases | 10.47 | 11.2 | ||
| Birds | 19.66 | 21.0 | ||
| Intra- and interspecies competition | 7.58 | 8.1 | ||
| Total | 83.26 | 89.0 | ||
| IV-V-age caterpillars | 10.24 | Parasites | 4.49 | 43.8 |
| Predators | 0.54 | 5.3 | ||
| Intra- and interspecies competition | 0.50 | 4.9 | ||
| Diseases | 1.64 | 16.0 | ||
| Total | 7.17 | 70.0 | ||
| Pupa | 3.57 | Predators | 0.92 | 25.7 |
| Diseases | 0.49 | 13.6 | ||
| Undefined causes | 0.11 | 3.2 | ||
| Total | 1.52 | 42.5 | ||
| Butterflies ♀ | 1.01 | Undefined causes | 0.65 | 64.5 |
| Total | 0.65 | 64.5 |
Speaking about the total mortality of brown-tail moth in the caterpillar phase, it should be noted that the younger caterpillars died much more often than the older ones. Statistical analysis of the influence of factors of the biotic environment on young caterpillars revealed an inverse relationship between their abundance and survival in this age interval (Fig. 2). In particular, (Iliiinskii, 1965; Ali et al., 2020; Tachi et al., 2021), considering the influence of the parasites of brown-tail moth, the authors established an inverse relationship of the effectiveness of their action on the survival of the pest.
This pattern was found during the parasitization of Eupteromalus nidulans and Apanteles lacteicolor Toer, tachin Zenillia libathrix Rapz, Blondelia nigripes Fall. The data of the listed authors are consistent with our studies. However, as further studies show, the percentage of infection of the caterpillars of brown-tail moth from the listed parasites was maximum during the crisis phase and in the first year of the depression. The research results made it possible to make a similar analysis of the influence of the factors of the dynamics of the brown-tail moth population using diagrams (Fig. 3-6).
Figure 2: Relationship in the number of I-III-age caterpillars per 100 growth points and their survival
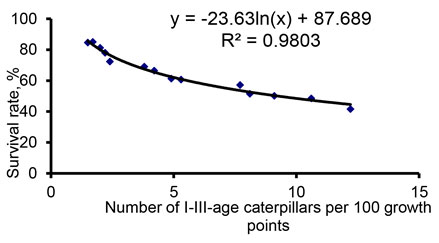
Figure 3: Mortality factors for caterpillars of I-III age
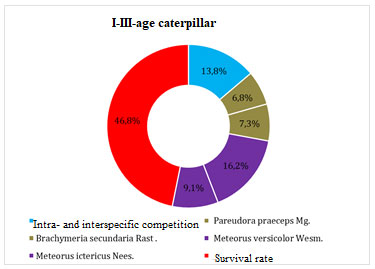
We found that during active feeding, mortality from intraspecific competition of young caterpillars that left the winter nest was 13.8%. The influence on the survival rate of caterpillars from parasites of the tachin family Pareudora praeceps Mg. – 6.8%, Brachymeria secundaria Rast – 7.3%, as well as the braconid family Meteorus versicolor Wesm. – 16.2%, Meteorus ictericus Nees. – 9.1 %, The role of birds in the decrease in the number of caterpillars in the nest was 21%. Mortality was also recorded from intraspecific and interspecific competition – 13.8% and diseases of Entomophthora aulicae Reich. Thus, the total mortality from the listed number of factors at this stage of the pest development was 88.0%.
Figure 4: Mortality factors for caterpillars of IV-V age
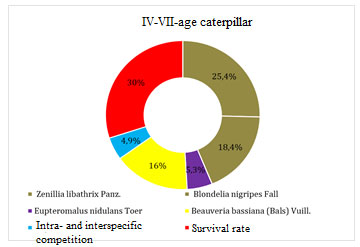
The total mortality of the pest for the given interval of development was 51.6%. A decrease in the number of caterpillars of older ages was caused by parasites of the tachin family Zenillia libathrix Papz. – 25.4%, Blondelia nigripes Fall – 18.4%, and the braconid family Eupteromalus nidulans Toer – 5.3%. Diseases of Beauveria bassiana (Bals) Vuill played an important role in the number of caterpillars – 16.0%. Their death from intra- and interspecific competition was 4.9%.
Figure 5: Mortality factors for caterpillars of IV-V age
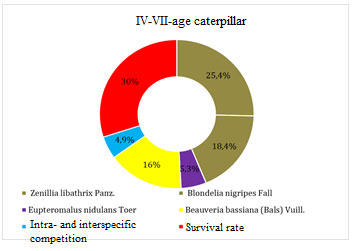
Figure 6. Pupa mortality factors
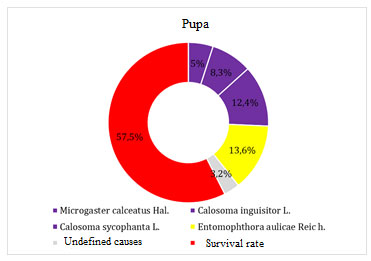
Microgaster calceatus Hal was bred from brown-tail moth pupae with a mortality rate of 5.0%. Predators of pupae Calosoma inguisitor L. and Calosoma sycophanta L. caused death in this phase – 25.7% of pupae. The death of pupae from diseases of Entomophthoraceae, Entomophthora aulicae Reic h. was about 13.6%, of unknown causes – 3.2%. The survival rate of brown-tail moth pupae was 57.5%
Figure 7: Egg mortality factor
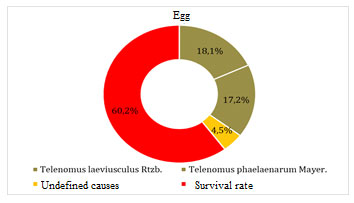
Chalcids Telenomus laeviusculus Rtzb and Telenomus phaelaenarum Mayer greatly influenced the survival rate of brown-tail moth in the egg phase. Total mortality from factors was 35.3%. Initial mortality from abiotic factors was 4.5%. The survival rate of caterpillars in egg-laying after overwintering was 60.2%.
CONCLUSION
Study of the population dynamics of brown-tail moth in the plantations of the Penza region identified 19 different factors of the biotic environment. The regulating role of each of them was established at a specific stage of development of brown-tail moth during ontogenesis. The data obtained will serve as the basis for further study of this pest in the plantations of the Penza region. They will provide an opportunity to plan and effectively carry out forest protection measures against phytophagous organisms.
Conflict of Interests: the authors state that there is no conflict of interest in this study.
REFERENCES
Ali L, Ali A, Prasanth C S and Abass M (2020). Natural enemies as biological control tools for highly infesting apricot pests of Ladakh region.
Dubrovin VV (2011). Phytosanitary monitoring methods for protecting plants from harmful insects. – Saratov, pp 232-250
Dubrovin VV (2016). Organization of plant protection from harmful organisms: a textbook – Saratov. pp. 380-387.
Dubrovin VV and Mladentsev VE (2019). An express method of recording and forecasting of brown-tail moth (Euproktis chrisorrhoea L.) in forest and garden plantations. Agrarian Scientific Journal. No 5 pp 14-17.
Iliiinskii AI (1965). Supervision, accounting, and forecast of mass reproduction of needle- and leaf-eating insects in the forests of the USSR / A.I. Iliiinskii, I.V. Tropin. – M.: Forest Industry. pp. 520- 525.
Ramachandran V and Rosen T (2020). Insecta Class: Caterpillars, Butterflies, Moths. In Dermatological Manual of Outdoor Hazards Springer, Cham. pp. 137-165.
Sajjadi A and Moosavi SM (2019). Synthesis of polymer-coated RDX/AP nano-composites using supercritical CO2. Journal of Medicinal and Chemical Sciences. Vol 1 No1 pp 9-10.
Sheikhshoaie I, Sheikhshoaei M and Ramezanpour S (2018). Synthesis and Characterization of Nano Sized ZnO and CdO by Direct Thermal Decomposition of Their Nano Sized Metal Schiff base Complexes. Chemical Methodologies, Vol 2 No 2 pp 103-113.
Tachi T, Huang Y Z, Komagata S, Araya K, Dawood M M, Pham, T H and Shima, H. (2021). Systematic study of the genus Compsilura Bouché in Southeast and East Asia with morphological and molecular data (Diptera, Tachinidae). Journal of Asia-Pacific Entomology, Vol 24 No 1 pp 285-296.
Wu Y, Bogdanowicz SM, Andres JA, et al., (2020). Tracking invasions of a destructive defoliator, the gypsy moth (Erebidae: Lymantria dispar): Population structure, origin of intercepted specimens, and Asian introgression into North America. Evolutionary Applications, Vol 13 No 8 pp 2056.


