1Amity Institute of Physiotherapy, Amity University, Noida, Uttar Pradesh, India
2Department of Neurology, Paras Hospital, Gurugram, Haryana, India
Corresponding Author Email: drankitjain@ymail.com
Article Publishing History
Received: 28/02/2022
Accepted After Revision: 12/05/2022
The noisy Galvanic vestibular stimulation (nGVS) is established to be a assuring tool to enhance vestibular functioning. Deterioration in vestibular functioning in the geriatric population results in reduced capacity to identify weakened signal which may result into reduction in balance and ultimately fall. Postural sway is produced when nGVS is given over mastoid process. In present study our aim is to find out whether nGVS can be utilized to maximize the outcome of balance training programme among elderly individuals. Community dwelling elderly (N=150, age 65.67±3.4 yrs) were randomly recruited to a control group (Group A, n=50, age 65.54±3.4 yrs), Placebo group (Group B, n=50, age 65.5±3.3 yrs) and a treatment group (Group C, n=50, age 65.98±3.5 yrs). No intervention was provided to Control group while placebo stimulation was given to group B along with balance training exercises and group C was provided with noisy galvanic vestibular stimulation along with balance training exercises.
Pre, mid and post data were recorded on Berg Balance Scale (BBS) for balance and Tinetti’s fall risk scale for risk of fall and analyzed. Compared to control group there was a significant improvement in balance and reduction in risk of fall in placebo and treatment groups. Significant difference was found in treatment group in comparison with placebo group for both, BBS and Tinetti’s fall risk scale. In treatment group early changes in Balance and risk of fall was observed while similar outcomes were not obtained in control and placebo groups. The findings of this study suggests that nGVS can be choosen to optimize the therapeutic efficacy of balance training exercises clinically.
Balance, Elderly, Galvanic Vestibular Stimulation, Rehabilitation & Risk of Fall.
Jain A, Sarkar A, Gupta M. Optimizing Balance Using Vestibular Electrical Stimulation to Study its Therapeutic Effect Among Elderly. Biosc.Biotech.Res.Comm. 2022;15(2).
Jain A, Sarkar A, Gupta M. Optimizing Balance Using Vestibular Electrical Stimulation to
Study its Therapeutic Effect Among Elderly. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3LNqgbV“>https://bit.ly/3LNqgbV</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
One of the greatest health concern among the people aging above 60 years is fall. Significant increase in number of episode of fall has been recorded with advancing age in both genders among all races (Tinetti and Kumar 2010; Thomas et al. 2019a). Fall and related injuries are among the leading cause of decreased ability to care for oneself, functional decline and greater dependence. Fall and related injuries are also found to be associated with prolonged hospitalization which add extra financial burden (WHO 2021).
Multiple intrinsic factors have been classified as a cause of imbalance, resulting in fall among geriatric population. Elderly peoples are prone to have multiple disease associated with vision, hearing, strength and proprioception (Verghese et al. 2006; Henry and Baudry 2019). Vestibular system and its roll in providing a key sensory inputs to stabilize the posture in variety of static and dynamic positions is very well understood. Specialised nerve endings in the vestobular canals detects the head position as well as movement and send signals to the area of brain responsible for processing, planning and coordinating with motor area for anticipatory action (Heuninckx et al. 2008; Mouthon et al. 2018; Ko et al. 2020; Anson and Jeka 2016; Coto et al. 2021).
Significant age associated degeneration has been seen in almost all type of vestibular structure including nerve results into the altered sensitivity (Anson and Jeka 2016; Coto et al. 2021). Other than physical therapy, no effective therapeutic techniques for vestibular system dysfunction have been found to yet (Fujimoto et al. 2016). However, Noisy Galvanic Vestibular Stimulation nGVS has lately demonstrated some promise in this area. Noisy Galvanic Vestibular Stimulation (nGVS) is a technique in which a small electrical current is delivered through electrodes put over the mastoids to stimulate the vestibular afferents nerve (Keywan et al. 2020; Coto et al. 2021).
The vestibular organ is stimulated with a mild noise current in this treatment, which has been found to improve vestibular perception and vestibulo-spinal reflex function. nGVS has been demonstrated to increase cognition in healthy people, enhance motor responsiveness in patients presenting neurodegenerative illnesses, and improve gait metrics and static balance in patients with vestibular abnormalities in earlier investigations (Keywan et al. 2018; Wuehr et al. 2018). In a study it was observed that bipolar biaural GVS to vestibular afferents (anode to the left and cathode to the right) stimulated network of the right hemisphere only while reversing the polarity resulted into bilateral activation (Coats 1972; Coats 1972; Utz et al. 2010). Sensitivity of the vestibular system to normal vestibular inputs can be increased by adding noise to subthreshold stochastic vestibular stimulation (Chen et al. 2021).
This increase in the sensitivity is important for postural stability in more challenging situations. In Humans 0.1-4 mA direct current is used to activate the vestibular afferents resulting in standing subject to lean in different directions depending upon the polarity of the electrodes. If this lean can be utilized upon combining with exercise is not yet studied (Nepveu et al. 2020; Chen et al. 2021).
Multiple evidences are available in support of the activation of otolith system and increase in sympathetic activity after GVS. A binaural application of sinusoidal variant of GVS has shown significant sympathetic nerve activity in lower limb muscles (Hammam and Macefield 2017). The low-frequency changes in vestibular input associated with postural changes, preferentially modify Muscle Sympathetic Nerve Activity (MSNA) (Grewal et al. 2009; Morita et al. 2020). Increase in peripheral vasoconstriction due to MSNA helps to maintain sufficient vascular supply to brain during upright position and this response could be associated with the Otolith system activation (Chen et al. 2021).
Another study suggestive of correlation between vestibular function and sympathetic nerve activity clearly demonstrated the increase in MSNA and calf muscle vascular resistance during head down rotation in prone position (Ray et al. 2002). nGVS can also alters visuomotor activity and motor circuit functioning. This sensorimotor integration and performance might be associated to change in oscillation related to processing of information and error. It has been seen that more erect posture is maintained after mechanical perturbation when appropriate galvanic current is given over the mastoid process (Ap et al. 2001; Lee et al. 2015).
If alteration in sympathetic response of vestibular system (achived thru stimulation) could be integrated along with voluntary control of posture then better postural control can be expected which might reduce the risk of fall. So far none of the study tried to fill this gap of integrating the nGVS to voluntary motor control for better outcome (Mitsutake et al. 2020). The aim of our study was to explore the effect of nGVS among elderly on balance and risk of fall by performing a randomized control trial and to elicit out whether nGVS can be choosen to augment the therapeutic efficacy of balance training exercises clinically among elderly individuals.
MATERIAL AND METHODS
In this study, a repeated measure design and randomized controlled trial were used. A recruitment of total of 150 subjects were done and each were assigned randomly to three groups at various Physiotherapy Centers in Noida. Individuals between 60 and 75 years of age, who could walk independently in the community, perform balancing tests without assistance and take part in several balancing exercise sessions. Our study excluded elderly with a history of any type of orthopaedic surgery in lower extremity, cognitive disorders, on psychoactive medications over the past six months, people with progressive neurological conditions that might have a serious effect on balance and gait, orthostatic hypotension, unstable medical conditions, uncontrolled diabetes, hearing loss, history of vertigo/tinnitus/fall in past twelve months, vision-less than 6/6 in either eye (even if not 6/6 in either eye) and patient with high risk of fall (Berg Balance Score<21, Timed up and Go >14 Sec and Tinetti<19).
A total of 546 volunteers were screened for recruitment in this study initially, of which 150 met the inclusion criteria. The first group was a control group of 50 subjects, 48 (2 drop-outs) were retested at 3rd and 6th-week intervals. Placebo Group B was allocated to 50 subjects who received sham stimulation together with balance training and to 50 other subjects in Group C, receiving (nGVS) along with balance training. A structured interview was conducted to gather socio-demographic information, including age and race. After the group allocation all the subjects carried out with pre, mid and post evaluation on Berg Balance Scale (BBS) and Tinetti’s Fall Risk scale for stability and risk of fall assessment. BBS is a five pointer scale intend to quantitatively assess the balance in older population. BBS Score less than 41 out of 56 is considered as moderate and less than 21 as severely effected balance. Tinetti’s fall risk scale is three pointer scale to assess risk of fall in elderly. Tinetti.s score less than 19 out of 28 indicate high risk of fall. Average assessment time was 45 minute including five minute of rest in between. There were no problem encountered while giving the balancing exercises and nGVS.
A 6-week program of active muscle stretching, endurance Walking, posture control and muscle coordination exercises were given in the group B and C interventions. Exercises began at a low intensity level and progressed slowly. The actual frequency, repetition and resistance of the exercises were modified following the individual ratings of the perceived exercise (equivalent to 11 Borg perceived exercise scale ratings) (Hunter et al. 2020). The follow-up training was conducted at a moderate intensity level (equivalent to 13 Borg perceived exercise scale ratings). These balance exercises were practiced thrice a week for the whole study duration.
Borg rating of perceived exertion (RPE) is used to study the perceived stress during any physical activity (pulse, breathing and excessive sweating); based upon maximum exertion of 20 and minimum of 6 points (12-14 considered as moderate intensity) (Paiva et al. 2019; Hunter et al. 2020).
Group C Participants additionally received bipolar binaural (left-cathodic / right-anodic) noisy Galvanic vestibular stimulation (nGVS) of subliminal intensity for 20 minutes during Exercise training session. A wet lint was placed over the mastoid while doing vestibular stimulation. All statistical analysis of the Berg Balance score and Tinetti’s Fal risk score performed with SAS version 9.4 software. The assumptions of normality are based on the Shapiro-Wilk test. All the individual data parameters have been tested for normality, and the test variables follow a normal population distribution of multiple variables (as assumed by the ANOVA Repeated Measures (RMA) as n>=25). For SAS programming, PROC MIXED was considered to generate results based on residual maximum likelihood (REML) considerations.
The REPEATED statement in PROC MIXED enables the estimation and testing of repeated measurement models with an arbitrary correlation structure for repeated observations. We have 50 subjects in Group-B and Group-C, and we have 48 subjects in Group-A, which has led to unbalanced data and may not turn to symmetric compounds. Since we have only two parameters for each group and visit, this may not lead to an intense computational matrix. Intra-class correlations are generaated from the same model between 14 each difference in treatment with-in and between treatment groups to test the reliability of the results.
Summary of demographic statistics presented based on descriptive statistics N, Mean and Standard deviation of three quartiles (Q1, Q2 and Q3) with minimum and maximum values. All the groups were presented as Control, Placebo and Treatment for Group A, Group B and Group C. All statistical analysis were performed at 95% confidence interval and alpha at 5% acceptance. This study was accepted at Amity University in Noida, Uttar Pradesh India by the Institutional Ethical Committee. All subjects were fully informed about the nature of the research and signed informed consent. The interests of all subjects were secured.
RESULTS AND DISCUSSION
In this study, we assessed the effect of vestibular stimulation on Balance and risk of fall among elderly subjects, randomized into experimental, control and placebo groups. We measured change in balance with Berg Balance scale and change in risk of fall with Tinetti’s fall risk scale. All subjects of both gender included in this study were between 60 to 74 years. The demographics of the subject are summarized in Table 1: age, gender, height, weight and BMI. The age and gender match was done among all the groups.
Table 1. Demographics Summary
| Control (n=50) |
Placebo (n=50) |
Treatment (n=50) |
Total (n=150) |
||
| Age (Years) | Mean (SD) | 65.54 (3.436) | 65.50 (3.388) | 65.98 (3.485) | 65.67 (3.420) |
| Gender | |||||
| Male | n (%) | 27 (54.0%) | 27 (54.0%) | 26 (52.0%) | 80 (53.3%) |
| Female | n (%) | 23 (46.0%) | 23 (46.0%) | 24 (48.0%) | 70 (46.7%) |
| Height (mts) | Mean (SD) | 1.65 (0.051) | 1.67 (0.049) | 1.66 (0.051) | 1.66 (0.050) |
| Weight (kgs) | Mean (SD) | 71.95 (3.081) | 72.23 (3.303) | 71.24 (4.917) | 71.81 (3.852) |
| BMI | Mean (SD) | 26.44 (1.685) | 26.01 (1.319) | 25.99 (1.321) | 26.15 (1.457) |
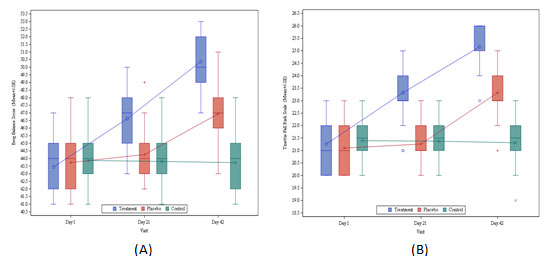
The Least squares Mean values for BBS score, test for Treatment and Placebo arms shows a good improvement from Day-1 to Day 21 and to Day-42 in both, which explains an improvement from baseline to the end visit. Like wise with tinetti’s assessments subjects from “Moderate Risk of Falls” shifted to “Low Risk of Falls” by Day42. The BBS score improved by 3.1 points from Day 1 to Day 21 and 3.7 points from Day 21 to Day 42 indicating a gain of nearly 7 points in the Treatment group. While in the placebo group a gain of 3.2 points was observed by Day 42, and with no change in the control group.
The treatment contrast based on LS Mean Estimate (Standard Error) is -6.940 (0.185) with 95% CI of [-7.31;-6.57], at Day 42 from Day-1. LS mean is higher in Day 42 with LS Mean Estimate (Standard Error) is 25.160 (0.124) with 95% CI of [24.91;25.41], and thus there is a 3.9 increase in Tinetti’s score at Day 42 from Day-1. The test is statistically significant as P‐value is less than 0.05 (p<0.0001). For follow‐up comparisons between pairs of time points, the Tukey’s-Kramer method is considered for the adjustment and it also shows statistical significance (p<0.001). The treatment and placebo effect show statistical significance (P<0.05) and thus we reject the null hypothesis and can say there is a significant difference for both Treatment and placebo groups within the visits (Chen et al. 2021).
Table 2. Pre, Mid and Post by shift difference – within Group – Berg Balance Score
| Estimate | SE | 95% CI | P-value | |
| Treatment With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | 0.580 | 0.177 | [ 0.23; 0.93] | 0.0015 |
| Mid-Post vs. Pre-Post Difference | 3.760 | 0.192 | [ 3.38; 4.14] | <0.0001 |
| Pre-Mid vs. Pre-Post Difference | 3.180 | 0.177 | [ 2.83; 3.53] | <0.0001 |
| Placebo With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | 2.160 | 0.100 | [ 1.96; 2.36] | <0.0001 |
| Mid-Post vs. Pre-Post Difference | 2.680 | 0.131 | [ 2.42; 2.94] | <0.0001 |
| Pre-Mid vs. Pre-Post Difference | 0.520 | 0.100 | [ 0.32; 0.72] | <0.0001 |
| Control With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | -0.021 | 0.047 | [-0.11; 0.07] | 0.6588 |
| Mid-Post vs. Pre-Post Difference | -0.083 | 0.056 | [-0.19; 0.03] | 0.1370 |
| Pre-Mid vs. Pre-Post Difference | -0.063 | 0.047 | [-0.16; 0.03] | 0.1872 |
Table 3. Pre, Mid and Post by shift difference – within Group – Tinetti’s Fall Risk Scale
| Estimate | SE | 95% CI | P-value | |
| Treatment With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | -0.220 | 0.127 | [-0.47; 0.03] | 0.0867 |
| Mid-Post vs. Pre-Post Difference | 1.840 | 0.138 | [ 1.57; 2.11] | <0.0001 |
| Pre-Mid vs. Pre-Post Difference | 2.060 | 0.127 | [ 1.81; 2.31] | <0.0001 |
| Placebo With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | 1.900 | 0.084 | [ 1.73; 2.07] | <0.0001 |
| Mid-Post vs. Pre-Post Difference | 2.060 | 0.105 | [ 1.85; 2.27] | <0.0001 |
| Pre-Mid vs. Pre-Post Difference | 0.160 | 0.084 | [-0.01; 0.33] | 0.0600 |
| Control With Group Difference | ||||
| Pre-Mid vs. Mid-Post Difference | -0.042 | 0.033 | [-0.11; 0.02] | 0.2052 |
| Mid-Post vs. Pre-Post Difference | -0.063 | 0.040 | [-0.14; 0.02] | 0.1218 |
| Pre-Mid vs. Pre-Post Difference | -0.021 | 0.033 | [-0.09; 0.04] | 0.5251 |
A table 2 and table 3 shows the evaluation of BBS and Tinetti’s fall risk score respectively for the three groups. The Pre-Post difference is high in Placebo group with respect to control group. The finding of this study is like those of many researchers who have identified lower limb strength and balance training as effective ways to clinically reduce the risk of falling. Almost all research that look at the risk of falling among the elderly concluded that physical activity, including leisure exercises, are efficient and productive ways to restore balance and prevent falls (Thomas et al. 2019b). Results obtained in the treatment group indicate the added improvement of balance and reducing risk of fall up on combining galvanic stimulation to the existing balance training program. Since the balance is directly associated postural sway which is a functionof vestibular system (Chen et al. 2021).
This finding is in support of those studies who reported improvement in vestibular function after giving nGVS. As patients with vestibular problems have longer postural sway route lengths and mean velocities, the data suggest that increased vestibular afferent function may have contributed to the reduced postural sway seen in this investigation (Talebi et al. 2016). The reduction of postural sway during nGVS may be due to the activation of cortical areas involved in multimodal input, including vestibular information (Piccolo et al. 2020; Chen et al. 2021). Interestingly we found increasing improvement over the whole duration of treatment nearing to the clinically significant levels in contrast to the study who found no improvement in balance after administering nGVS. The clinical improvement of balance in our study can be understood as we have used subliminal intensity induces imperceptible vestibular stimulation along with voluntary motor task in contrats to the higher intensity of unpleasant perception delivered for short duration before the functional exercises (Hassan et al. 2021).
Furthermore, GVS-induced more afferent vestibular excitement can activate brain areas related to multisensory input (areas 2, 3a/b, and 7, as well as the parieto-insula vestibular cortex) via delivering direct current through the vestibular nuclei in the brainstem and vestibular thalamus (Inukai et al. 2018). GVS with an alternating current can also activate parts of the brain that interpret vestibular information for head and body positioning in space (i.e. the supramarginal gyrus, posterolateral thalamus, cerebellar vermis, posterior insula and hippocampus). Stimulating brain areas along with peripheral voluntary contraction during exercise has been offered as a possible explanation (Ferreira et al. 2019; Helmchen et al. 2020; Chen et al. 2021).
Table 4. Between the groups shift difference – Treatment estimates – Berg Balance Score
| Estimate | SE | 95% CI | P-value | |
| Between Treatment group effects | ||||
| Treatment Pre-Mid vs Placebo Pre-Mid | -2.660 | 0.158 | [-2.97;-2.35] | <0.0001 |
| Treatment Pre-Mid vs Control Pre-Mid | -3.239 | 0.162 | [-3.56;-2.92] | <0.0001 |
| Placebo Pre-Mid vs Control Pre-Mid | -0.579 | 0.160 | [-0.89;-0.26] | 0.0003 |
| Treatment Mid-Post vs Placebo Mid-Post | -1.080 | 0.158 | [-1.39;-0.77] | <0.0001 |
| Treatment Mid-Post vs Control Mid-Post | -3.842 | 0.162 | [-4.16;-3.52] | <0.0001 |
| Placebo Mid-Post vs Control Mid-Post | -2.762 | 0.160 | [-3.08;-2.45] | <0.0001 |
| Treatment Pre-Post vs Placebo Pre-Post | -3.740 | 0.158 | [-4.05;-3.43] | <0.0001 |
| Treatment Pre-Post vs Control Pre-Post | -7.085 | 0.162 | [-7.40;-6.77] | <0.0001 |
| Placebo Pre-Post vs Control Pre-Post | -3.345 | 0.160 | [-3.66;-3.03] | <0.0001 |
Table 5. Between the groups shift difference – Treatment estimates – Tinetti’s Fall Risk Scale
| Estimate | SE | 95% CI | P-value | |
| Between Group Difference | ||||
| Treatment Pre-Mid vs Placebo Pre-Mid | -1.900 | 0.112 | [-2.12;-1.68] | <0.0001 |
| Treatment Pre-Mid vs Control Pre-Mid | -2.089 | 0.114 | [-2.31;-1.86] | <0.0001 |
| Placebo Pre-Mid vs Control Pre-Mid | -0.189 | 0.113 | [-0.41; 0.03] | 0.0959 |
| Treatment Mid-Post vs Placebo Mid-Post | 0.220 | 0.112 | [-0.00; 0.44] | 0.0503 |
| Treatment Mid-Post vs Control Mid-Post | -1.905 | 0.114 | [-2.13;-1.68] | <0.0001 |
| Placebo Mid-Post vs Control Mid-Post | -2.125 | 0.113 | [-2.35;-1.90] | <0.0001 |
| Treatment Pre-Post vs Placebo Pre-Post | -1.680 | 0.112 | [-1.90;-1.46] | <0.0001 |
| Treatment Pre-Post vs Control Pre-Post | -3.984 | 0.114 | [-4.21;-3.76] | <0.0001 |
| Placebo Pre-Post vs Control Pre-Post | -2.304 | 0.113 | [-2.53;-2.08] | <0.0001 |
Findings (Table 4 and 5) of this study suggest the consistent and added improvement during two halves of the study period in the treatment group indicate no adaptation and carry over effect as we have used small, repeated session considering neural adaptation as in previous studies relatively negligible difference in effects were found after vestibular stimulation for longer duration of three hours compared to thirty minutes (Fujimoto et al. 2016; McLaren et al. 2021). Appropriateness of nGVS for repetitive treatment sessions for bringing change in balance can be understood due to long lasting effects, non-invasion and absence of adverse effects. Though this study does not determine that small duration of stimulation can bring the optimal therapeutic effects.
But we advocate the further exploration of factors which helps to identify appropriate duration to bring lasting effects among various subject population and with various vestibular disorders. No difference in the first half of study duration among control and placebo group indicationg balance training exercise alone is insufficient to bring early detectable change in balance. This could be explained as lack of cortical excitability during balance training exercise may delay the changes to reflect clinically. Cortical excitability achived in treatment group resulted in enhancement in motor evok potential targeting the lower limb muscles elicited resting motor threshold could be the possible explanation (Fleming et al. 2018; Kudo et al. 2022).
Figure 1: Box plot – By groups across the visits – (A) Berg Balance Scale (B) Tinetti’s fall risk score
We observed (Fig. 2) early improvement upon adding noise to the stimulation which is further supported by the findings of other studies who reported Stochastic resonance, a process in which a signal that is too weak to exceed a specific threshold is amplified by adding noise, is thought to be the reason for these ameliorating effects of nGVS (M et al. 2017). The sensory system’s information processing appears to be aided by stochastic resonance. Proscessing of subthreshold signals is augmented by lowering the vestibular detection threshold upon adding noise to GVS (Wuehr et al. 2018). Identification and processing of the subthreshold signal which helps to modulate MSNA can result into the motor firing in the muscles of the lower limb (Fleming et al. 2018; Kudo et al. 2022).
If this rmotor recruitment is integrated with exercises including static and dynamic voluntary control of different posture and repeated regularly, it could have been resulted into the extensive neuroplasticity in the vestibular system. Stimulating Vestibular end organs excite the ipsilateral extensor motor neuron and inhibit the reciprocal flexor motor neuron thru vestibular nuclei via lateral vestibulospinal tract (Puyal et al. 2003). The signal from vestibular nuclei then passes to the vestibular thalamus resulting in recruitment of brain areas associated with multisensory input (Utz et al. 2010). Activation of brain areas associated with vestibulospinal relay and sensory vestibular input might have resulted into the improvement in the balance during various task involved in balance assessment. A study on animal model demonstrate that the stimulating vestibular neuron can induce long term potentotiation (LTP) and long term depression (LTD) of vestibular nuclei field potential (Grassi and Pettorossi, 2001; Smith et al. 2020; Kudo et al. 2022).
In general there are multiple evidences available which establish the benefits of nGVS for improving balance and reducing risk of fall, but excat mechanism is still lacking a strong evidence. Due to the complexity of the functioning of the vestibular organ, sometime it is also thought to be the involvement of multiple mechanism. Evidence for modulation of vestibular hair cell activity is available but further what frequency and intensity is appropriate is still yet to know.
Exercises included in the groups were advised based upon the rate of perceived exertion which was variable for individuals considering age and no prior involvement in any exercise regime, but it is important to explore further in future about the effects of nGVS keeping exercises regime constant for all or selecting the subjects with similar level of physical activity at the stage of inclusion. Although various methods of delivering galvanic stimulation is used by different researchers, we considered transcranial delivery of current to be more appropriate due to non invasive and no side effects, but consensus is lacking which method is most effective. No episode of fall had been reported during the study period (Steinhardt and Fridman 2021; Kudo et al. 2022).
CONCLUSION
The findings of this study suggest that vestibular electrical stimulation can significantly improve balance and reduce risk of fall among elderly individual. We can also conclude from the findings of this study that vestibular electrical stimulation also brings the early improvement in balance and can be used as therapeutic tool among elderly with impaired balance. Findings suggest that the improvement in the balance and reduction in risk of fall is not a placebo effect of vestibular stimulation. The finding of this study may be useful in further exploring what frequency, intensity and duration is appropriate to have optimal benefit.
Conflict of Interests: Authors declare no conflict of interests to disclose.
ACKNOWLEDGEMENTS
The valuable contributions for this study were provided by Prof. Jasobanta Sethi, Director Amity Institute of Physiotherapy, Amity University, Uttar Pradesh, India.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
Author Ethical Statement: This study was approved at Amity University in Noida, Uttar Pradesh by the University Ethics Committee on 25 July 2016. All subjects were fully informed about the nature of the research and signed informed consent. The interests of all subjects were secured.
REFERENCES
Anson E and Jeka J (2016). Perspectives on Aging Vestibular Function. Frontiers in Neurology 6, 269. https://doi.org/10.3389/fneur.2015.00269
Scinicariello A, Eaton K, Inglis J et al. (2001). Enhancing human balance control with galvanic vestibular stimulation. Biol Cybern 84, 475–480. https://doi.org/10.1007/PL00007991
Chen H, Hu Z, Chai Y, et al. (2021). Galvanic vestibular stimulation with low intensity improves dynamic balance, Translational Neuroscience, vol. 12, no. 1, pp. 512-521. https://doi.org/10.1515/tnsci-2020-0197
Chen PY, Jheng YC, Wang CC et al. (2021). Effect of noisy galvanic vestibular stimulation on dynamic posture sway under visual deprivation in patients with bilateral vestibular hypofunction. Sci Rep 11, 4229. https://doi.org/10.1038/s41598-021-83206-z
Coats AC (1972). The sinusoidal galvanic body-sway response. Acta Otolaryngol 74, 155–162. https://doi.org/10.3109/00016487209128436
Coats AC, (1972). Limit of Normal of the Galvanic Body-Sway Test. Ann Otol Rhinol Laryngol 81, 410–416. https://doi.org/10.1177/000348947208100312
Coto J, Alvarez CL, Cejas I et al. (2021). Peripheral vestibular system: Age-related vestibular loss and associated deficits. Journal of Otology 16, 258–265. https://doi.org/10.1016/j.joto.2021.06.001
Fleming MK, Theologis T, Buckingham R et al. (2018). Transcranial direct current stimulation for promoting motor function in cerebral palsy: a review. Journal of NeuroEngineering and Rehabilitation 15, 121. https://doi.org/10.1186/s12984-018-0476-6
Fujimoto C, Yamamoto Y, Kamogashira T et al. (2016). Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep 6, 37575. https://doi.org/10.1038/srep37575
Grassi S and Pettorossi VE (2001). Synaptic plasticity in the medial vestibular nuclei: role of glutamate receptors and retrograde messengers in rat brainstem slices. Prog. Neurobiol. 64, 527–553. https://doi.org/10.1016/s0301-0082(00)00070-8
Grewal T, James C and Macefield VG (2009). Frequency-dependent modulation of muscle sympathetic nerve activity by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res 197, 379–386. https://doi.org/10.1007/s00221-009-1926-y
Hammam E and Macefield VG (2017). Vestibular Modulation of Sympathetic Nerve Activity to Muscle and Skin in Humans. Frontiers in Neurology 8, 334. https://doi.org/10.3389/fneur.2017.00334
Hassan FM, Elaziz AA, Obaya HE et al. (2021). The impact of galvanic vestibular stimulation on elderly balance. European Journal of Molecular & Clinical Medicine 8, 1833–1846.
Helmchen C, Machner B, Rother M et al. (2020). Effects of galvanic vestibular stimulation on resting state brain activity in patients with bilateral vestibulopathy. Human Brain Mapping 41, 2527–2547. https://doi.org/10.1002/hbm.24963
Heuninckx S, Wenderoth N and Swinnen SP (2008). Systems Neuroplasticity in the Aging Brain: Recruiting Additional Neural Resources for Successful Motor Performance in Elderly Persons. J. Neurosci. 28, 91–99. https://doi.org/10.1523/JNEUROSCI.3300-07.2008
Hunter GR, Neumeier WH, Chandler-Laney PC et al. (2020). Ratings of Perceived Exertion During Walking Predicts Endurance Independent of Physiological Effort in Older Women. J Strength Cond Res 34, 1340–1344. https://doi.org/10.1519/JSC.0000000000003268
Inukai Y, Masaki M, Otsuru N, et al. (2018). Effect of noisy galvanic vestibular stimulation in community-dwelling elderly people: a randomised controlled trial. Journal of NeuroEngineering and Rehabilitation 15, 63. https://doi.org/10.1186/s12984-018-0407-6
Keywan A, Badarna H, Jahn K, et al. (2020). No evidence for after-effects of noisy galvanic vestibular stimulation on motion perception. Sci Rep 10, 2545. https://doi.org/10.1038/s41598-020-59374-9
Keywan A, Wuehr M, Pradhan C, et al. (2018). Noisy Galvanic Stimulation Improves Roll-Tilt Vestibular Perception in Healthy Subjects. Front. Neurol. 9, 83. https://doi.org/10.3389/fneur.2018.00083
Ko LW, Chikara RK, Chen PY et al. (2020). Noisy Galvanic Vestibular Stimulation (Stochastic Resonance) Changes Electroencephalography Activities and Postural Control in Patients with Bilateral Vestibular Hypofunction. Brain Sciences 10, 740. https://doi.org/10.3390/brainsci10100740
Kudo D, Koseki T, Katagiri N et al. (2022). Individualized beta-band oscillatory transcranial direct current stimulation over the primary motor cortex enhances corticomuscular coherence and corticospinal excitability in healthy individuals. Brain Stimulation 15, 46–52. https://doi.org/10.1016/j.brs.2021.11.004
Lee S, Kim D, Svenkeson D, et al. (2015). Multifaceted Effects of Noisy Galvanic Vestibular Stimulation on Manual Tracking Behavior in Parkinson’s Disease. Frontiers in systems neuroscience 9, 5. https://doi.org/10.3389/fnsys.2015.00005
Wuehr M, Decker J and Schniepp R (2017). Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol 264, 81–86. https://doi.org/10.1007/s00415-017-8481-4
McLaren R, Smith PF, Lord S et al. (2021). Noisy Galvanic Vestibular Stimulation Combined With a Multisensory Balance Program in Older Adults With Moderate to High Fall Risk: Protocol for a Feasibility Study for a Randomized Controlled Trial. JMIR Research Protocols 10, e32085. https://doi.org/10.2196/32085
Mitsutake T, Sakamoto M, Ueta K et al. (2020). Standing postural stability during galvanic vestibular stimulation is associated with the motor function of the hemiplegic lower extremity post-stroke. Topics in Stroke Rehabilitation 27, 110–117. https://doi.org/10.1080/10749357.2019.1667662
Morita H, Kaji H, Ueta Y et al. (2020). Understanding vestibular-related physiological functions could provide clues on adapting to a new gravitational environment. J Physiol Sci 70, 17. https://doi.org/10.1186/s12576-020-00744-3
Mouthon A, Ruffieux J, Mouthon M, et al. (2018). Age-Related Differences in Cortical and Subcortical Activities during Observation and Motor Imagery of Dynamic Postural Tasks: An fMRI Study. Neural Plasticity 2018, e1598178. https://doi.org/10.1155/2018/1598178
Nepveu JF, Mikhail Y, Pion CH et al. (2020). Assessment of vestibulocortical interactions during standing in healthy subjects. PLOS ONE 15, e0233843. https://doi.org/10.1371/journal.pone.0233843
Paiva PC, Figueiredo CA, Reis-Silva A et al. (2019). Acute and Cumulative Effects With Whole-Body Vibration Exercises Using 2 Biomechanical Conditions on the Flexibility and Rating of Perceived Exertion in Individuals With Metabolic Syndrome: A Randomized Clinical Trial Pilot Study. Dose-Response 17(4), 1559325819886495. https://doi.org/10.1177/1559325819886495
Piccolo C, Bakkum A, Marigold DS (2020). Subthreshold stochastic vestibular stimulation affects balance-challenged standing and walking. PLOS ONE 15(4), e0231334. https://doi.org/10.1371/journal.pone.0231334
Puyal J, Grassi S, Dieni C et al. (2003). Developmental shift from long-term depression to long-term potentiation in the rat medial vestibular nuclei: role of group I metabotropic glutamate receptors. J Physiol 553, 427–443. https://doi.org/10.1113/jphysiol.2003.051995
Ray CA and Monahan KD, (2002). The vestibulosympathetic reflex in humans: neural interactions between cardiovascular reflexes. Clin Exp Pharmacol Physiol 29, 98–102. https://doi.org/10.1046/j.1440-1681.2002.03614.x
Ferreira IS, Costa BT, Ramos CL et al. (2019). Searching for the optimal tDCS target for motor rehabilitation. J NeuroEngineering Rehabil 16, 90. https://doi.org/10.1186/s12984-019-0561-5
Smith PF, Truchet B, Chaillan FA et al. (2020). Vestibular Modulation of Long-Term Potentiation and NMDA Receptor Expression in the Hippocampus. Frontiers in Molecular Neuroscience 13. https://doi.org/10.3389/fnmol.2020.00140
Steinhardt CR and Fridman GY (2021). Direct current effects on afferent and hair cell to elicit natural firing patterns. iScience 24 (3), 102205. https://doi.org/10.1016/j.isci.2021.102205
Talebi H, Karimi MT, Abtahi SHR et al. (2016). Static Balance in Patients with Vestibular Impairments: A Preliminary Study. Scientifica (Cairo) 2016, 6539858. https://doi.org/10.1155/2016/6539858
Thomas E, Battaglia G, Patti A et al. (2019a). Physical activity programs for balance and fall prevention in elderly. Medicine (Baltimore) 98, e16218. https://doi.org/10.1097/MD.0000000000016218
Thomas E, Battaglia G, Patti A et al. (2019b). Physical activity programs for balance and fall prevention in elderly. Medicine (Baltimore) 98, e16218. https://doi.org/10.1097/MD.0000000000016218
Tinetti ME and Kumar C (2010). The Patient Who Falls. JAMA 303, 258–266. https://doi.org/10.1001/jama.2009.2024
United Nations (2019). World Population Ageing. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
Utz KS, Dimova V, Oppenländer K et al. (2010). Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology–a review of current data and future implications. Neuropsychologia 48, 2789–2810. https://doi.org/10.1016/j.neuropsychologia.2010.06.002
Wuehr M, Boerner JC, Pradhan C et al. (2018). Stochastic resonance in the human vestibular system – Noise-induced facilitation of vestibulospinal reflexes. Brain Stimul 11, 261–263. https://doi.org/10.1016/j.brs.2017.10.016


