Department of Environmental Science, Lucknow University, Lucknow India
Corresponding author email: shwetayadav2008@gmail.com
Article Publishing History
Received: 11/10/2019
Accepted After Revision: 30/12/2019
Metallic entities with quiet a heavier molecular weight have been a part of environment with a subtle existence, but their haphazard use for mankind purposes has amended the natures balance in two of the ways, whether it may be biochemical or geochemical. Outcome from these activity speaks loudly about their excess release in the form nickel, zinc cadmium, copper, lead, and others into the environment. Exposure with them for longer durations and with hugely growing accumulation rates portrays deleterious effects on aquatic forms of life as well as human life forms. Microorganisms with their versatile metabolic activities are somehow indulged in their remediation this has been well studied and implemented till now. The present review is an attempt to scrutinize the capability of microorganisms and as well as plants to remediate the compilation of heavy metals. In accordance to build up a referable literature this review will also show insight to physical and chemical ways. A microorganism when studied at the molecular level gives insight that they accumulate heavy metals and denigrate them at their sites where they are present. The present literature and work review will discuss benevolent strategies to tackle compilations of heavy metals. Microbial life forms which are used for the removal of heavy metals from the water bodies include bacteria, fungi, algae and yeast. Some important antioxidants such as flavonoids, pectin and phytic acid are also used for the elimination of the heavy metals from the human body. The present article is an extensive review that will offer a number of strategies and possible mechanisms for the heavy metals removal both from environment as well as from human body.
Metallic, Flavonoids, Physical, Bioremediation
Yadav S. On the Physical Chemical and Bioremediation Methods for the Removal of Certain Heavy Metals. Biosc.Biotech.Res.Comm. 2019;12(4).
Yadav S. On the Physical Chemical and Bioremediation Methods for the Removal of Certain Heavy Metals. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2p5tdAn
INTRODUCTION
When the density of metallic constitution is 5, i.e., heaviest it belongs to the category of heavy metals. They are important and effective in various practical applications. Sometimes they are part of our diet just because they are very essential to be consumed in trace amounts. Blood iron is usually 0.06-0.6 mg/l, while zinc is 4-8 mg/l , Molybdenum 5-157 micrograms/ Litre, cobalt is 0.09-0.46 µg/ litre, chromium0.05-0.5 µg/ litre, manganese 6.7-10.4 µg/ litre Caroli et al. (1994); Minola et al. (1990). Several other elements called as ultra-trace element are present in very lesser amount like 1µg/g of a 40-290 µg/ litre of lead 1 – 1.14 µg/ litre of Nickle, vanadium 0.1-0.9 µg/ litre, ( Nielsen et al. 1984; Rodushkin et al. 1999, Ontarion et al., 2018, Rether and Schuster (2019) and Magda (2019).
Few heavy metals which are either metals or semi metallic components (Arsenic) have toxic level as follows:-
Table 1: The MCL standards for the most hazardous heavy metals
| Heavy metals | Toxicities | MCL(mg/l) |
| Arsenic | Skin manifestations , Visceral Cancers ,Vascular diseases | 0.050 |
| Cadmium | Kidney damage , renal disorder, human Carcinogen | 0.01 |
| Chromium | Headache, diarrhoea , nausea, vomiting, carcinogenic | 0.05 |
| Copper | Liver damage, Wilson disease, insomnia | 0.25 |
| Nickel | Dermatitis , nausea, chronic asthama,human carcinogen | 0.20 |
| Zinc | Depression, Lethargy,neurological signs and increased | 0.80 |
| Lead | Damage the fetal brain, diseases of the kidneys, circulatory system and nervous system | 0.0060 |
| Mercury | Rheumatoid arthritis and diseases of the kidneys circulatory systems and nervous systems | 0.00003 |
The above MCL standards were given by Babel and Kurniawan, (2003).To eliminate the trace of heavy metals from any sort of components on the basis of suitable conditions can be categorized as:
Physical methods
These methods are being used by numerous researchers to remove the heavy metals. These are primarily efficient and applied on particulate form of metals, distinct particles or metal containing particles, Dermont et al. (2008). The technique consist mechanical screening, hydrodynamic categorization, gravity concentration, magnetic separation, flotation, electrostatic separation, and attrition scrubbing (Dermont et al. (2008). The effectiveness of physical separation is result of different soil characteristics such as particle size distribution, shape of particle , clay content, humidity content , moisture content , density between soil matrix and metal contaminants, heterogeneity of soil matrix, magnetic properties, and hydrophobic properties of particle surface Smith et al.(1995); Williford et al. (2000) Rether and Schuster (2019).
Coagulation and Flocculation
This mechanism is based on zeta potential (ζ) measurement as the condition to define the electrostatic interaction between pollutants and coagulant – flocculants agents (Maldonado et al. (2014). Coagulation process reduces the net surface charge present on the colloidal particles to stabilize via electrostatic repulsion process Benefield et al. (1982). In flocculation process, the particle size increases frequently due to collisions and interaction of various inorganic polymers formed by addition of organic polymers Tripathy (2006). After the changing of distinct particles into huge particles, they can be filtered easily or removed by the process of straining or floatation.
Drawback of this procedure could be production of sludge, chemical applications and application of toxic compounds into solid phase.
Electrochemical Treatments Electrolysis
one of the technologies used for removal of metals is electrolytic recovery. In this process, electric current is passed through an aqueous metal having solution by the help of an indissoluble anode and a cathode plate. The movement of electrons from cathode to anode generates electricity. The heavy metals got precipitated in a weak acidic or neutralized catholyte by forming hydroxides. Electro-deposition, electro-flotation, electro-oxidation and electro-coagulation are some types of electrochemical treatment of water Shim et al. (2014 ) Magda (2019).
Electro destabilization : Some solids can adsorb both positively and negatively charged ions from an electrolyte solution, and then releases other ions with the same charge in an equal amount into the solution, such as ion exchange resins, in which ions like sodium and hydrogen are interchanged with positively charged ions like nickel, copper and zinc ions present in the solution. In the same way, the negatively charged ions in the resins like hydroxyl and chloride are replaced by negatively charged ions such as chromate, nitrate, cyanide, sulphate and dissolved organic carbon (DOC).
Membrane filtration
This technique is mostly considered and used for the inorganic effluent’s treatment. Suspended solids, organic and inorganic components (heavy metals) can be removed by the process. There are so many types of membrane filtration techniques such as nano-filtration, reverse filtration and ultra filtration can be used depending upon the size of the particles for the elimination of heavy metals from wastewater. In ultra filtration technique, heavy metals, suspended solids and macromolecules can be filtered depending on the pore size and (5-20 micrometres) and molecular weight of the separating compounds (1000 – 100,000 Da). Ultra filtration is capable of removing more than the 90% of metals concentration ranging from 10 to 112mg/L at PH from 5 to 9.5 at 2.5 Barr of pressure. Ultra filtration have some advantages due to its high packing density such as lower driving force and little space requirement.
Polymer- supported ultrafiltration (PSU)
In these techniques, water soluble polymeric ligands are bounded to metal ions, which form macromolecular complexes. They produce a free targeted metal ions effluent Rehter et al. (2003). The PSU technology is advantageous as it requires low energy in ultrafiltration, the reaction kinetics is very fast and thus is higher selectivity of separation of selective bonding agents in aq. solution.
Complexation- ultrafiltration is another similar technique, that can be used, which is also based on ion exchange and precipitation. In this method, the water soluble metal- binding polymers are combined with ultra-filtration (UF) to filter heavy metals from a solution Petrov and Nenov et al. (2009).
Electro dialysis(ED)
Is a technique in which the solution containing ionized species are passed via an ion exchange membrane by action of an electric potential. These membranes are thin plastic sheets and have either anionic or cationic characteristics. When the solution (having ionic species) is passed through the cell components, the anions are attracted towards cathode, crossing the cation- exchange and anion exchange membranes. Its disadvantages are membranes replacement and the corrosion process Kumiavan et al. (2006). Use of membranes that have higher ion exchange capacity, yields better cell performance. The effect of flow rate, voltage and temperature were studied at different concentrations, using two types of commercial membrane, a laboratory and ED cell, for removal of lead Mohammadi et al. (2004) Magda (2019).
Chemical method of bioremediation
Chemical precipitation: In this process, the pH pf the effluent is increased by using caustic soda (NaOH) or (MgO) and lime (CaO) for precipitating and so, inactivates the heavy metals by changing them to hydroxides Esmaelie et al. (2009). To remove the heavy metal ions from their aqueous solution, magnesium oxide was found as a better absorber, at low pH, Asadzadeh et al. (2018).
Various coagulants like magnesia, caustic soda, lime, cationic poly electrolyte (CPE) and their combinations were applied to choose the suitable one having most appropriate removal efficiency. In removing the metals, magnesium oxide is found as very effectual even when used small doses.It started precipitating the metals by adsorbing them, before the pH was increased. Situation differs in CaO and NaOH, as they are pH dependent. This proves the MgO to be but precipitator of iron and cobalt.To calculate the removal efficiency of the metals from its solution :(Ci- C) / Ci * 100%,
Where, Ci is the initial concentration of metals, before the addition of coagulant and C is the final concentration of metals, after the addition of the coagulant.In the case pf chromium metals, the CaO reacts with Cr (III) and gives out CaCrO4, which is soluble in water have, the Cr (III) remains in the solution. Whereas MgO reacts with Cr (III) to form MgCrO4, which precipitates in solution (Faon 2006). The precipitation can start with even a little close of MgO. But no adsorption takes place in the case of CaO and NaOH, as they have soluble in water (High TDS) and depends totally on increasing pH attained by chemicals (hydroxide precipitation).
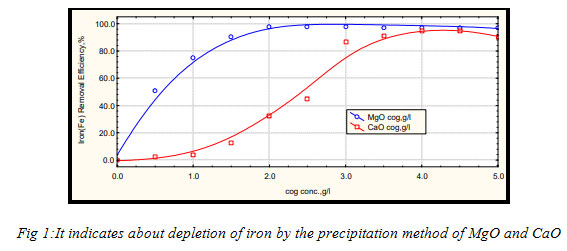 |
Figure 1: Fig 1:It indicates about depletion of iron by the precipitation method of MgO and CaO |
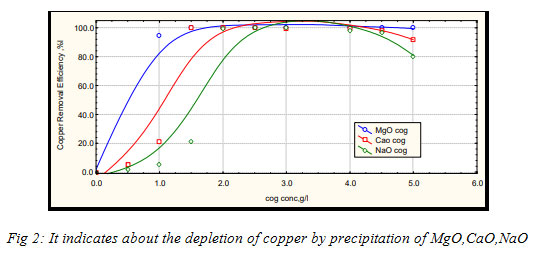 |
Figure 2: It indicates about the depletion of copper by precipitation of MgO,CaO,NaO |
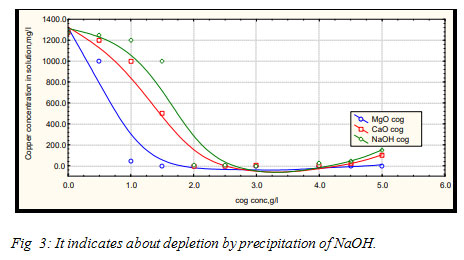 |
Figure 3: It indicates about depletion by precipitation of NaOH. |
Mechanism of Bioremediation
Micro – organisms are ubiquitous that lies mostly in heavy metal contaminated soil and can easily change heavy metals into non-toxic forms. The organic wastes are converted into mineral end products such as water and CO2 or as metabolic intermediates which can be later used as primary substrates for growth of cell microbes, can produce degradative enzymes for the target pollutants as well as resistance to relevant heavy metals, so they are two- way protected. Many types of bioremediation techniques are known, these includes metal – microbe interactions, bio mineralisation, bio sorption bioaccumulation bioleaching and biotransformation, (Magda 2019).
Microbes have a unique property to consume the heavy metals and produce some of their metabolites using this as a catalyst, in order to grow and develop properly. Their capabilities vary from dissolving metals to oxidising transition metals. Various methods are used by microbes to restore the environment such as immobilizing, binding, oxidizing, transformation and volatizing. By the application of microbes and controlling their growth and activity at the contaminated sites, and by controlling their metabolic and response to environmental changes, one can succeed in bioremediation of a derived location. Their plasma membrane may be disrupted by certain defence mechanisms like forming outer cell membrane protective materials, other hydrophobic or solvent efflux pumps, cells can survive Sikkena et al. (1995). Many bacteria are resistant to As, Cr and Cd as they have plasmid encoded and energy dependent metal efflux systems consisting of ATPase and chemiosmosis ion/proton pumps Roane and Pepper (2000) Ontarion et al 2018 ).
Bioremediation by adsorption
Microbes have some binding sites in their cellular structure, which can bisorb the heavy metals by using energy. In bacterial cell walls, the extracellular polymeric substances have major effects on acid base properties and metal adsorption, (Guine (2006). EPS or extracellular polymeric substances can compound heavy metals through different mechanism, that are proton exchange and micro precipitation metals, (Guine 2006; Farg et al. 2010).Studies have expressed the proton and adsorbed metals on bacterial cells and cells without EPS to find the importance of EPS molecule in removal of metals, (Fang et al. 2011).The era of researches in the sector of bioremediation has not yet got to an end because there are still chances of developments in the genetic composition of microbes .The possible framework for the development of microbe to remove heavy metals has still been under progress,( Gan et al. (2009, Haritash and Kaushik 2009; Onwubuya et al. 2009; Carter et al., 2006; Kinya et al. 1996 Magda 2019) .
Bioremediation by Physio-Bio-Chemical Mechanism
Biosorption is the process which consist higher affinity of a bio sorbent towards sorbate (metal ions), continues till equilibrium is attained between the two components, (Das et al. 2008). Saccharomyces cerevisiae acts as a bio sorbent and remove the Zn (II) and Cd (II) through the ion exchange mechanism Chen and Wang (2007) Talo et al. (2009). Cunninghamella elegans emerged as a perfect sorbent in against of heavy metals released by textile wastewater. Heavy metal degradation involves energy for possessing metabolic cycle in a cell. The combined active and passive modes of toxic metal bioremediation can be called bioaccumulation Brierly (1990). Fungi have emerged as potential biocatalysts to access heavy metals and transform them into less toxic compounds Pinedo-Rivella, (2009).
Some fungi such as Klebsiella oxytoca, Allescheriella sp., Stachybotrys sp., Phlebia sp. Pleurotus pulmonarius, and Botryosphaeria rhodina have metal binding potential Annibale et al. (2007). Pb (II) contaminated soils can be biodegraded by fungal species like Aspergillus parasitica and Cephalosporium aphidicola with bio sorption process Tunali et al. (2006); Akar et al. (2007). Hg resistant fungi (Hymenoscyphus ericae, Neocosmospora vasinfecta and Verticillum terrestre) were able to bio transform Hg (II) state to a nontoxic state Kellu et al. (2006). Many of the contaminants are hydrophobic, and these substances appear to be taken up by microbes through the secretion of some biosurfactant and direct cell-contaminant association Lajszner et al. (2018). Biosurfactants form stronger ionic bonds with metals and form complexes before being desorbed from soil matrix to water phase due to low interfacial tension Jhavasi et al. (2011).
Molecular Mechanisms Involved in Bioremediation Process
Various mechanisms involved in the removal of heavy metals by microorganisms are known. In a genetically engineered bacterium Deinococcus geothemalis, Hg reduction has been reported at high temperatures due to the expression of mer operon from E. coli coded for Hg2+ reduction. Mercury resistant bacteria Cupriavidus metallidurans strain MSR33 was modified genetically by introducing a plasmid that provided genes (merB and merG) regulating Hg biodegradation along with the synthesis of organ mercurial lyase protein (MerB) and mercuric reductase (MerA) [. Modification of Pseudomonas strain with the pMR68 plasmid with novel genes (mer) made that strain resistant to mercury, (Magda 2019). Two different mechanisms for Hg degradation by bacteria (Klebsiella pneumonia M426) are mercury volatilization by reduction of Hg (II) to Hg (0) and mercury precipitation as insoluble Hg due to volatile thiol (H2S). Genetic engineering of Deinococcus radiodurans (radiation resistant bacterium) which naturally reduces Cr (IV) to Cr (III) has been done for complete toluene (fuel hydrocarbon) degradation by cloned genes of tod and xyl operons of Pseudomonas putida. Microbial metabolites like metal bound coenzymes and siderophores mainly involved the degradation pathway, ( Dixit et al. 2015 and Magda (2019).
CONCLUSION
Since, the impact of bioremediation at present or in future is not well known, it can be better alternately compared to chemical and physical methods. Meanwhile, better technical implementations, continuous surveillance, continuous developmental methodologies are necessities .Bioremediation has great advantages as it is more effective in not only destruction but also the complete removal of remaining debris. The filtration techniques are safe to handle and the microorganisms are present naturally as well as can be easily cultured Despite being better, the studies have shown that degradation of most of the metals are carried out completely. The advantages include easy handling, cost effective, more natural, completely destroys wide variety of contaminants. Also, there is very lesser chance of future liability related to treatments. It does not disrupt the normal activities. The limitations include the limited effectiveness of bioremediation on some organic contaminants. Also, the microbes are more or less affected by the various environmental conditions like pH, temperature, etc. The bioremediation process is capable of removing the contaminants that made the approach cost effective and time saving; there are some studied indigenous organisms which are worth exploiting for the eco-friendly management for the treatment of effluents containing the multiple contaminants. Bioremediation with its certain pros and less cons have been explored and shall be explored with the change in chronology.
REFERENCES
Akar, T., Tunali, S., Cabuk, A. (2007) Study on the characterization of lead (II) biosorption by fungus Aspergillus parasiticus. Appl. Biochem. Biotech. 2007, 136, 389–406.
Asadzadeh F, Maleki-Kaklar M, Soiltanalinejad N, Shabani F(2018). Central composite design optimization of zinc removal from contaminated soil, using citric acid as biodegradable chelant. Sci Rep.;8(1):2633.
Aziz, H.A., M.N. Adlan, and K.S. Ariffin (2008) Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr (III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresource Technology, 99 (6): p. 1578-1583.
Babel, S., Kurniawan, T.A. (2003). Various treatment technologies to remove arsenic and mercury from contaminated groundwater: an overview. In: Proceedings of the First International Symposium on Southeast Asian Water Environment, Bangkok, Thailand, 24–25 October, pp. 433–440.
Benefield, L.D., J.F. Judkins, and B.L. Weand, (2006) Process chemistry for water and wastewater treatment: Prentice Hall Inc. 25. Tripathy, T. and B.R. De, Flocculation: a new way to treat the waste water.
Boros-Lajszner E, Wyszkowska J, Kucharski J. (2018) Use of zeolite to neutralise nickel in a soil environment. Environ Monit Assess. 190(1):54.
Brierley, C.L. (1990) Bioremediation of metal-contaminated surface and groundwater. Geomicrobiol. 8, 201–223.
Brim, H., Osborne, J.P., Kostandarithes, H.M., Fredrickson, J.K., Wackett, L.P., Daly, M.J.(2006) Deinococcus radiodurans engineered for complete toluene degradation facilities Cr(IV) reduction. Microbiology, 152, 2469–2477.
Caroli, S., Alimonti, A., Coni, E., Petrucci, F., Senofonte, O., (1994) the assessment of reference values for elements in human biological tissues and fluids A systemic review. Crit Rev Anal Chem 24: 363-398. 2.
Carter, P., Cole, H., Burton, J. (2006) Bioremediation: Successes and Shortfalls. In Proceedings of Key International Conference and Exhibition for Spill Prevention, Preparedness, Response and Restoration (Interspill), London, UK, 23 March.
Chen, C., and Wang, J.L. (2007) Characteristics of Zn2+ biosorption by Saccharomyces cerevisiae. Biomed. Environ. Sci. 20, 478–482.
Comte, S., Guibaud, G., and Baudu, M. (2008) Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater, 151, 185–193.
D’Annibale, A., Leonardi, V., Federici, E., Baldi, F., Zecchini, F. and Petruccioli, M. (2007) Leaching and microbial treatment of a soil contaminated by sulphide ore ashes and aromatic hydrocarbons. Appl. Microbiol. Biotechnol. , 74, 1135–1144.
Das, N., Vimla, R. and Karthika, P. (2008) Biosorption of heavy metals –An Overview .Indian J Biotechnol. 7,159-169.
Dizge, N., Keskinler, B., and Barlas, H. (2009) Sorption of Ni (II) ions from aqueous solution by Lewatit cation-exchange resin. Journal of hazardous materials, 167(1): p. 915- 926.
Esmaeili A., Mesdaghi A. and Vazirinjad, R. (2005). Chromium (III) Removal an Recovery from Tannery Wastewater by Precipitation Process, American Journal of Applied Science 2(10): pp.1471-1473.
Essa, A.M.M., Macaskie, L.E. and Brown, N.L. (2002) Mechanisms of mercury bioremediation. Biochem. Soc. Trans. 2002, 30, 672–674.
Fang, L., Wei, X., Cai, P., Huang, Q., Chen, H., Liang, W., and Rong, X.(2015) Role of extracellular polymeric substances in Cu (II) adsorption on Bacillus subtilis and Pseudomonas putida. Bioresource Technol.
Fang, L.C., Huang, Q.Y., Wei, X., Liang, W., Rong, X.M., Chen W.L., Cai, P. (2010) Microcalorimetric and potentiometric titration studies on the adsorption of copper by extracellular polymeric substances (EPS), minerals and their composites. Bioresour. Technol. 101, 5774–5779.
Gan, S., Lau, E.V. and Ng, H.K. (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. , 172, 532–549.
Guiné, V., Spadini, L., Sarret, G., Muris, M., Delolme, C., Gaudet, J.P., and Martins, J.M. (2006) Zinc sorption to three gram-negative bacteria: Combined titration, modeling and EXAFS study. Environ. Sci. Technol. 2006, 40, 1806–1813.
Hamdaoui, O. (2009) Removal of copper (II) from aqueous phase by Purolite C100-MB cation exchange resin in fixed bed columns: Modeling. Journal of hazardous materials, 161 (2): p. 737-746.
Haritash, A.K. and Kaushik, C.P.(2006) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs) : A review j. Hazard Toxic Radioact Waste Manag 2006,10,59-72
Kelly, D.J.A., Budd, K. and Lefebvre, D.D (2006).The biotransformation of mercury in pH-stat cultures of microfungi. Can. J. Bot. 84, 254–260.
Kinya, K.; Kimberly, L.D. (1996) Current use of bioremediation for TCE cleanup: Results of a survey Remediat. J. 1996, 6, 1–14.
Kurniawan, T.A. (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. Chemical engineering journal, 2006. 118(1): p. 83-98. 38.
López-Maldonado, E. (2014) Coagulation– flocculation mechanisms in wastewater treatment plants through zeta potential measurements. Journal of hazardous materials, 279: p. 1-10.
Magda M. Aly. (2019) Bioremediation of hazardous Heavy Metals from solutions or soil using living or dead microbial biomass IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) 13.6 (2018): 75-80.
Mahmood M., Brboot, I., Balasim, A., Abi, D. and Najah, M., Al-Shuwaik, I. (2011) Removal of Heavy Metals Using Chemicals Precipitation Eng. & Tech. Journal, Vol.29, No. 3, 2011.
Minoia, C., Sabbioni, E., Apostoli, P., Pietra, R., Pozzoli, L., (1990) Trace element reference values in ƟƐƐƵĞƐ from inhabitants of the European Community. I. A study of 46 elements in urine, blood and serum of Italian subjects. Sci Total Environ 95: 89-105.
Mohammadi, B. and Pironneau, O. (2004) Shape optimization in fluid mechanics. Annu. Rev. Fluid Mech., 36: p. 255-279.
Mollah, M.Y.A. (2001) Electrocoagulation (EC)—science and applications. Journal of hazardous materials, 2001. 84(1): p. 29-41.
Nayan, K S. S. Panda, A. Basu, and N. K. Dhal (2018) Enhancement of toxic Cr(VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Velti veria of phytoremediation, Journal of Phytoremediation, vol. 20, no. 7, pp. 682–691, 2018.
Nielsen F.H. (1984) Ultra trace elements in nutrition. Annu Rev Nutr 4: 21-41.
Ontañon OM, M. Fernandez, E. Agostini, and P. S. González, (2018) Identification of the main mechanisms involved in the tolerance and bioremediation of Cr(VI) by Bacillus sp. SFC 500-1E,” Environmental Science and Pollution Research, vol. 25, no. 16, pp. 16111–16120, 2018.
Onwubuya, K., Cundy, A., Puschenreiter, M., Kumpiene, J. and Bone, B. (2009) Developing decision support tools for the selection of “gentle” remediation approaches. Sci. Total Environ. 2009, 407, 6132–6142.
Petrov, S. and Nenov, V. (2004) Removal and recovery of copper from wastewater by a complexation-ultrafiltration process. Desalination, 162: p. 201-209.
Pinedo-Rivilla, C., Aleu, J. and Collado, I.G. (2009) – Pollutants biodegradation by fungi. Curr. Org. Chem. 2009, 13, 1194–1
Rether, A. and Schuster, M. (2019) Selective separation and recovery of heavy metal ions using water-soluble N-benzoylthiourea modified PAMAM polymers. Reactive and Functional Polymers, 57(1): p. 13-21. 33.
Roane, T.M.; Pepper, I.L. (2000) Microorganisms and metal pollution. In Environmental Microbiology; Maier, I.L., Pepper, C.B., Eds.; Gerba, Academic Press: London, UK, p
Rodushkin, I., Odman, F. and Branth, S. (1999) multi element analysis of whole blood by resolution inductively coupled plasma mass spectrometry. Fresenius J Anal Chem 364: 338-34
Rojas, L.A., Yanez, C., Gonzalez, M., Lobos, S., Smalla, K. and Seeger, M. (2011) Characterization of the metabolically modified heavy metal-resistant Cupriavidus metallidurans strain MSR33 gener-ated for mercury bioremediation. PLoS One 6, e17555.
Ruchita Dixit, Wasiullah, Malaviya, D., Pandiyan, k., Singh, U.B., Sahu, A., Shukla, R., Singh, B.P., Rai, J.P., Sharma, P.K., Lade, H. and Pau, D.(2015) Bioremediation of Heavy metals from soil and aquatic environment : An overview of principles and criteria for fundamental processes .
Shim, H.Y. (2014) Application of Electrocoagulation and Electrolysis on the Precipitation of Heavy Metals and Particulate Solids in Washwater from the Soil Washing. Journal of Agricultural Chemistry and Environment, 3(04): p. 130.
Sikkema, J., de Bont, J.A. and Poolman, B. (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59, 201–222.
Smith, L. (1995) Contaminants and remedial options at selected metal-contaminated sites. Technical resource report, Battelle, Columbus, OH (United States).
Sone, Y., Mochizuki, Y., Koizawa, K., Nakamura, R., Pan-Hou, H., Itoh, T. and Kiyono, M. (2013) Mercurial resistance determinants in Pseudomonas strain K-62 plasmid pMR68. AMB Express 2013, 3,Article 41
Talos, K., Pager, C., Tonk, S., Majdik, C., Kocsis, B., Kilar F. and Pernyeszi, T. (2009) Cadmium biosorption on native Saccharomyces cerevisiae cells in aqueous suspension. Acta Univ. Sapientiae Agric. Environ. , 1, 20–30.
Thavasi, R. Microbial biosurfactants: From an environment application point of view. J. Bioremed. Biodegrad. 2011, 2, Article 104e.
Tigini, V., Prigione, V., Giansanti, P., Mangiavillano, A., Pannocchia, A. and Varese, G.C. (2000) fungal biosorption, an innovative treatment for the decolourisation and detoxification of textile effluents Water 2, 550–565.
Tunali, S., Akar, T., Oezcan, A.S., Kiran, I. and Oezcan, A.(2004) Equilibrium and kinetics of biosorption of lead(II) from aqueous solutions by Cephalosporium aphidicola. Sep. Purif. Technol., 47, 105–112.
Vigneswaran, R (2004) Cerebral palsy and placental infection: a case-cohort study. BMC pregnancy and childbirth, 4(1): p. 1. 32.
Williford, C., R.M. Bricka, and I. Iskandar (2000), Physical separation of metal-contaminated soilsCRC Press LLC, Boca Raton.


