1Department of Biochemistry, Patna University, Patna, Bihar
2Department of Botany, Patna University, Patna, Bihar
Corresponding author email: bb2mishra@gmail.com
Article Publishing History
Received: 08/12/2020
Accepted After Revision: 23/03/2021
Oxidative stress induced by the rise in free radicals is the pivotal cause of many dreadful diseases in which Diabetes mellitus is one of them. Diabetes mellitus results in hyperglycemia leading to an increase in oxidative stress in the body due to the generation of free radicals that cause complications such as nephropathy due to oxidative damage. Many plant-derived phytomedicines are known to reduce diabetes-related complications. Asparagus is one such medicinal plant, which is widely used as phytomedicine for many diseases by traditional healers. In the present study, Asparagus racemosus crude methanolic root extract (ACMRE) was assessed for its hypoglycemic and antioxidant properties. The crude methanolic root extract of Asparagus was prepared using soxhlet apparatus. Wister albino rats were divided into three groups viz. Normal control, Diabetic and Asparagus treated diabetic rats. Diabetes was induced by administering Alloxan (100 mg/kg body weight) in the tail vein.
Diabetic rats were orally treated with 500 mg per kg body weight dose of crude methanolic root extract of Asparagus racemosus for 30 days using gavage. Blood glucose, creatinine and tissue antioxidants levels were analyzed at an interval of 10, 20 and 30 days respectively. Enzymic antioxidants such as Superoxide dismutase (SOD), Glutathione-S-Transferase (GST), Glutathione Peroxidase (GPx), Glutathione Reductase (GR), Catalase (CAT) and nonenzymic antioxidant molecule like Reduced Glutathione (GSH) were analysed along with serum creatinine and blood sugar using UV-Vis Spectrophotometer. Treatment with crude root extract significantly reduced the blood glucose and increased the enzymic and non enzymic antioxidant significantly (p< 0.05) of kidney tissue as compared to the diabetic rat group which was further confirmed by the decrease in the level of serum creatinine. Thus, the results indicate that the plant has hypoglycemic as well as antioxidant potential.
Antioxidant, Asparagus Racemosus, Diabetes Mellitus, Hypoglycemic, Oxidative Stress.
.
Mishra B. B, Padmadeo S. R. On the Hypoglycemic and Antioxidant Activities of Root Extract of Asparagus racemosus in Alloxan-Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2021;14(1).
Mishra B. B, Padmadeo S. R . On the Hypoglycemic and Antioxidant Activities of Root Extract of Asparagus racemosus in Alloxan-Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3kWpbTq“>https://bit.ly/3kWpbTq</a>
Copyright © Mishra and Padmadeo This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Oxidative stress occurs in the living body when reactive species outnumbers the antioxidant buffering capacity, Reactive species include Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). It causes several degenerative diseases such as Parkinson’s, Rheumatoid arthritis, cardiovascular, Diabetes mellitus etc. Diabetes mellitus, commonly known as Diabetes, is a metabolic disorder that is marked by symptoms such as hyperglycemia, glycosuria, etc. resulting from lack of insulin or action (Dandekar, 2002; Galli et al., 2005; Amira, 2010). Hyperglycemia-induced oxidative stress is reported to be associated with the initiation and progression of Diabetes and its complications (Maritim, 2003; Matough et al., 2012 and Asmat et al, 2016).
In addition to this, the generation of free radicals associated with diabetes is reported to cause oxidative damage in organs such as the kidney, liver, eyes, gastrointestinal, cardiovascular system.. Insufficient glycemic control a major public health concern and therefore needs research on new complementary medicine derived from plants. Phytomedicine has been reported to ameliorate secondary complications of diabetes such as kidney damage, oxidative stress etc. The World Health Organization has reported that 80% of the developing countries population is beyond the reach of pharmaceutical drugs relies on plant-based traditional medicines for their health care needs (Juarez-Rojop et al., 2012; Khatune et al., 2016, Ramar et al., 2012; Buko et al., 2018; Yin et al., 2018).
In India, several plant products have been reported to be used by the tribal community and practitioners of the Ayurvedic system of medicine to treat diabetes and other diseases; one such plant is Asparagus, Asparagus racemosus Willd. (family Asparagaceae; Liliaceae), is commonly called Satavari, Satawar or Satmuli in Hindi; Satavari in Sanskrit; Shatamuli in Bengali; Shatavari or Shatmuli in Marathi; Satawari in Gujarati; Toala-gaddalu or Pilli-gaddalu in Telegu; Shimaishadavari or Inli-chedi in Tamil; Chatavali in Malayalam; Majjigegadde or Aheruballi in Kannada; Kairuwa in Kumaon; Narbodh or Satmooli in Madhya Pradesh; and Norkanto or Satawar in Rajasthan (opana et al., 2007; Alok et al., 2013; Tanwar et al., 2017; Tanwar et al., 2017).
The plant grows throughout the tropical and subtropical parts of India up to an altitude of 1500 m. The plant is a spinous under-shrub, with tuberous, short rootstock bearing numerous succulent tuberous roots (30–100 cm long and 1–2 cm thick) that are silvery-white or ash coloured externally and white internally. It has been reported that these roots are used in various medicinal preparations and possess various pharmacological activities such as antioxidant and free radical scavenging activity, anti-inflammatory property etc. (Bopana et al., 2007; Vadivelan et al., 2018).However, the effects of methanolic root extract on the various antioxidant enzyme in animal models have been meagrely reported. The present study is designed to evaluate the in-vivo hypoglycemic and antioxidant activity of crude methanolic root extract of Asparagus racemosus to understand how the extract acts against diabetes-induced oxidative stress (Vadivelan et al., 2018).
MATERIAL AND METHODS
The Plant part was authenticated by Prof. S. R. Padmadeo, Former Head of the Department of Botany, Patna University. The roots of Asparagus racemosus were purchased from the local market. Roots of A. racemosus were carefully washed with distilled water 3-6 times to remove dirt and other contaminating material. The plant materials were shade dried at ambient temperature and pressure until no moisture was left in it. The plant material was converted to fine powder using a kitchen grinder followed by sieving with the help of muslin cloth to remove coarse particles. The powdered form of roots of Asparagus racemosus was stored in a well-labeled airtight container for further use. The methanolic crude extract of roots of Asparagus racemosus was prepared using a Soxhlet apparatus (Riviera, India). 100 grams of fine powder of plant material was weighed using a digital weighing machine (Wensar, India) and placed in the cellulose thimble using gloves. The thimble was carefully placed in the extraction chamber of the Soxhlet apparatus while 500 ml of Methanol (100%) was placed in the boiling flask attached to the heating mantle (Nafisa et al., 2007).
The Soxhlet apparatus was run for 48 hours at 60oC to ensure that all phytochemicals in the plant material have dissolved in methanol. After 48 hrs cycle, the methanolic extract was collected from the Soxhlet apparatus and was further filtered using Whatman filter paper to get rid of any solid particle. The methanolic extract was concentrated by Rotavapour (Popular, India) at 60 o C and reduced pressure to one-twentieth volume (5 ml). it was further lyophilized to get thick yellowish-brown coloured residue which was stored in a well-labeled vial at 4oC. Alloxan monohydrate used in this study was a product of Sigma Chemical Company, St Louis, U.S.A. Gluco-one glucometer was a product of Dr.Morepen, Delhi, India. UV-Vis Spectrophotometer (Systronics, India) was used to analyse enzymes and molecules. All other chemicals and assay kits used were products of Sigma-Aldrich Inc. and Merck, Germany, respectively. Healthy Wistar male albino rats (100–150 g) were kept under well-ventilated standard environmental conditions (temperature 25±2 °C, relative humidity 50±5 %) with a 12 h light / dark cycle. Animals were allowed to acclimatize for 7 days before the commencement of the experiment (Nafisa et al., 2007).
The experiments were designed and conducted as per the current ethical norms and guidelines approved by the Ministry of Social justices and Environment, Government of India. The rats were fed on Laboratory prepared pellet having the composition suggested by Subcommittee on Laboratory animal nutrition, National Research Council, USA and water ad libitum to ensure proper growth and nourishment. The extra supplement that was given was carrot, sprouted Bengal gram and Green gram. Alloxan monohydrate 100 mg/kg body weight dissolved in 0.9% sterilized NaCl solution of pH 7.0 was administered in the tail vein of rats to induce diabetes mellitus. After 48 hours, their fasting blood glucose levels were monitored using a glucometer by collecting blood from the tail artery of animals. Those rats having fasting glucose levels in the range of 250 and 400 mg/dl were considered diabetic and used for the experiment (Nafisa et al., 2007). The pure breed rats were kept in new polypropylene cages and were categorized into the following groups:Group I – Normal Control, Group II – Alloxan treated Diabetic rats, Group III – Asparagus racemosus Crude Methanolic root extract (ACMRE) treated diabetic rats.
ACMRE of 500mg/kg.body weight was prepared from the stock solution according to the weight of the rats by dissolving in olive oil. Oral administration of the desired herbal extract was made through oral gavages for 10,20 and 30 days. For the present research work blood sample were collected by tail clipping for fasting glucose estimation and after an interval of 10, 20, and 30 days rats were sacrificed for organ collection and preservation. For the entire research work, tissue samples of the kidney for the antioxidant assay of different parameters were kept in Tris-buffer at -20o C. The kidney tissue was isolated, washed in 0.2 M Tris buffer solution, blotted dry and weighed. A 10% tissue homogenate was prepared in 0.2 M Tris buffer solution by a Potter–Elvehjem Homogenizer. The tissue homogenate was centrifuged at 10,000 g for 20 min, to remove cell debris and then the supernatant was centrifuged at 35,000 g for 30 min. The supernatant obtained was used for various antioxidant assays. The tissues collected at each interval were immediately processed and each tissue sample was analyzed separately. Superoxide Dismutase (SOD) activity was measured by the method of Marklund and Marklund (Marklund and Marklund, 1974) based on the inhibition of the autoxidation of pyrogallol.
Catalase (CAT) activity was determined by measuring the rate of decomposition of H2O2 by the method of Claiborne, 1985. The Glutathione Peroxidase (GPx) activity was determined using H2O2 as a substrate according to the method of Rotruck et al., 1973. Glutathione Peroxidase enzyme catalyzes the decomposition of H2O2 or other peroxides (-OH) with the simultaneous oxidation of GSH into GSSH (Rotruck et al., 1973). The tissue GSH content was estimated by the method of Beutler (Beutler et al., 1967) based on the development of a stable yellow coloured complex, with 5,5’-dithio, bis-2, nitrobenzoic acid (DTNB) or Ellman’s reagent. The activity of GSH-R was measured by the oxidation of NADPH as described by Horn,1963. The activity of GST was determined using 1-chloro 2,4-dinitrobenzene (CDNB) as substrate (Habig et al., 1974). Data were expressed as the Mean ± SEM. For statistical analysis of the data, group means were compared by analysis of variance (ANOVA) followed by Tukey and Duncan post hoc test for multiple comparisons using Graph Pad Prism 8 software. P < 0.05 was considered to be statistically significant (Habig et al., 1974).
RESULTS AND DISCUSSION
Hyperglycemia is the major cause of structural changes (Somania et al., 2012). In the present study, Alloxan treated rats at a dose of 100 mg/kg body weight caused elevation in blood glucose up to 489% as compared to control leading to loss of weight and lethargic activity. Nevertheless, when the crude Asparagus methanolic root extract at a dose of 500 mg/kg body weight was administered to diabetic rats caused a significant decline in blood glucose level up to -70% (Fig.1) which is in agreement with the findings of Taepongsorat et al., (2018) and Vadivelan et al., (2011). The antioxidant effects of crude Asparagus were studied in terms of antioxidant enzymes like SOD, GST, GPx, Catalase and Glutathione Reductase along with antioxidant molecules like Reduced Glutathione (GSH).
Superoxide dismutase plays a key role during oxidative stress. It catalyses the dismutation of superoxide radicals. In the diabetic rat group, SOD level considerably decreased (-84%), Glycosylation of proteins may be responsible for degradation in SOD activity in the diabetic group (Satheesh et al., 2004; Jabeen et al., 2006). Nonetheless, ACMRE treatment leads to a significant increase in enzyme activity (+121%) on the 30th day (p<0.005) as compared to the diabetic group. (Table.1). Glutathione S-transferases (GSTs) is another significant antioxidant enzyme that helps to overcome oxidative stress. GST catalyse addition or substitution reactions of the substrate through the nucleophilic attack of the tripeptide glutathione to electrophilic substrates (Armstrong, 1997; Jabeen et al., 2006).
GST activity in the alloxan-induced diabetes group illustrated -49% decrease as compared to normal on day 30. However, on treatment with methanolic crude Asparagus root extract, there was a noticeable elevation (p<0.05) in enzyme activity by 1.5 fold from day 10 to day 30 showing the beneficial effect of the extract (Table 2). GPxs have been reported to catalyze the reduction of H2O2 or organic hydroperoxides to water or the corresponding alcohols, respectively, typically using Glutathione (GSH) as a reducing agent (Brigelius-Flohé et al., 2013). Its activity decreased substantially by 90% in the diabetic group, nonetheless, on treatment with crude extract caused recovery of enzyme activity by 3.47 fold on day 30 (p<0.05) with respect to the diabetic group (Table 3).
Catalase which is a significant antioxidant enzyme against H2O2 was analyzed and there was a marked decline in catalase activity up to 98% in the diabetic group as compared to normal although when treated with ACMRE, enzyme activity increased 3.46 times as compared to the diabetic group showing recovering trend (P<0.05) (Table 4) (Tehrani et al., 2018). Glutathione reductase which helps to maintain a consistent supply of reduced glutathione in the cell was observed to follow the declining trend in the case of the diabetic rat group (-58%) (Couto et al., 2016). Nevertheless, on treatment with ACMRE, there was 59% increase in enzyme activity as compared to the diabetic group on day 30 (P<0.05) (Table 5) (Tehrani et al., 2018).
Reduced Glutathione (L-ɣ -glutamyl-L-cysteinyl glycine, GSH) is at the core of one of the most significant antioxidant enzyme systems of the cell which in its reduced form is efficient of neutralizing reactive oxygen and nitrogen species, thus assisting in the control of redox homeostasis. Treatment with ACMRE leads to 3.07 fold increase in GSH level in the diabetic rat (p<0.05) in contrary to the diabetic group without treatment, where GSH level fell drastically to -24% as compared to Normal (Table 6). High production of reactive oxygen species is a significant reason behind the advancement of diabetic complications like diabetic nephropathy and some reports illustrated the regulation of oxidative stress using antioxidants to reduce diabetic complexities (Kataya and Hamza, 2008; Couto et al., 2016).
In the present study, diabetic rats showed an overall decreasing level of enzymic antioxidants such as SOD, GPx, GR, GST, CAT and non-enzymic antioxidant like GSH however on treatment with crude methanolic Asparagus root extract elevated the enzymic as well as non-enzymic antioxidant which is in concordance with the findings of Vadivelan et al., (2011) and Purena et al., (2018). The result is also in congruence with the findings of Kamat et al., (2000) and Acharya et al., (2012) which were reported in liver tissue suggesting the protective role of crude root extract of Asparagus on SOD enzyme against free radicals (Acharya et al., 2012; Purena et al., 2018).
Serum creatinine is the most widely used endogenous renal biomarker which increased 36.96 fold in the diabetic group. Elevated Creatinine levels may be due to the damage caused to the podocyte foot process leading to degradation of filtration (Ahmed et al., 2015; Hokamp et al., 2016). In contrast to this, when treated with crude asparagus extract there was -37 % decrease in creatinine level on the 30th day as compared to the diabetic group (p<0.05) (Table 7). This finding suggests that Asparagus root extract provides nephroprotection against renal deterioration and is in corroboration with the findings of Somania et al., (2012) (Sachdeva et al., 2014; Hokamp et al., 2016). Possibly, the phytoconstituents of Asparagus root extract reduced the blood glucose through its insulin secretory activity and complemented the activity of enzymic and non-enzymic antioxidant. Thus establishing its hypoglycemic and antioxidant properties (Hannan et al., 2007).
Figure 1: Effect of Crude Asparagus methanolic root extract on blood glucose.
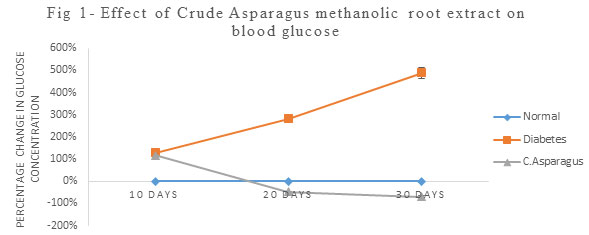
Table 1. Effect of ACMRE on SOD (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 172.156± 0.494 | 130.396± 0.471* | 25.686± 1.055*# |
| 20 days | 172.156± 0.494 | 97.0633± 0.087* | 42.94± 0.390*# |
| 30 days | 172.156± 0.494 | 27.5966± 0.8* | 61.11± 0.061*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 2. Effect of ACMRE on GST (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 0.612± 0.004 | 0.481± 0.001* | 0.694± 0.001*# |
| 20 days | 0.612± 0.004 | 0.462± 0.003* | 0.949± 0.001*# |
| 30 days | 0.612± 0.004 | 0.311± 0.002* | 1.046± 0.008*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 3. Effect of ACMRE on GPx (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 12.286± 0.014 | 4.26± 0.05* | 1.386± 0.026*# |
| 20 days | 12.286± 0.014 | 2.093± 0.027* | 2.13± 0.011* |
| 30 days | 12.286± 0.014 | 1.23± 0.0057* | 4.813± 0.001*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 4. Effect of ACMRE on Catalase (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 | 413.756± 57.748 | 271.85± 1.668 | 95.596± 1.189# |
| 20 | 413.756± 57.748 | 51.48± 0.931 | 168.04± 0.843# |
| 30 | 413.756± 57.748 | 8.703± 0.275 | 330.886± 1.206# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 5. Effect of ACMRE on GR (U/ mg of protein) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 0.774± 0.001 | 0.693± 0.003* | 0.192± 0.0005*# |
| 20 days | 0.774± 0.001 | 0.462± 0.0005* | 0.281± 0.001*# |
| 30 days | 0.774± 0.001 | 0.323± 0.001* | 0.515± 0.001*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 6. Effect of ACMRE on GSH (µg/ml of sample homogenate) in Kidney Tissue
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 11.569±0.024 | 10.216±0.006* | 6.666± 0.041*# |
| 20 days | 11.569± 0.024 | 9.613± 0.024* | 9.057± 0.001*# |
| 30 days | 11.569± 0.024 | 8.767± 0.041* | 20.482± 0.024*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
Table 7. Effect of ACMRE on Creatinine (mg/dl) in blood serum
| Days | Normal | Diabetes | Crude Asparagus |
| 10 days | 0.011± 0.001 | 0.03± 0.001* | 0.91± 0.004*# |
| 20 days | 0.011± 0.001 | 0.12± 0.002* | 0.62± 0.005*# |
| 30 days | 0.011± 0.001 | 1.109± 0.005* | 0.58± 0.002*# |
| Values indicate mean ± SEM (n=3)
*p<0.05, compared with normal control values, # p<0.05, compared with Diabetic values |
|||
CONCLUSION
Based on our result it may be concluded that roots of Asparagus racemosus possess the hypoglycemic and antioxidant potential hence can be utilized as a significant source of antioxidant that has the ability to deal with diabetic complications such as nephropathy. Therefore may be promoted as a food supplement in the treatment of diabetes. However further researches are needed to analyse its actual therapeutic capability and compounds involved in its medicinal value.
ACKNOWLEDGEMENTS
The authors are thankful to the Department of Biochemistry, Patna University for providing necessary equipment along with chemicals and Mr. Ravinder for helping in research activities in the Department.
Conflict of Interest: The authors declare that there are no conflicts of interest regarding publication or any other activity related to this article.
REFERENCES
Acharya, S. R., Acharya, N. S., Bhangale, J. O., Shah, S. K. and Pandya, S. S. (2012) Antioxidant and hepatoprotective action of Asparagus racemosus Willd. root extracts, Indian journal of experimental biology, 50(11), 795–801.
Ahmed, W., Moselhy, W. and Nabil, T. (2015) Bisphenol A toxicity in adult male rats: haematological, biochemical and histopathological approach, Glob. Vet., 14 (2), 228–238.
Alok, S., Jain, S. K., Verma, A., Kumar, M., Mahor, A. and Sabharwal, M. (2013) Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review, Asian Pacific Journal of Tropical Disease, 3(3), 242–251.
Amira, A.M.A. (2010) Oxidative stress and disease: An updated review, Research Journal of Immunology, 3(2), 129-145.
Armstrong, K.N. (1997) Structure, catalytic mechanism and evolution of the glutathione transferases, Chem Res Toxicol, 10, 2–18.
Asmat, U., Abad, K., and Ismail, K. (2016) Diabetes mellitus and oxidative stress-A concise review, Saudi pharmaceutical journal:s SPJ: the official publication of the Saudi Pharmaceutical Society, 24(5), 547–553.
Beutler, E., Duron, O. and Kelly, B.M. (1967) Improved method for the determination of blood glutathione, J Lab Clin Med, 61, 882-888.
Bopana, N. and Saxena, S. (2007) Asparagus racemosus-ethnopharmacological evaluation and conservation needs, Journal of ethnopharmacology, 110(1), 1–15.
Brigelius-Flohé, R. and Maiorino, M. (2013) Glutathione peroxidases, Biochimica et biophysica acta, 1830(5), 3289–3303.
Buko, V., Zavodnik, I., Kanuka, O., Belonovskaya, E., Naruta, E., Lukivskaya, O., Kirko, S., Budryn, G., Z˙ yz˙ elewicz, D. and Oracz, J. (2018) Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats, Food Funct., 9, 1850–1863.
Claiborne, A. (1985) Catalase activity. In: Greenwald, R.A. (Ed.), Handbook of Methods for Oxygen Radical Research, CRC Press, 283–284.
Couto, N., Wood, J., and Barber, J. (2016) The role of glutathione reductase and related enzymes on cellular redox homeostasis network, Free radical biology & medicine, 95, 27–42.
Dandekar S.P. (2002) Medical biochemistry, 2nd ed. India: Elsevier.
Galli, F.M., Piroddi, C., Anneti, C., Aisa, E., Floridi and Floridi, A. (2005) Oxidative stress and reactive oxygen species, Contrib Nephrol., 149,240-260.
Habig, W.H., Pabst, M.J. and Jakoby, W.B. (1974) Glutathione S transferase-The first enzymatic step in mercapturic acid formation, J Biol Chem, 249, 71301-09.
Hannan, J. M., Marenah, L., Ali, L., Rokeya, B., Flatt, P. R. and Abdel-Wahab, Y. H. (2007) Insulin secretory actions of extracts of Asparagus racemosus root in perfused pancreas, isolated islets and clonal pancreatic beta-cells, The Journal of endocrinology, 192(1), 159–168.
Hokamp, J. A. and Nabity, M. B. (2016). Renal biomarkers in domestic species, Veterinary clinical pathology, 45(1), 28–56.
Horn, H.D. (1963) Glutathione reductase. In: Bergmeyer HU.ed. Methods in Enzymatic Analysis.New York: Academic Press, 875-879.
Jabeen, R and Saleemudin, M. (2006) Polyclonal antibodies inhibit the glycation-induced inactivation of bovine Cu, Zn-superoxide dismutase, Biotechnol Appl Biochem, 43, 49 – 53.
Juárez-Rojop, I. E., Díaz-Zagoya, J. C., Ble-Castillo, J. L., Miranda-Osorio, P. H., Castell-Rodríguez, A. E., Tovilla-Zárate, C. A., Rodríguez-Hernández, A., Aguilar-Mariscal, H., Ramón-Frías, T. and Bermúdez-Ocaña, D. Y. (2012). Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats, BMC complementary and alternative medicine, 12, 236.
Kamat, J. P., Boloor, K. K., Devasagayam, T. P. and Venkatachalam, S. R. (2000) Antioxidant properties of Asparagus racemosus against damage induced by gamma-radiation in rat liver mitochondria, Journal of ethnopharmacology, 71(3), 425–435.
Kataya, H. A. and Hamza, A. A. (2008) Red Cabbage (Brassica oleracea) Ameliorates Diabetic Nephropathy in Rats, Evidence-based complementary and alternative medicine: eCAM, 5(3), 281–287.
Khatune, N. A., Rahman, B. M., Barman, R. K. and Wahed, M. I. (2016) Antidiabetic, antihyperlipidemic and antioxidant properties of ethanol extract of Grewia asiatica Linn. bark in alloxan-induced diabetic rats, BMC complementary and alternative medicine, 16, 295.
Maritim, A.C., Sanders, R.A. and Watkins, J.B. (2003) Diabetes, oxidative stress and antioxidants: a review, J. Biochem. Mol. Toxicol. 17 (1), 24–38.
Marklund, S. and Marklund, G. (1974) Involvement of superoxide anion radical and a convenient assay of superoxide dismutase. Eur J Biochem 47, 469-474.
Matough, F.A., Budin, S.B., Hamid, Z.A., Alwahaibi, N. and Mohamed, J. (2012) The role of oxidative stress and antioxidants in diabetic complications, Sult. Qaboos Univ. Med. J., 12, 5–18.
Nafisa, P.C., Chakradnar, V.L., Vandana, S.P. and Suresh, R.N. (2007) An experimental evaluation of the antidiabetic and antilipidaemic properties of a standardized Momordica charantia fruit extract, BMC Complement Alternat Med, 7, 29–55.
Purena, R., Seth, R. and Bhatt, R. (2018) Protective role of Emblica officinalis hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats, Toxicology reports, 5, 270–277.
Ramar, M., Manikandan, B., Raman, T., Priyadarsini, A., Palanisamy, S., Velayudam, M., Munusamy, A.,Marimuthu Prabhu, N. and Vaseeharan, B. (2012) Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice, Eur. J. Pharmacol., 690, 226–235.
Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W.G., (1973) Selenium: biochemical role as a component of glutathione peroxidase, Science 179(4073),588-590.
Sachdeva, H., Sehgal, R. and Kaur, S. (2014). Asparagus racemosus ameliorates cisplatin induced toxicities and augments its antileishmanial activity by immunomodulation in vivo, Parasitology International, 63(1), 21–30.
Satheesh, M. A. and Pari, L. (2004). Antioxidant effect of Boerhavia diffusa L. in tissues of alloxan induced diabetic rats, Indian journal of experimental biology, 42(10), 989–992.
Somania, R., Singhai, A. K., Shivgunde, P. and Jain, D. (2012). Asparagus racemosus Willd. (Liliaceae) ameliorates early diabetic nephropathy in STZ induced diabetic rats, Indian journal of experimental biology, 50(7), 469–475.
Taepongsorat, L. and Phadungkit, M. (2018) Effects of Asparagus racemosus Root Extracts on Serum Lipid Profiles, Lipid Peroxidation and Superoxide Dismutase in Ovariectomized Rat, Polymer Journal, 10, 1036-1041.
Tanwar, R. S., Sharma, S. B. and Prabhu, K. M. (2017). In vivo assessment of antidiabetic and antioxidative activity of natural phytochemical isolated from fruit-pulp of Eugenia jambolana in streptozotocin-induced diabetic rats, Redox report: communications in free radical research, 22(6), 301–307.
Tehrani, H.S. and Moosavi-Movahedi, A. A. (2018) Catalase and its mysteries, Progress in biophysics and molecular biology, 140, 5–12.
Vadivelan, R., Gopala Krishnan, R. and Kannan, R. (2018) Antidiabetic potential of Asparagus racemosus Willd. leaf extracts through inhibition of α-amylase and α-glucosidase, Journal of traditional and complementary medicine, 9(1), 1–4.
Yin, P., Wang, Y., Yang, L., Sui, J. and Liu, Y. (2018) Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid, Kaempferol and Their Derivatives, Molecules (Basel, Switzerland), 23(5), 1046.


