1Department of Biochemistry, Rajah Serfoji Government College (Autonomous), Thanjavur.
2Department of Biochemistry, Periyar E.V.R. College (Autonomous), (Affiliated to Bharathidasan University), Tiruchirappalli
3Department of Biochemistry and Biotechnology, Annamalai University, Annamalainagar, Tamil Nadu, India
4Post Graduate and Research Department of Biochemistry, Theivanai Ammal College for Women (A), Villupuram
5Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha University, SIMATS, Chennai, Tamil Nadu, India
Corresponding author email: aiyavu@yahoo.com
Article Publishing History
Received: 07/10/2020
Accepted After Revision: 10/12/2020
Diosmin, Neuroprotective, Parkinson’s Disease
Balraj S, Aiyavu C, Manivasagam T, Kalaimathi J, Rajeshkumar S. Neuroprotective Role of Diosmin on Rotenone Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Biosc.Biotech.Res.Comm. 2020;13(4).
Balraj S, Aiyavu C, Manivasagam T, Kalaimathi J, Rajeshkumar S. Neuroprotective Role of Diosmin on Rotenone Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3en1HDx
Copyright © Balraj et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Parkinson disease (PD) is the most common neurodegenerative and movement disorder, that is characterized by resting tremor, rigidity, postural abnormalities, stooped posture, bradykinesia, akinesia, and festinating gait. These motor deficits have been mainly attributed to the progressive loss of neuromelanin containing dopaminergic neurons in substantia nigra (SN) and lead to the loss of dopamine (DA) in the striatum and manifest as motor disabilities that are characteristics of PD (De Lau and Breteler, 2006). The etiology of PD remains elusive and comprises the involvement of both genetic and environmental factors. Agricultural pesticide use has been well-established causes of parkinsonism (Tanner and Goldman, 1996). One among them is the neurotoxin rotenone; a lipophilic molecule that easily crosses the cellular membranes and blood-brain barrier. Exposure of rotenone mimics numerous pathological features of PD, including dopaminergic neuronal death, mitochondrial dysfunction, oxidative stress, inflammation, and behavioral changes that possess in vitro and in vivo studies (Hoehn and Yahr, 1967; Al-Adawi et al. 2000; Shulman et al. 2001).
By inhibiting mitochondrial complex, I activity, rotenone generates reactive oxygen species (ROS), inhibits adenosine triphosphate (ATP) synthesis, depolarizes the mitochondrial membrane, and finally leads to the death of neurons in SN (Pollanen et al. 1993). ROS produced from mitochondrial dysfunction causes changes in the mitochondrial membrane permeability, leading to the activation of apoptotic caspases (Kuzuhara et al. 1988). Plants and based bioactive compounds play an important role in the production of medicine for various diseases and disorders (Rajeshkumar et al. 2019, Renukadevi Balusamy et al. 2020, Thangavelu et al. 2020, Mehta et al. 2020, Tamizhselvi et al. 2020, Rajakumari et al. 2020).
Currently available pharmacological interventions provide symptomatic relief for patients with PD and have little efficacy in reversing the underlying neuropathological changes associated with the disease. Therefore, there is a clinical need to identify therapeutic agents that can ameliorate, or slow down the deleterious processes associated with PD. One such paradigm is to explore the possible contribution of natural products that might interfere with PD pathology (Al-Maskari et al.2011). Natural products have been increasingly found to have specific molecular or pharmacological effects that are likely to contribute to the development of neuroprotective agents against PD. Diosmin, (3′, 5, 7-Trihydroxy-4′-methoxy flavone 7-rutinoside) is found naturally in numerous plants, including citrus fruits, especially lemons, green Meyer lemons, and Buddha’s finger fruits Caucasian vetch, Hyssopus officinalis, and Hyssopus.
Also, it is found quite effective in mitigating hyperglycemia in diabetic rats (Pari and Srinivasan, 2010). It is also speculated that diosmin has neuroprotective potential in the treatment of Alzheimer’s disease (Mustafa, et al. 2010; Essa et al. 2012), and against lipopolysaccharide-induced neurotoxicity in PC 12 cells (Halliwell and Whiteman, 2004). However, the neuroprotective effect of diosmin against experimental PD is not investigated so far. Because of this light, the present study was aimed to evaluate the neuroprotective effect of diosmin against rotenone-induced in vitro model of PD.
MATERIAL AND METHODS
Chemicals: Rotenone, Diosmin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2-7-diacetyl dichlorofluorescein (DCFH-DA), rhodamine 123 (Rh-123), heat-inactivated fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), antibiotic/ antimycotic, EDTA, and Trypsin-EDTA were procured from Sigma Chemicals Co. (St. Louis, USA). Anti-Bcl-2, anti-Bax, caspase-3, caspase-8, caspase-9, Cyt-c, and anti-JNK and anti-P38 MAPK antibodies were obtained from Cell Signaling (USA) and -actin, anti-mouse, and anti-rabbit secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (USA).
Cell Culture: SH-SY5Y cells were obtained from National Center for Cell Science (NCCS), Pune, India. The cells were grown in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution. Cultures were maintained in a humidified incubator at 37℃ in an atmosphere of 5% CO2 and 95% air. The cell culture medium was changed every 2 days.
Cell Viability Assay: Cell viability assay was determined by MTT assay, as described previously (Johnson et al. 1980). SH-SY5Y cells were collected and seeded in 96-well plates, at a density of 3 ×103 cells/well. To determine the toxicity of rotenone, cells were incubated with different concentrations of rotenone (5, 10, 50,100, and 200 nM) and Diosmin (5 nM, 10 nM, 20 nM, 50 nM,100 nM, 200 nM, 500 nM, 1 M, 10 M, 100 M, 200 M, and 500 M) for 24 h and MTT assay was performed to detect IC50 value of rotenone and Diosmin. To assess the therapeutic efficacy of Diosmin against rotenone toxicity, cells were pre-treated with different concentrations of Diosmin (5,10, 20, 50, 100, and 200nM) for 2h and then incubated with rotenone (effective dose) for 24 h. Diosmin was also present during rotenone treatment for an additional 24 h. Then all the cells were incubated with MTT final concentration (1mg/mL of serum-free DMEM medium) at 37·C for 4h. After the incubation, the medium was removed, and 100 L of DMSO was added to dissolve the formazan crystals. The absorbance of the formazan product was evaluated by a spectrophotometer at 570 nm using a microplate reader. Four independent experiments were performed from each group.
Based on the results obtained from cell viability assay, the effective dose of Diosmin against rotenone toxicity was utilized to study the effect of Diosmin by assessing ROS, MMP, apoptosis, and apoptotic markers protein expression.
Experimental Design ( = 4 Experiments): Group I: untreated control cells, Group II: rotenone (effective dose: 100 nM), Group III: Diosmin (100 nM) + rotenone (100 nM), Group IV: Diosmin (100 nM).
Measurement of Intracellular ROS: The levels of endogenous ROS formed in control and experimental cells were estimated by using fluorescence dye (DCFH-DA) (Jayaraj et al. 2013). After pre-treatment with Diosmin (100 nM/mL) for 2 h, the cells (1 ×105 cells/well in 6-well plates) were incubated with rotenone(100nM/mL) for 24 h and then incubated with 100 L DCFHDA for 30 min at 37·C and washed twice with PBS to remove the excess probe; the cells were suspended in glucose enriched PBS and transferred and visualized using a fluorescent microscope. Fluorescent measurements were made with excitation and emission filters set at 485 ±10 nm and 530 ± 12.5 nm, respectively (Shimadzu RF-5301PC spectrofluorimeter), and the images were captured using a fluorescence microscope (Hu et al. 2009).
Measurement of Mitochondrial Transmembrane Potential (MMP): MMP changes were determined by the mitochondrial-specific, incorporation of a cationic fluorescent dye Rh-123 (Bernheimer et al. 1973). After treatment with Diosmin for 2 h and rotenone for 24 has previously been described, the cells (1 ×105 cells/well in 6-well plates) were changed to fresh medium containing 1 L of fluorescent dye Rh-123 (5mmol/L) and kept for 30min at 37·C. The cells were then collected, washed twice with PBS, and estimated by using a blue filter (450–490 nm) (Shimadzu RF-5301 PC spectrofluorimeter).
Apoptosis Analysis Using Dual Staining: Dual staining method is used to analyze the apoptotic morphological changes by treating the control and experimental cells with fluorescent
probes acridine orange and ethidium bromide (AO and EB) and using a fluorescence microscope (Lirdprapamongkol et al. 2005). After the treatment schedule as described in previous experiments, the medium was removed from the plates; cells (1 × 105) were washed with PBS twice and then fixed with 4% paraformaldehyde for 20 min and stained with 100 g/mL AO and EB. These cells were incubated for 20min at room temperature and washed with warm PBS to remove excess dye. Cellular morphology was examined using fluorescence microscopy and photographed and quantified.
Data Analysis: Statistical analysis was performed by one-way analysis of variance followed by Duncan’s multiple range test (DMRT) using Statistical Package for the Social Science (SPSS) software package version 12.0. Results were expressed as mean ± SD for four experiments in each group. < 0.05 were considered significant.
RESULTS AND DISCUSSION
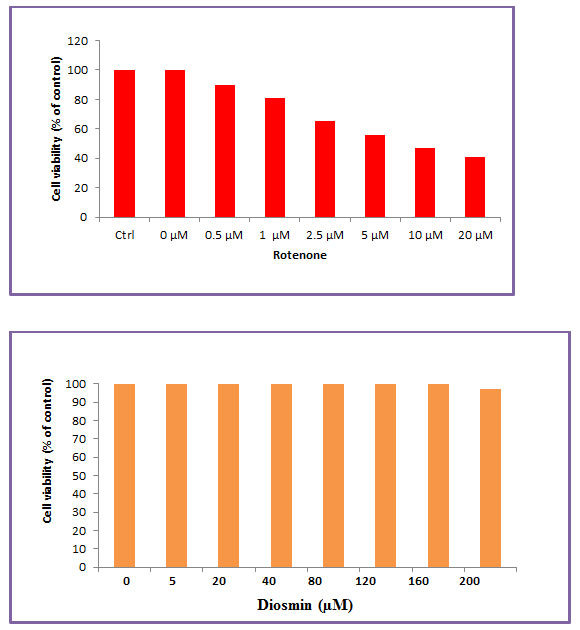
Cytotoxicity of Rotenone in SH-SY5Y Cells: A dose-dependent cytotoxic effect of rotenone was evaluated by MTT assay in SH-SY5Y human neuroblastoma cells, which measures mitochondrial function or integrity with a dose of 100 nM which caused ~50% of cell death as compared with controls and was taken as inhibitory dose (Figures 1(a) and1(b)).
Figure 1: Effect of Diosmin on rotenone-induced cytotoxicity in SH-SY5Y neuroblastoma cells were assessed by MTT assay. (a) shows the dose-dependent effect of rotenone (5, 10, 50, 100, and 200 nM) induced cell toxicity after 24 h. An approximately half-maximal inhibition of cell viability was obtained at 100 nM rotenone concentration. (b) shows the dose-dependent effect of Diosmin at various concentrations. Low concentrations (5, 10, 20, 50, 100, and 200 nM) did not induce any toxicity after 24 h treatment, whereas slight toxicity was induced at 500 M concentration. Values are expressed as the percentage of the untreated control and represented as mean ± SD of four independent experiments in each group.
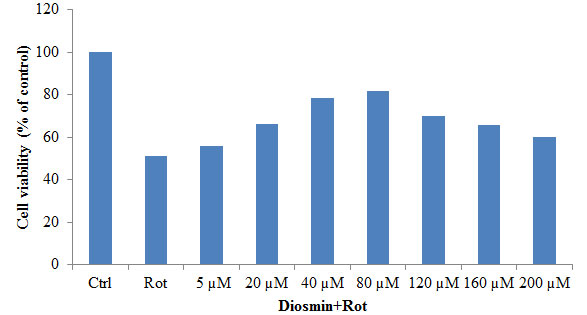
3.2. Diosmin Protects Rotenone Induced SH-SY5Y Cell Death: Figure 2 shows the protective effect of diosmin against rotenone-induced injury (100 nM) with cell viability increasing to 84 ± 6.7% of control in the presence of 100 nM Diosmin. So, based on the dose-response data, the treatments of 100 nM Diosmin and 100 nM rotenone were chosen for further experiments (Figure 2).
Figure 2: The protective effect of Diosmin (5, 10, 20, 50, and 100 nM) against rotenone-induced cell death was determined by the MTT assay. Values are expressed as the percentage of the untreated control and represented as mean ± SD of four independent experiments in each group.
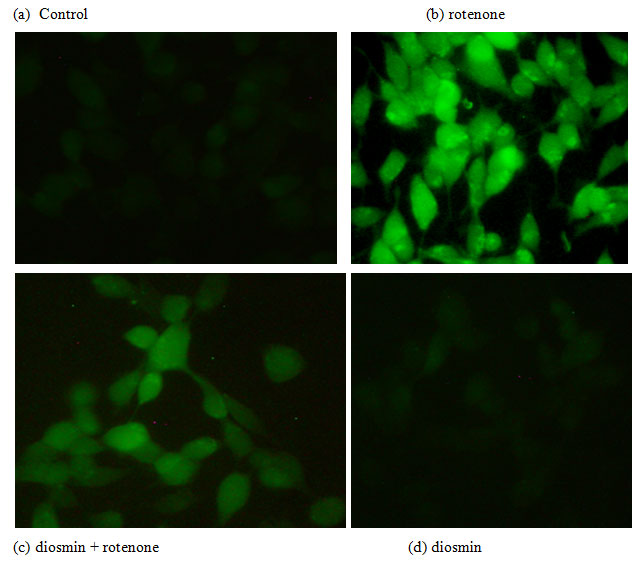
Diosmin Attenuates Rotenone Induced ROS Generation: To analyze the effect of Diosmin on free radical generation, the levels of intracellular ROS formed were quantified by fluorescence with H2DCF-DA. Rotenone treatment enhanced the green fluorescence, an indicator of high levels of ROS, and pre-treatment of Diosmin to rotenone exposed cells revealed reduced green color intensity, an indicator of decreased ROS formation (Figures 3).
Figure 3: Diosmin reduced ROS formation as stained by 1 M CM-H2 DCFDA. (a) Photo-micrograph showing the preventive effect of diosmin (100 nM) against rotenone induced ROS generation. (A)Control, (B) rotenone, (C) diosmin + rotenone, and (D) diosmin.
Diosmin Ameliorates Rotenone Induced Mitochondria membrane potential
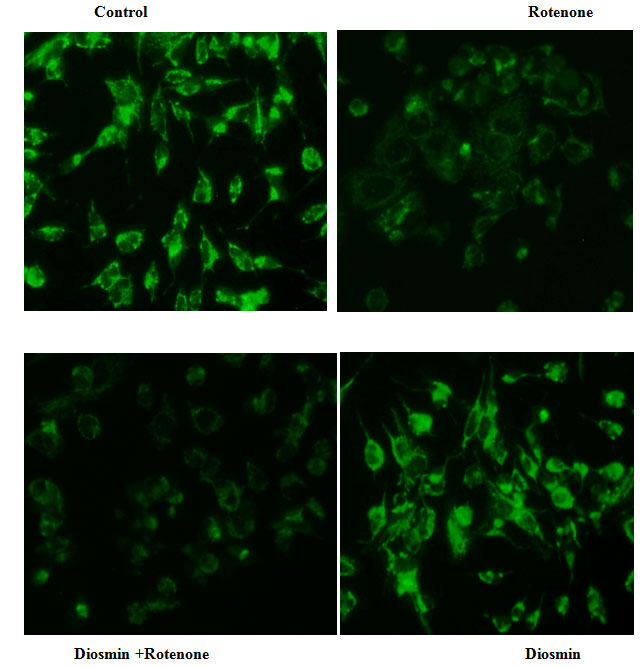
(ΔΨm): Alteration in the MMP is considered to be one of the important events related to apoptosis. The effect of Diosmin on MMP in rotenone-induced toxicity was by measuring the uptake of Rh-123. In normal cells, Rh-123 steadily penetrates the cells, stains mitochondria, and exhibits high fluorescent intensity. The depolarization of MMP due to rotenone treatment results in the loss of Rh-123from the mitochondria and a decrease in intracellular green fluorescence. Cell cultures pre-treated with diosmin before rotenone treatment partially reduced this decline in fluorescence and approached control levels (Figure 4).
Figure 4: Diosmin stabilizes MMP as stained by Rh-123. Rotenone (100 nM) significantly decreased mitochondria membrane potential, while cells that were pre-treated with diosmin (100 nM) significantly increased MMP. Values are given as mean ± SD of four independent experiments in each group. < 0.05 compared to control; # <0.05 compared to rotenone groups (DMRT).
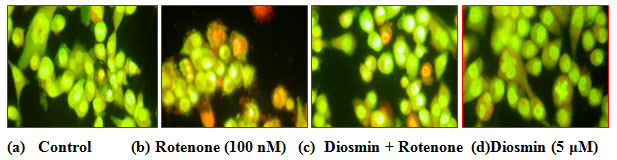
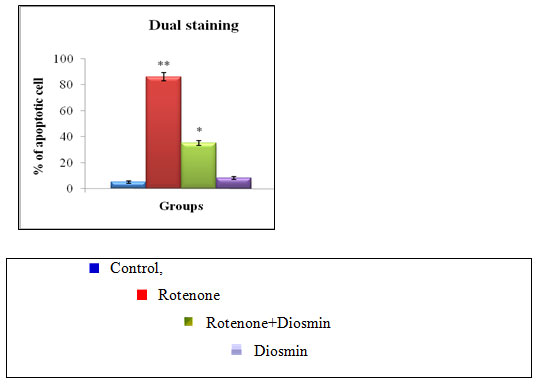
Effect of Diosmin on rotenone-induced apoptotic features in SK-N-SH cells by dual staining: Apoptotic morphological changes were measured in terms of fluorescence by Acridine orange (AO) and Ethidium bromide (EtBr). Our results indicated that the Control cells which fluoresced brightly with green nuclei and normal morphology were showed in Figure 6(a) and the rotenone (100 nM) treated cells exhibited significant nuclear fragmentation and destruction which is characteristic of Apoptosis (bright orange color) and necrosis (red color), respectively. However, the amount of fragmentation and destruction of rotenone treated cells were dramatically reduced when the cells were pre-treated with Diosmin (5 µM) (Fig. 5a &b).
Figure 5 (A): Photo micrograph illustrates apoptotic morphological changes in SK-N-SH cells treated with diosmin and rotenone.
Figure 5 (B): Depicts apoptotic morphological changes in control, TF and rotenone treated SH-SY5Y cells. Values are given as mean±SD of four experiments in each group. **P<0.05 compared to non-treated cells; *P < 0.05 compared to rotenone-treated cells.
Figure 5 clearly shows the diosmin protects SH-SY5Y cells against rotenone-induced apoptosis. (a) Photomicrograph showing the antiapoptotic effect of (100 nM) against rotenone at a concentration of 100nM effective dose. (A) Control, (B) rotenone, (C) diosmin + rotenone, and (D) diosmin. (b) Rotenone (100 nM) treatment induced cell apoptosis compared to control cells; pre-treatment with diosmin (100 nM) suppresses these apoptotic features. Values are given as mean ± SD of four independent experiments in each group. < 0.05 compared to control and
# < 0.05 compared to rotenone group (DMRT). Results of the present study indicated that the rotenone treatment for 24 h destroyed SH-SY5Y cells in a dose-dependent manner and approximately half-maximal inhibition of cell viability (54.41%) was obtained at 100 nM rotenone concentration, which corroborated our previous studies (Kim H. J et al. 2007). The observed condition mimics the situation at the time of initial diagnosis of PD, when approximately 50% of neurons in the substantia nigra are alive, although many of them may be undergoing subcellular stress (Bender et al. 2006).
In the present study, 24 hours prior exposure of diosmin significantly enhanced cell viability in a dose-dependent manner. In this study, the IC50 of diosmin was found at 100 nM. In the present study, increased levels of ROS observed in the rotenone model indicated that oxidative stress was induced by rotenone and is attenuated by treatment of diosmin which might be because of its free radical scavenging activity. Though diosmin is a potent antioxidant, its alone treatment to SH-SY5Y cells triggers the levels of ROS non significantly.
Results of the present study corroborate previous experiments, in which the addition of celastrol, a triterpenoid (Seaton et al. 1997), rutin, a quercetin glycoside (Talpade et al. 2000), and baicalein, a flavonoid (Koopman et al. 2010), alone increased the levels of ROS. Treatment with diosmin ameliorated 3-nitropropionic acid-induced impaired mitochondrial enzyme complexes (I, II, and IV) in an experimental model of Huntington’s disease (Turrens, 2003). Further, it could inhibit singlet oxygen-induced protein and lipid oxidation (Turrens. 1997; Kamat et al. 2000; Santosh Kumar et al. 2002; Song et al. 2012; Choi et al. 2014; Park et al. 2014).
CONCLUSION
In conclusion, this study demonstrates that Diosmin, a black tea polyphenol exhibited neuroprotective effects on rotenone-induced apoptosis in SH-SY5Y cells. These antiapoptotic effects of Diosmin appeared to be related to its ability to reduce oxidative stress and maintain the MMP stability in SH-SY5Y cells against rotenone toxicity. Hence, Diosmin may be a potential agent for the treatment of neurodegenerative disorders such as PD, though further research in in vitro and experimental models (in vivo) are needed to prove its neuroprotective effects.
ACKNOWLEDGEMENTS
The authors would like to thank our college management for support.
Conflict of Interests: Herewith all the authors declare that they do not have any conflict of interests.
REFERENCES
Al-Adawi S (2000) Aboulia: Neurobehavioral dysfunction of dopaminergic system? Medical Hypotheses; 54:523-530.
Al-Maskari MY (2011) Basil: A Natural Source of Antioxidants and Neutraceuticals. In: Natural Products and their Active Compounds. Nova Science Publishers, Inc.
Bender A (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons imaging and Parkinson disease, Nature Genetics, vol. 38, no. 5, pp.515–517.
Bernheimer H (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations, Journal of the Neurological Sciences, vol., no. 4, pp. 415–455.
Choi B.-S (2014) Celastrol from ‘Thunder God Vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 mapk in a rotenone model of Parkinson’s disease, Neurochemical Research, vol. 39, no. 1, pp. 84–96.
De Lau LM, and Breteler MM. (2006) Epidemiology of Parkinson’s disease. Lancet Neurol; 5:525–35.
Essa MM (2012) Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res; 37:1829-42.
Halliwell B and Whiteman. M (2004) Measuring reactive species and damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology, vol. 142, no. 2, pp. 231–255.
Hoehn MM, and Yahr MD. (1967) Parkinsonism: onset, progression and mortality. Neurology; 17:427-42.
Hu, M L. (2009) Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function, Molecular Pharmacology, vol.75, no. 1, pp. 27–34.
Jayaraj R. L (2013) Neuroprotective effect of CNB-001, a novel pyrazole derivative of curcumin on biochemical and apoptotic markers against rotenone-induced SK-N-SH cellular model of Parkinson’s disease, Journal of Molecular Neuroscience, vol. 51, no. 3, pp. 863–870.
Johnson L. V (1980) Localization of mitochondria in living cells with rhodamine 123, Proceedings of the National Academy of Sciences of the United States of America, vol. 77, no. 2, pp. 990–994.
Kamat J. P (2000) Vanillin as an antioxidant in rat liver mitochondria: inhibition of protein oxidation and lipid peroxidation induced by photosensitization, Molecular and Cellular Biochemistry, vol. 209, no. 1-2, pp. 47–53.
Kim H. J (2007) Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia, Brain Research, vol. 1181, no. 1, pp. 130–141.
Koopman W. J. H (2010) Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation, Antioxidants and Redox Signaling, vol. 12, no. 12, pp. 1431–1470, 2010.
Kuzuhara S (1988) Lewy bodies are ubiquitinated: a light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl); 75:345-353.
Pari L. and Srinivasan S. (2010) Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats, Biomedicine & Pharmacotherapy, Volume 64, Issue 7, September, Pages 477-481.
Lirdprapamongkol K (2005) Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells, European Journal of Pharmaceutical Sciences. 25, no. 1, pp. 57–65.
Mehta, M (2020) Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: an update. Inflammopharmacol (2020). https://doi.org/10.1007/s10787-020-00698-3.
Park S.-E (2014) Rutin from dendropanax morbifera leveille protects human dopaminergic cells against rotenone induced cell injury through inhibiting JNK and p38 MAPK signaling, Neurochemical Research, vol. 39, no.4, pp. 707–718.
Pollanen MS (1993) Pathology and biology of the Lewy body. J Neuropathol Exp Neurol; 52:183-191.
Rajakumari R (2020) Grape Seed Extract-Soluplus Dispersion and its Antioxidant Activity, Drug Development and Industrial Pharmacy, DOI: 10.1080/03639045.2020.1788059.
Rajeshkumar S (2019) Anticariogenic Activity of Fresh Aloe Vera Gel Mediated Copper Oxide Nanoparticles Indian Journal of Public Health Research & Development 10 (11) 3664-3667.
Renukadevi Balusamy S (2020) Citral Induced Apoptosis through Modulation of Key Genes Involved in Fatty Acid Biosynthesis in Human Prostate Cancer Cells: In Silico and In Vitro Study BioMed Research International, Volume 2020, Article ID 6040727, 15 pages, https://doi.org/10.1155/2020/6040727.
Santosh Kumar S (2002) Free radical scavenging activity of vanillin and o-vanillin using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, Redox Report, vol. 7, no. 1, pp. 35–40.
Seaton T. A (1997) Free radical scavengers to protect dopaminergic cell lines from apoptosis induced by complex I inhibitors, Brain Research, vol. 777, no.1-2, pp. 110–118.
Shulman LM (2001) Comorbity of the nonmotor symptoms of Parkinson’s disease. Movement Disorders; 16:507–510.
Sirlak (2010) Micronized purified flavonoid fraction in pre-treating CABG patients, Texas Heart Institute Journal. 37 (2): 172–177. ISSN 1526-6702. PMC 2851420 . PMID 20401289.
Song J.-X (2012) Baicalein antagonizes rotenone-induced apoptosis in dopaminergic SHSY5Y cells related to Parkinsonism, Chinese Medicine, vol. 7, article 1.
Talpade D. J (2000) In vivo labeling of mitochondrial complex I (NADH: oxidoreductase) in rat brain using [3H] dihydrorotenone, Journal of Neurochemistry, vol. 75, no. 6, pp. 2611–2621.
Tamizhselvi A (2020) Cardioprotective Effect of Ethanolic Flower Extract of Clitoria Ternatea on Doxorubicin Induced Cardiotoxicity in Rats, International Journal of Research in Pharmaceutical Sciences, 11(2), pp. 1604-1611.
Tanner CM, and Goldman SM. (1996) Epidemiology of Parkinson’s disease. Neurol Clin; 14:317-335.
Thangavelu, L (2020) Evaluation of the sub-acute toxicity of Acacia catechu Wild seed extract in a Wistar albino rat model. Regulatory Toxicology and Pharmacology, p.104640.
Turrens J. F. (1997) Superoxide production by the mitochondrial respiratory chain, Bioscience Reports, vol. 17, no. 1, pp. 3–8.
Turrens J. F. (2003) Mitochondrial formation of reactive oxygen species, The Journal of Physiology, vol. 552, no. 2, pp. 335–344.