1Department of Biochemistry and Biotechnology, Annamalai University, Annamalainagar, Tamil Nadu, India.
2Department of Chemistry, Government College of Arts and Science, Tittagudi, Tamil Nadu, India
3Department of Biochemistry, School of Biological Sciences, Madurai Kamaraj University, Madurai, Tamil Nadu, India.
Corresponding author email: justinthenmozhi@gmail.com
Article Publishing History
Received: 21/11/2021
Accepted After Revision: 25/03/2022
According to the report of World Health Organization, it is expected that depression will be one of the major causes for disability and disease in the world, by 2030. Currently used drugs will not be effective to the depressive patients, due to their lower efficiency with side effects. Hence, there is a need to concentrate on natural products to overcome therapeutic hassles. Morin, a bioflavonoid found in fruits, vegetables, some herbs and wine is reported to have an antidepressant-like effect against acute stress conditions. It is reported to possess various pharmacological properties such as antioxidant, anti-diabetic, hepatoprotective, anti-cancer and anti-inflammatory activities. To understand the antidepressant effect of morin against unpredicted chronic mild stress (UCMS), rats were divided into normal, UCMS alone, UCMS and morin (30 & 60 mg/kg) and morin alone (60 mg/kg) groups. Serum corticosterone levels, expression of brain derived neurotrophic factor (BDNF), apoptotic and endoplasmic reticulum (ER) stress related indices were compared among the groups. UCMS alone exposed rats showed less crossings with diminished activities in open field test (OFT), increased serum corticosterone levels and enhanced expression of BDNF signaling and ER stress related markers associated with apoptosis as compared to control. In contrast, morin (60 mg/kg b.w.) cotreatment attenuated UCMS induced abnormalities as compared to UCMS alone exposed animals. The present results indicated the antidepressant-like actions of morin against UCMS in rats were partially due to its anti-apoptotic effects by regulating BDNF/Trk-B and ER stress related markers. Moreover the results of the present study indicated that the morin may act as therapeutic agent for the management of depression alone or with other currently used antidepressants.
Brain Derived Neurotrophic Factor, Chronic Mild Stress, Corticosterone, Endoplasmic Reticulum Stress, Morin.
Kiruthika R, Prema A, Devi S. A, Manivasagam T, Thenmozhi A. J. Morin mitigates Chronic and Unpredictable Mild Stress-Induced Depression by Regulation of Endoplasmic Reticulum Stress and Brain-Derived Neurotrophic Factor-Mediated Apoptosis. Biosc.Biotech.Res.Comm. 2022;15(1).
Kiruthika R, Prema A, Devi S. A, Manivasagam T, Thenmozhi A. J. Morin mitigates Chronic and Unpredictable Mild Stress-Induced Depression by Regulation of Endoplasmic Reticulum Stress and Brain-Derived Neurotrophic Factor-Mediated Apoptosis. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3twoGUE“>https://bit.ly/3twoGUE</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Stress is the unavoidable condition affecting the quality of human life in which the system can acclimatize or fail to acclimatize day-today hassles, abuse or diseased state (Sakr et al. 2015). The failure of adaptation or exposure to prolonged stress leads to development of depression, ultimately leads to various diseases including Alzheimer’s, Parkinson’s and cardiovascular diseases, diabetes mellitus and rheumatoid arthritis due to the disturbance in brain functions and body physiology (Chakravarty et al. 2013). The World Health Organization predicted that depression will be the second most contributing source of disability and disease in the world by (2030) (Albert and Fiori 2014; Sheng et al. 2021).
Chronic stress exposure induces numerous reactions inside the body via activating HPA axis, thereby stimulating hypothalamic-derived corticotropin-releasing factor (CRF) secretion. CRF enhances the adrenocorticotropic hormone secretion from the pituitary gland, which in turn activates adrenal cortex to release cortisol (Charney 2003; Sheng et al. 2021). Protein folding and secretion is one of the main functions of endoplasmic reticulum. Under various pathological state such as oxidative stress, a high‑fat diet, hypoxia, hypoglycemia, calcium depletion and stress could impair the protein folding and results in their accumulation, a condition called as ER stress (Hetz and Papa 2018).
It is shown that both the chronic social defeat stress and UCMS triggers ER stress and depressive behaviors in rodents (Zhao et al. 2013; Tan et al. 2015). ER stress and apoptosis are the key features underlying in the pathology of depression, as the functions of ER are vigorously impaired under stress conditions, apoptotic events are stimulated (Hetz et al. 2015). BDNF is the most studied neurotrophin in depressed conditions, reported to maintain the homeostasis of neuronal proliferation and death that acts via the activation of two receptors, (i) tyrosine kinase receptor-B (Trk-B, and (ii) p75 (Peng et al. 2020).
Unpredictable chronic mild stress (UCMS) exposure to rats mimics the similar behavioural and neuroendocrine changes as that of depressive patients (Lee et al. 2015) and hence used to analyze stress pathology. Hippocampus, an important brain region, reported to play a key role in cognitive function that is mainly affected by depression and anxiety (Biala and Kruk 2009). Incomplete knowledge about the etiology and progression of depression leads to unsuccessful treatment. Currently used drug could not used successfully to the depressive patients, due to their lower efficiency with side effects (Bschor et al. 2012). Hence, there is a need to focus on natural products to overcome therapeutic hassles (Munir et al. 2020).
Morin, a ubiquitous bioflavonoid possesses antioxidant, anti-diabetic, cardioprotective, nephroprotective, hepatoprotective, anti-cancer and anti-inflammatory properties (Rajput et al. 2021). Further, morin exhibited its neuroprotective effect against amyloid, acrylamide, haloperidol, glutamate and 1-methyl-4-phenylpyridinium ion induced toxicity in rodents (Khamchai et al. 2020; Singaravelu et al., 2021). Previous experiment from our lab demonstrated that the oral administration of morin attenuated the UCMS induced behavioural impairments and oxidative stress by its potent antioxidant activity (Kiruthika et al. 2021). However to elucidate further molecular mechanism and confirm its neuroprotective effect, the antiapoptotic role of morin via ER stress and BDNF/TrkB mediated pathways were evaluated in this study.
MATERIAL AND METHODS
Male Albino wistar rats (200–225 g) were obtained and kept in standard conditions at Central Animal House, Rajah Muthiah Medical College & Hospital, Annamalai University with food and water ad libitum. The Institutional Animal Ethics Committee (Reg. No. 160/1999/CPCSEA, Ethical Clearance No. AU-IAEC/1212/4/18) approved the protocols of the work. Morin and other chemicals of analytical grade used in this study were procured from Sigma-Aldrich, Bangalore, India. Primary antibodies against glucose-regulated protein 78 (GRP78), spliced X-box-binding protein-1 (XBP-1), CCAAT/enhancer binding protein homologous protein (CHOP), cytochome c, cleaved caspases 3and 12, bax, BDNF, TrkB, p-TrkB, p75, β-actin and secondary antibodies were purchased from Cell Signaling Technology Inc (Beverly, MA, USA). After the acclimatization phase of one week, thirty rats were randomized into five groups (n = 6). Group I animals were kept without disturbance for 42 days under standard condition. Group II rats were subjected to UCMS for 6 weeks (Lucca et al. 2009; Yang et al. 2017).
Group III and IV rats were exposed to UCMS and oral administration of morin (30 and 60 mg/kg) (Ola et al. 2014) for 42 days. Group V rats were administered with morin (60 mg/kg) alone as group IV for 42 days. After performing the open field test (Rajasankar et al. 2009) animals were decapitated and blood was collected and centrifuged for serum separation. It was stored in frozen condition until biochemical assay was carried out. Circulatory corticosterone level was quantified by enzyme immunoassay using a commercial kit procured from Assay Designs, Inc., Ann Arbor, USA. Hippocampus was procured, ground in an ice-cold RIPA buffer and centrifuged to collect the supernatant. Levels of protein were quantified by Lowry et al. (1951) method. Cellular proteins (50 µg) were separated using SDS–PAGE. It was blotted to PVDF membrane, which were developed with blocking buffer and incubated with primary immunoglobulins of cyto c, caspases 3 and 12, bax, BDNF, TrkB, p-TrkB, GRP78, XBP-1, CHOP and p75 with shaking overnight.
The membranes were then incubated with secondary antibodies for 2 h at 37˚C and washed. Protein bands were quantified by using chemiluminescence’s method and obtained bands were scanned and estimated by gel image analysis program. Data were expressed as mean ± Standard Error (SEM) of six rats for behavioural and biochemical studies and of three rats for western blot analysis. The statistical significance was calculated by one-way analysis of variance (ANOVA) using SPSS version 15.0 using Duncan’s Multiple Range Test (DMRT). A value of p< 0.0.5 was considered as a significant difference between groups and the values not sharing common alphabet differ significantly with each other.
RESULTS AND DISCUSSION
The protective effect of morin on UCMS induced behavioral despair was assessed by the OFT (Figure 1). In OFT, there was a significant reduction in the number of peripheral and central lines crossed, grooming and rearing actions in the rats subjected to UCMS as compared to control animals. However, more crossings with enhanced grooming and rearing activities were exhibited by UCMS and morin (30 and 60 mg/kg) co-treated groups as compared to UCMS alone rats. Liu et al. (2009) indicated that the rats exposed to UCMS for 42 days showed depressive behavioral indices like reduced locomotion and activity. The open field test is used to measure the deleterious effect of UCMS. The enhanced score in OFT represents the increased movement, grooming and rearing activities and lowered state of anxiety (Duan et al. 2008). In our study, diminished locomotion and activities were found in the UCMS exposed and morin co-administrated rats as compared to UCMS alone exposed animals, which is corroborated with previous studies (Ben-Azu et al. 2019; Hassan et al. 2020).
Figure 1: Effect of morin on movements (A, B) and activities (C) of rats exposed to UCMS.
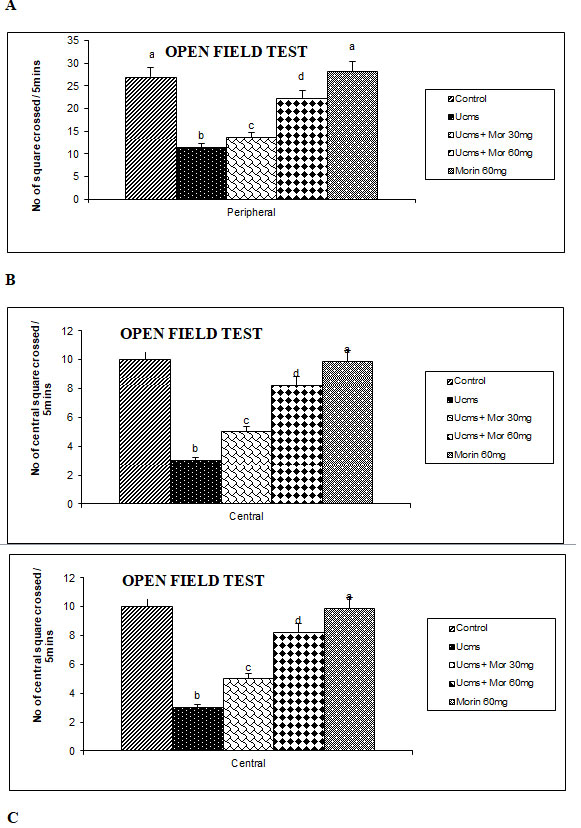
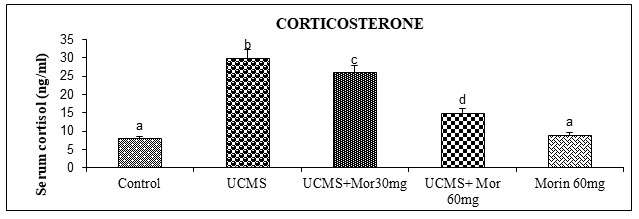
The protective effect of morin on UCMS persuaded dysfunctions in feedback mechanism regulating endocrine secretion was indicated by estimating serum corticosterone levels (Figure 2). UCMS rats showed an increase in the serum corticosterone levels than the non-stressed control rats. Morin (30 and 60 mg/kg) administration to UCMS rats significantly depleted the levels of serum corticosterone as compared to UCMS exposed rats. Hyperactivation of the HPA axis causes more secretion of corticosteroids from the adrenal cortex. Hence corticosterone is considered as an important marker of the HPA axis hyperactivation that plays a vital function in the determination of therapeutic efficacy of antidepressant drugs (Dean and Keshavan 2017; Nandam et al.2020).
The UCMS induced enhancement of corticosterone levels were observed in this study is consistent with previous results having deleterious effect on health as suggested by Teague et al. (2007). Pariante and Miller (2001) shown that an increased levels of glucocorticoid is found in cerebrospinal fluid, plasma and urine of depressive patients. Checkley (1996) suggested that the enhanced level of glucocorticoid is one of the main causes of depression in animal models.
Prolonged glucocorticoid administration induced alterations in both the morphology and functions in the brain regions thereby inducing depressive like behaviors (Gregus et al. 2005). Moreover neuronal atrophy stimulated by glucocorticoids resembles the same as that of depressed patients (Sapolsky 2000). Successful antidepressant therapies are connected with the normalization of HPA axis dysfunction by the reducing serum cortisol levels and enhancing monoamine levels in UCMS rats (Parker et al. 2003; Nandam et al.2020).
Figure 2: Effect of morin on the levels of circulatory corticosterone in rats exposed to UCMS.
To investigate the antiapoptotic effect of morin on UCMS exposed rats, the expression of apoptotic (bax, caspase 3, cyto c), neurotrophin (BDNF, TrkB, p-TrkB and p75) and ER stress (GRP78, spliced XBP-1, CHOP, cleaved caspase 12) related markers were studied. The protein expression studies indicated that the elevated expression of bax, cleaved caspases 3 and 12, cyto c, GRP78, spliced XBP-1, CHOP and p75, diminished expression of BDNF and p-TrkB with un-altered expression of total TrkB were found in the UCMS-exposed rats (p < 0.05). Administration of morin attenuated UCMS induced alterations in the expression of apoptotic, neurotrophin and ER stress related markers (Figure 3, 4, 5). Levels of BDNF in brain were found to be diminished in experimental models of depression and postmortem samples of patients with depression, while the administration of antidepressants enhanced BDNF expression in hippocampus and cortex (Song et al. 2020).
Kimpton (2012) indicated that the reduced expression of BDNF in stressed animals results in diminished neurogenesis and development of depressive and impaired cognitive symptoms, which is corroborated with our present results. BDNF exert pivotal effect through activation of TrkB receptor (facilitating viability, differentiation and synaptogenesis of neurons) and p75 (leading to apoptosis) receptors. The diminished expression of TrkB receptors and enhanced expression of p75 found in UCMS rats thus favored apoptosis, whereas oral treatment of morin ameliorated these alterations by its neuroprotective function (Celik et al. 2020).
Figure 3: A. Effect of morin on the expression of apoptotic markers in rats exposed to UCMS. B.
Immunoblot data are quantified by using β-actin as an internal.
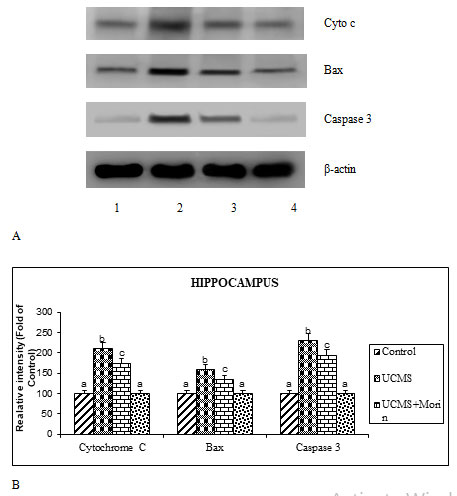
Figure 4: A. Effect of morin on the expression of BDNF, pTrkB and P75 in rats exposed to
UCMS. B. Immunoblot data are quantified by using β-actin as an internal.
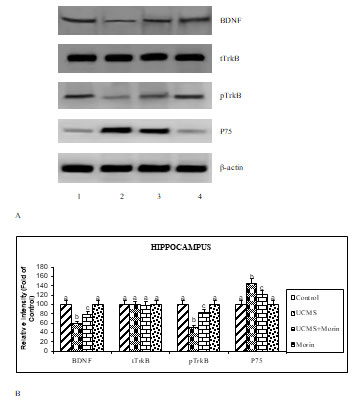
Figure 5: A. Effect of morin on the expression of ER stress related markers in rats exposed to
UCMS. B. Immunoblot data are quantified by using β-actin as an internal.
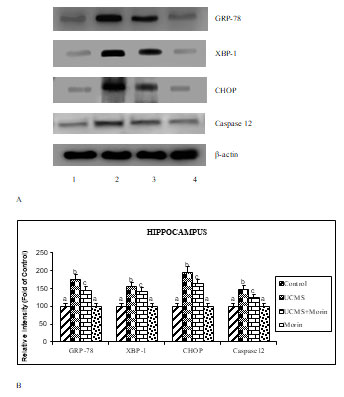
Previous studies indicated that neurological disorders like depression, Parkinson’s and Alzheimer’s disease occurred due to the progression of excessive apoptosis. We found that the increased bax, cytosolic cytochrome c and caspase 3 expression indicating the enhanced apoptosis of hippocampal neurons in CUMS rats. Activation/inactivation of apoptosis is primarily regulated by the Bcl-2 family proteins (Elmore 2007). The activation of proapoptotic factor Bax, enhances mitochondrial membrane permeability after their entry into mitochondria from cytosol, thereby resulted in mitochondrial cytochrome c release and activation of caspases 3. This eventually leads to apoptotic cell death (Eskes et al. 1998; Lidsky and Schneider 2003). Previous studies demonstrated that morin has been showed to offer the neuroprotective effects against several metabolic disorders and neurodegenerative diseases through its anti-apoptotic effect (Komirishetty et al. 2016; Sharma et al. 2020).
During UCMS exposure, increased expression of GRP78 is reported and considered as the hall mark of ER stress because of its translocation from the membrane receptors and stimulate the unfolded protein response (UPR) (defensive process) in neurons (Zhang and Zhang 2010). The UPR induced apoptosis occurred by the activation of CHOP and caspase-12. CHOP is classified in the C/EBP family which maintains the homeostasis of pro-apoptotic BCl2 proteins to induce apoptosis (McCullough et al. 2001). Similar to GRP78, the upregulated CHOP expression is a key symbol of ER stress-induced apoptosis and its inhibition is considered as the restoration of ER function (Nishitoh 2012).
The caspase-12, a sole member of caspase family found in ER membrane, and its down regulation was shown to prevent ER stress-induced cell death (Ferri and Kroemer 2001). Lee et al. (2003) indicated that the genes of spliced XBP-1, play a key role in the restoration of ER function by regulating the transcription factors involved in protein folding and degradation. Morin attenuated various toxins induced ER stress related indices in several in vivo and in vitro studies, which is corroborated with our results (Sharma et al. 2020).
CONCLUSION
The findings of the present experiment indicated that morin offered neuroprotective effects on UCMS-induced apoptosis in rat model of depression. The above said antiapoptotic role of morin is seems due to the regulation of BDNF pathway and ER stress. The obtained molecular results were supported by the biochemical and behavioral studies. Hence, morin may act as therapeutic agent for the management of depression with other currently used antidepressants. However further studies are needed for exploring its clinical efficacy and safety.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Albert PR and Fiori LM. (2014). Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Current Pharmaceutical Design 20: 3738-3750.
Ben-Azu B, Aderibigbe AO, Ajayi AM, et al. (2019). Morin decreases cortical pyramidal neuron degeneration via inhibition of neuroinflammation in mouse model of schizophrenia. International Immunopharmacology 70: 338-353.
Biala G and Kruk M. (2009). Effects of co-administration of bupropion and nicotine or D-amphetamine on the elevated plus maze test in mice. Journal of Pharmacy and Pharmacology 61: 493-502.
Bschor T, Ising M, Erbe S, et al. (2011). Impact of citalopram on the HPA system. A study of the combined DEX/CRH test in 30 unipolar depressed patients. Journal of Psychiatric Research 46: 111-117.
Celik H, Kucukler S, Comakli S, et al. (2020). Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of oxidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology 76: 126-137.
Chakravarty S, Reddy BR, Sudhakar SR, et al. (2013).Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a Zebra fish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS One 8: e63302.
Charney DS (2003). Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatrica Scandinavica, 417: 38-50.
Checkley S (1996). The neuroendocrinology of depression and chronic stress. British Medical Bulletin 52: 597-617.
Dean J and Keshavan M. (2017). The neurobiology of depression: An integrated view. Asian Journal of Psychiatry 27: 101-111.
Duan DM, Tu, Y and Chen LP. (2008). Effects of electroacupuncture at different acupoint groups on behavior activity and p- CREB expression in hippocampus in the rat of depression. Chinese Acupuncture and Moxibustion 28: 369-373.
Elmore S. (2007). Apoptosis: a review of programmed cell death. Toxicologic Pathology, 35, 495-416.
Eskes R, Antonsson B, Osen-Sand A, et al. (1998). Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol 143: 217-224.
Ferri KF and Kroemer G. (2001). Organelle-specific initiation of cell death pathways. Nature Cell Biology 3: 255-263.
Gregus A, Wintink AJ, Davis AC, et al. (2005). Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in rats. Behav Brain Res 156: 105-114.
Hassan MM, Gad AM, Menze ET et al. (2020). Protective effects of morin against depressive-like behavior prompted by chronic unpredictable mild stress in rats: Possible role of inflammasome-related pathways. Biochem Pharmacol 180:114140
Hetz C, Chevet E, and Oakes SA. (2015). Proteostasis control by the unfolded protein response. Nature Cell Biology 17: 829 838.
Khamchai S, Chumboatong W, Hata J et al. (2020). Morin protects the blood-brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep 10,13379.
Kimpton J. (2012). The brain derived neurotrophic factor and influences of stress in depression. Psychiatria Danubina 24: 169-171.
Kiruthika R, Hema T, Thenmozhi AJ, et al. (2021). Neuroprotective effect of morin against unpredictable chronic mild stress induced oxidative stress and behavioural deficits in Wistar rats. International Journal of Research in Pharmaceutical Sciences 12(2): 1-7.
Komirishetty P, Areti A, Sistla R, et al. (2016). Morin mitigates chronic constriction injury (CCI)-induced peripheral neuropathy by inhibiting oxidative stress induced PARP over-activation and neuroinflammation. Neurochemical Research 41: 2029-2042
Lee AH, Iwakoshi NN and Glimcher LH. (2003). XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular Cell Biology 23: 7448-7459.
Lee TH, Kim K, Shin MS, et al. (2015). Treadmill exercise alleviates chronic mild stress-induced depression in rats. Journal of Exercise Rehabilitation 11: 303-310.
Lidsky TI and Schneider JS. (2003). Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126: 5-19.
Liu Q, Li B, Zhu HY, et al. (2009). Clomipramine treatment reversed the glial pathology in a chronic unpredictable stress-induced rat model of depression. European Neuropsychopharmacology 19: 796-805.
Lowry OH, Rosebrough NJ, Farr AL, et al. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 193(1): 265-275.
Lucca G, Clarissa M, Samira CS, et al. (2009). Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochemistry International 54: 358-362.
McCullough KD, Martindale JL, Klotz LO, et al. (2001). Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Molecular Cell Biology 21: 1249-1259.
Munir S, Shahid A, Aslam B, et al. (2020). The therapeutic prospects of naturally occurring and synthetic indole alkaloids for depression and anxiety disorders. Evidence-Based Complementary and Alternative Medicine 8836983: 1-11.
Nandam LS, Brazel M, Zhou M et al. (2020). Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front Psychiatry 10: 974.
Nishitoh H (2012). CHOP is a multifunctional transcription factor in the ER stress response. Journal of Biochemistry 151: 217-219.
Ola MS, Aleisa A, Al-Rejaie SS, et al. (2014). Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurological Sciences 35: 1003-1008.
Pariante CM and Miller AH. (2001). Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry 49: 391-404.
Parker KJ, Schatzberg AF and Lyons DM. (2003). Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior 43: 60-66.
Peng Z, Zhang C, Yan L, et al. (2020). EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippocampal apoptosis signaling in a chronic stress-induced rat model of depression. International Journal of Molecular Sciences 21: 1769.
RajaSankar S, Manivasagam T and Surendran S. (2009). Ashwagandha leaf extract: a potential agent in treating oxidative damage and physiological abnormalities seen in a mouse model of Parkinson’s disease. Neuroscience Letters 454: 11-15.
Rajput SA, Wang X, and Yan H. (2021). Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomedicine & Pharmacotherapy 38 (in press)
Sakr HF, Abbas AM, Elsamanoudy AZ, et al. (2015). Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. Journal of Physiology and Pharmacology 66: 515-527.
Sapolsky RM. (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry 57: 925-935.
Sharma SH, Kumar JS, Madhavan S, et al. (2020). Morin supplementation modulates PERK branch of UPR and mitigates 1,2-dimethylhydrazine-induced angiogenesis and oxidative stress in the colon of experimental rats. Toxicology Mechanisms and Methods 30(4): 306-315.
Sheng JA, Bales NJ, Myers SA, et al. (2021). The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front Behav Neurosci 14: 601939.
Singaravelu A, Venkatachalam K, Jayaraj RL, et al. (2021). Morin treatment for acute ethanol exposure in rats. Biotech Histochem 96: 230-241.
Song AQ, Gao B, Fan JJ, et al. (2020). NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. Journal of Neuroinflammation 17: 178.
Tan H, Zou W, Jiang J, et al. (2015). Disturbance of hippocampal H2S generation contributes to CUMS induced depression-like behavior: Involvement in endoplasmic reticulum stress of hippocampus. Acta Biochimica et Biophysica Sinica 47(4): 285-291.
Teague CR, Dhabhar FS, Barton RH, et al. (2007). Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague-Dawley rats. Journal of Proteome Research 6: 2080-2093.
Yang X, Song S and Xu Y. (2017). Resveratrol ameliorates chronic unpredictable mild stress induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatric Disease and Treatment 13: 2727-2736.
Zhang LH and Zhang X. (2010). Roles of GRP78 in physiology and cancer. Journal of Cellular Biochemistry 110: 1299-1305.
Zhao T, Huang GB, Muna SS, et al. (2013). Effects of chronic social defeat stress on behavior and choline acetyltransferase, 78-kDa glucose-regulated protein, and CCAAT/enhancer-binding protein (C/EBP) homologous protein in adult mice. Psychopharmacology 228: 217-230.


