1Department of Prosthetic Dental Sciences, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
2General Dental Practitioner, Riyadh, Saudi Arabia.
3Postgraduate Student, Doctorate Program of Restorative Dental Sciences, College of Dentistry, King Saud University. Riyadh, Saudi Arabia
Corresponding author email: haalotaibi@ksu.edu.sa
Article Publishing History
Received: 10/12/2020
Accepted After Revision: 20/03/2021
Computer-aided design and Computer-aided manufacturing (CAD/CAM) has emerged as a new approach for the fabrication of removable prosthesis offering many advantages over the conventional fabrication methods. The pre-polymerized polymethyl Methacrylate (PMMA) pucks used for the fabrication of CAD/CAM removable prosthesis has a significantly enhanced physical and mechanical properties. This study aims to evaluate the effect of different salivary pH values on monomer leakage from heat-cured and CAD/CAM denture acrylic materials. Two groups of 60 discs were fabricated from heat-cured and CAD/CAM acrylic materials. These acrylic samples were subjected to mechanical brushing and thermocycling according to a standardized protocol. The discs of the two acrylic materials were immersed and incubated in three salivary solutions with different pH values (acidic, 5.7; neutral, 7; basic, 8.3) for 30 days, after which the amount of leaked monomer in the saliva solution in the two groups was determined using high-performance liquid chromatography (HPLC). Both the acrylic material type and salivary pH value had a significant effect on monomer leakage. An acidic salivary pH caused the most monomer leakage in both acrylic material groups (P < 0.05). The heat-cured acrylic material leaked less monomer than the CAD/CAM acrylic materials. The acidic salivary pH values were associated with higher amounts of monomer leakage in both heat-cured and CAD/CAM denture acrylic materials. In-laboratory immersion of newly fabricated heat-cured and CAD/CAM acrylic dentures in an acidic solution might be recommended to allow most unreacted monomers to leak before delivering the denture to the patient.
Acrylic, CAD/CAM, Denture, Monomer Leakage, Salivary pH.
Al-Otaibi H. N, Alshaalan N. S, Alqarni R, AlMutairi R. M, Altaweel S. M, Alshehri H. A, Alfouzan A. F. Monomer leakage Behavior of Conventional and CAD/CAM Denture Acrylic Materials Under Different Salivary pH Values. Biosc.Biotech.Res.Comm. 2021;14(1).
Al-Otaibi H. N, Alshaalan N. S, Alqarni R, AlMutairi R. M, Altaweel S. M, Alshehri H. A, Alfouzan A. F. Monomer leakage Behavior of Conventional and CAD/CAM Denture Acrylic Materials Under Different Salivary pH Values. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/2MhS8wd”>https://bit.ly/2MhS8wd</a>
Copyright © Al-Otaibi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Despite advances in preventive dentistry, edentulism is still a major public health problem and is considered an important indicator of the oral health of elderly population where the loss of some or all remaining teeth has a negative impact on the health-related quality of life (Cunha-Cruz et al 2007; Emami et al 2013; Batista et al 2014; Silva-Junior et al 2017; Batista et al 2018). Accordingly , the use of removable dental prostheses has increased among older patients who are the primary wearers of dentures in the general population (Dye et al 2012 Kassebaum et al 2014; Kassebaum et al 2017). Several materials have been used for the construction of removable prosthesis and dentists have long been searching for ideal materials for the construction of dentures. Nowadays, Polymethyl methacrylate (PMMA) resin is considered the material of choice for the fabrication of removable prostheses. Despite its weak flexural and impact strength and low fatigue resistance, it has many advantageous properties, including good mechanical features, ease of fabrication and repair, aesthetic properties and stability in the oral cavity (Dogan et al 2007; Nakamura et al 2007; Mohamed 2008 ; Alla et al 2013; Gad et al 2017; Zafar 2020).
Similar to any other dental materials used inside the oral cavity, PMMA resin denture base materials are subjected to changing wet oral environment which is physiologically characterized by natural saliva and its components (Zidan et al 2020). Potential harmful effects may arise from pH changes due to cariogenic biofilms in the oral ecology, diet intake and different enzymes (Turssi et al 2003). These phenomena can lead to the leaching out of plasticizers and soluble components from the acrylic over extended periods (Mohamed 2008; Marsh and Zaura 2017; Du et al 2020). It is widely reported in the literature that substances leaching out from denture base acrylic resins can cause cytotoxic effects (Koutis and Freeman 2001; Gonçalves et al 2006; Mörmann et al 2013; Rashid et al 2015). Unreacted residual monomers are the main substances that leach out from acrylic resins by the process of diffusion, the quantity of which is highly related to the polymerization reaction of acrylic resins (Chaves et al 2012; Iça et al 2014; Nik et al 2014). Unreacted monomers may cause toxic effects, adverse allergic reactions and significant damage at the cellular level (Drozdz et al 2011; Goiato et al 2015; Çakırbay et al 2018; Degirmenci et al 2020).
Several studies have aimed to quantify the amount of diffusing monomer and other leachable components from acrylic-based materials into the saliva. One study found that the maximum concentration of residual monomer leaching into the saliva of patients wearing complete dentures in their post-insertion period peaked one day after the insertion and that despite this amount of released monomer being at a none toxic levels, it could still potentially sensitize complete denture patients and induce an allergic reaction (Singh et al 2013). Another study attempted to quantify the residual monomer elution of conventional and computer-aided design/computer-aided manufacturing (CAD/CAM) dental acrylic-based materials during
artificial aging and it found that both CAD/CAM and conventional polymers eluted residual monomer within different aging time (Engler et al 2020).
Another important factor to be considered in the diffusion of monomers from acrylic-based materials is the salivary pH value which is known to affect biodegradation of the material and it was found that the amount of monomer released from different denture base acrylic material processed by different polymerization methods and stored in different storage conditions is higher when stored in an acidic saliva environment in comparison to neutral saliva (Bettencourt et al 2010; Tuna et al 2013; Akay et al 2017; Sá et al 2020).
In recent years, CAD/CAM technology has become an alternative to conventional methods in the fabrication of removable prostheses. In 1994, the first scientific article discussing the use of CAD/CAM in the fabrication of complete dentures was published (Maeda et al 1994). Since then, numerous CAD/CAM denture systems have been introduced into the market (Kattadiyil et al 2013; Steinmassl et al 2017). CAD/CAM-fabricated complete dentures have several advantages over conventionally fabricated complete dentures, such as decreased porosity, enhanced predictability of the desired outcomes and excellent fitting accuracy ( Bidra et al 2013; de Mendonça et al 2016). Because the acrylic used for the fabrication of dentures using CAD/CAM technology is pre-polymerized, the prosthesis seems to contain less residual monomer and is more hydrophobic than the conventionally processed one, resulting in a more bio-hygienic prosthesis (Masri and Driscoll 2015). A recent research that studied CAD/CAM dentures and aimed at evaluating the color stability of it when immersed in different beverages found that milled denture blocks had greater resistance to stain accumulation in comparison to the conventional one (Al-Qarni et al 2020). However, limited data are available on the properties related to the monomer leakage of CAD/CAM processed denture material when the salivary pH values alternate between acidic and basic conditions.
This study has aimed to evaluate the effect of different salivary pH values on monomer leakage from conventional and CAD/CAM acrylic denture base materials, with a null hypothesis that there is no difference between the two types of acrylic denture base materials in terms of the effect of the salivary pH values on monomer leakage.
MATERIAL AND METHODS
Two types of acrylic resin materials were used: a CAD/CAM-manufactured resin (IvoBase® CAD; Zenotec, Wieland Dental, Germany) and a heat-cured resin (SR Ivocap High Impact®; Ivoclar Vivadent AG, Liechtenstein). Two groups of 60 discs were fabricated. The dimensions of the discs were 10 mm (diameter) × 3 mm (thickness). Each of the two groups was divided into three subgroups, with 10 discs each.The CAD/CAM Acrylic discs were designed with predetermined dimensions using Zenotec® CAD software (Wieland Digital Denture; Ivoclar Vivadent, Schaan, Liechtenstein). PMMA blocks were used (Opera system, Principauté de Monaco, French), and the milling procedure was performed using Zenotec® selection (Wieland Digital Denture; Ivoclar Vivadent, Schaan, Liechtenstein). The discs were then finished and polished using a dental laboratory polishing machine with a vacuum cleaner (Aspyclean+ M2V®, Manfredi, Italy), pumice (Interdent, Slovenia) and a rag polishing wheel (Rag muslin wheel; Kerr, USA).
For the fabrication of Heat-Cured acrylic resin discs, a putty molds of the preferred disc dimensions were fabricated using a polyvinyl siloxane putty material (Express STD®; 3 M ESPE, United States). The silicone molds were filled with melted base plate wax. A Bantam flask was filled with a plaster mix with a powder : water ratio of 100 g:47 cc (Lab Plaster Fast Set®; Dentsply, Canada), and then the putty mold was immersed in the plaster mix so that the top of the mold was flushed with the top of the plaster mix. After the plaster was set, a thin layer of petroleum jelly (Vaseline) was applied to the top. The upper half of the flask was then fixed to the bottom half and filled with plaster mix, and then the lid of the flask was placed on the top. After that, the flask was placed in a wax elimination machine (Wapo-Ex®; Wassermann, Germany) for 30 minutes at 90 °F to 100 °F. The flask was then opened, and the melted wax was washed away using boiling water.
A thin layer of separating fluid (Ivoclar Vivadent; Schaan, Liechtenstein) was applied to the plaster surface. The heat-cured acrylic provided as a single capsule containing premeasured polymer and monomer (SR Ivocap High Impact®; Ivoclar Vivadent AG, Liechtenstein) was then mixed for 5 minutes using a cap vibrator (Cap vibrator®; Ivoclar Vivadent, Schaan, Liechtenstein). The mixture was poured into the putty mold and pressed using a pressure apparatus (OL 463, Manfredi, Italy). Next, the flask assembly was placed in a polymerization bath (100 °C water) for 35 minutes (Electronic Denture Curing System; Nevin Labs™; USA). The discs were finished and polished using a dental laboratory polishing machine with pumice (Interdent, Slovenia) and a rag polishing wheel (Rag Muslin wheel®; Kerr, USA).
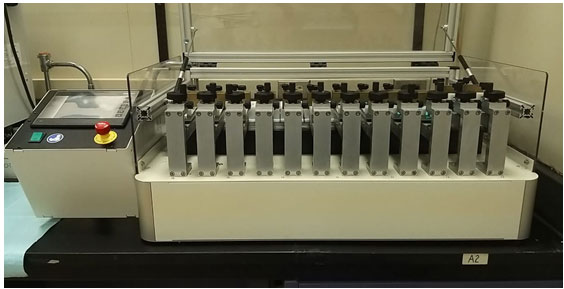
A Mechanical brushing was performed according to the recommendations of the International Organization for Standardization (ISO). The specimens were brushed with soft toothbrushes mounted on a toothbrush simulator (ZM-3.12; SD Mechatromik GmbH, Germany) (Figure 1). The specimens were subjected to linear toothbrush abrasion movement at a rate of 356 brush strokes (back and forth) per minute. The machine provides a 200-g vertical load over each specimen and a 5-mm path starting from the center of each specimen and brushes six specimens simultaneously. The total brushing time was 50 minutes, with 17,800 cycles (representing one year). Brushing was performed in distilled water (23+3 °C) and dentifrice (Crest Cavity Protection Regular Paste; P&G, Germany) (Figure 2).
Using an SD Mechatronik GmbH thermocycler (SD Mechatronik, Germany), all the specimens were stored in distilled water and subjected to thermocycling between 5 °C and 55 °C, with a dwell time of 30 seconds and a transfer time of 12 seconds for 1,000 cycles (Pusz et al 2010).
Figure 1: Tooth brushing Simulator Machine
Figure 2: Brushing of the PMMA discs sample.
For the process of artificial saliva preparation and incubation of the samples, an artificial saliva was prepared at three different pH values (5.7, 7 and 8.3). An electrolyte composition similar to that of human saliva was used in this study, as shown in Table 1 (Kostić et al 2015):
Table 1: Chemical Composition of Artificial Human Saliva
| Na2HPO4 | 0.260 g/l |
| NaCl | 0.700 g/l |
| KSCN | 0.330 g/l |
| KH2PO4 | 0.200 g/l |
| NaHCO3 | 1.500 g/l |
| KCl | 1.200 g/l |
A buffer solution comprising KH2PO4 and Na2HPO4 was prepared by dissolving each solution in 1 liter of deionized distilled water. Basic saliva was prepared by taking 500 ml of Na2HPO4 and adding KH2PO4 gradually until the desired pH was reached. Next, the other salts (NaCl, KSCN, NaHCO3 and KCl) were added to the saliva, and the volume was completed to 1 liter using deionized distilled water. Neutral and acidic saliva solutions were prepared by taking 500 ml of KH2PO4 and adding Na2HPO4 gradually until the desired pH was reached. Next, the other salts (NaCl, KSCN, NaHCO3 and KCl) were added in the same manner as described above. For neutral saliva, a greater amount of Na2HPO4 was added to reach the desired pH (Pusz et al 2010).The discs were assorted into 6 groups, with 10 discs in each group, and then were stored in artificial saliva in an incubator (Blanket warming cabinet; Malmet, Australia) at 37 °C for 30 days.
High-Performance Liquid Chromatography (HPLC) was used to determine the quantity of residual methyl methacrylate (MMA) monomer following the immersion of the two types of acrylic materials in artificial saliva at three different pH values. A UV PerkinElmer Series 200 HPLC system (PerkinElmer, Shelton, USA) equipped with a C18 column was used to perform HPLC analysis. Ten milliliters (ml) of each sample solution was injected and analyzed at 40 °C and a flow rate of 1.0 ml/min (revolutions per minute) with acetonitrile in water (50/50). One reading was obtained from each milliliter of the 10 ml sample. Fifteen minutes after sample injection, the content of MMA was calculated from the area under the peak. The average of 10 readings for each sample was calculated (Mohamed 2008).
Statistical Analysis: The data were analyzed using the SPSS statistical software (v16; SPSS Inc., Chicago, IL, USA). The effect of the acrylic material type and pH and their interaction on monomer leakage were analyzed by two-way ANOVA. Tukey’s post hoc multiple comparison was used to evaluate the differences in monomer leakage among the three pH values under each type of acrylic material.
RESULTS AND DISCUSSION
At α = 0.05 and a sample size equal to 10 under each pH value used (acidic, neutral, basic), the power of the study was estimated to be 88%. Two-way ANOVA was performed to evaluate the effect of salivary pH on monomer leakage into the saliva. Both the material type and pH of the saliva significantly affected monomer leakage (P = 0.03 and P= 0.00, respectively) (Table 2).
The mean and standard deviation (SD) of monomer leakage when the two acrylic material types were soaked in salivary solutions with different pH values are presented in table 3. The highest amount of monomer leaked from the CAD/CAM material when the material was soaked in an acidic salivary solution, while the least amount of monomer leakage occurred in the basic solution (table 3). Post-hoc multiple-comparison analysis revealed that the monomer leakage of the CAD/CAM material soaked in an acidic solution was significantly higher than that of the neutral and basic pH solutions (P = 0.01 and P = .00, respectively;(table 4).
Similarly, heat-cured acrylic exhibited most of the monomer leakage when the material was soaked in an acidic salivary solution; however, the lowest monomer leakage was observed when the material was soaked in neutral pH solution (table 3). Post-hoc multiple-comparison analysis revealed that the monomer leakage of the heat-cured acrylic material soaked in an acidic solution was significantly higher than that of the two other salivary solutions. (P = 0.00; table 4).
Table 2. Two-way ANOVA of the effect of two independent variables material type and pH value on monomer leakage (uV.sec).
| Source | Type III Sum of Squares | Df | Mean Square | F | Sig. |
| Disc Material | 426398.26 | 1 | 426398.26 | 4.86 | 0.03 |
| pH | 2635911.05 | 2 | 1317955.52 | 15.03 | 0.00 |
| Disc Material * pH | 440815.82 | 2 | 220407.91 | 2.51 | 0.09 |
| Error | 4735157.61 | 54 | 87688.10 | ||
| Total | 49121084.98 | 60 | |||
| Corrected Total | 8238282.73 | 59 |
Table 3. Monomer leakage (uV.sec) from the three different acrylic materials (CAD/CAM and heat-cured) when soaked in three solutions with different pH values (acidic, neutral, basic).
| Acrylic material | pH | Mean | Std. Deviation |
| CAD/CAM | Acid | 1320.52 | 159.31 |
| Neutral | 750.54 | 146.11 | |
| Basic | 658.21 | 122.36 | |
| Heat-Cured | Acid | 922.57 | 65.43 |
| Neutral | 628.57 | 138.56 | |
| Basic | 672.34 | 73.43 |
Table 4. Post hoc multiple-comparisons analysis to compare the effect of salivary pH value on monomer leakage for each acrylic material type.
| Disc material | (I) pH | (J) pH | Mean Difference (I-J) | Sig. | |
| CAD/CAM | Acidic | Neutral | 569.98 | 0.01* | |
| Basic | 662.31 | 0.00* | |||
| Neutral | Acid | -569.98 | 0.01* | ||
| Basic | 92.33 | 0.87 | |||
| Basic | Acid | -662.31 | 0.00* | ||
| Neutral | -92.33 | 0.87 | |||
| Heat-Cured | Acidic | Neutral | 294.00 | 0.00* | |
| Basic | 250.23 | 0.00* | |||
| Neutral | Acid | -294.00 | 0.00* | ||
| Basic | -43.77 | 0.59 | |||
| Basic | Acid | -250.23 | 0.00* | ||
| Neutral | 43.77 | 0.59 |
* The mean difference is significant at the .05 level.
Acrylic-based resins are frequently used in daily dental practice. These acrylic resins are used to replace lost tissue and transfer masticatory forces from the denture to the residual ridges because they can provide essential properties and have the necessary characteristics for use in diverse functions. Although acrylic resins have many desirable properties, one of their main drawbacks is that they contain residual monomers that may leach out and trigger undesirable side effects (Oliveira et al 2010; Ivković et al 2013; Kostić et al 2015). Diffusion is the mechanism that underlies residual monomer leakage from acrylic resins in which the constant contact of saliva with the material causes expansion of the openings present between the polymer chains, causing the unreacted monomer to diffuse out. Thus, the substances that are leached out from the denture bases into the saliva are transferred to the oral structures, causing adverse allergic reactions (Urban et al 2009; Kopperud et al 2011;Chaves et al 2012; Gautam et al 2012; Nik et al 2014; Choudhary et al 2020).
Based on the results obtained from this study, the null hypothesis was rejected indicating that the variation in salivary pH values had a significant effect on the monomer leakage from the acrylic materials used in the study. The results demonstrated that, when different acrylic resin materials were soaked in saliva with different pH values, the greatest amount of monomer leakage occurred in the acidic salivary solution, a finding that was in agreement with other studies (Faltermeier et al 2007; Bettencourt et al 2010; Akay et al 2017; Sá et al 2020). One study evaluated the residual monomer using high performance liquid chromatography (HPLC) for microwave-cured, conventional heat and injection-technique acrylic materials that were stored in neutral and acidic artificial saliva for 24 hours and it was found that all three materials exhibited higher monomer release into the acidic saliva (Tuna et al 2013). The chemical structure of the monomers used to prepare the resins could directly affect the amount of eluted monomer. Lefebvre et al (1995) studied the pattern of release of cytotoxic substances from four light-polymerized denture base resins and suggested that different components may leach out at different rates and that the release of cytotoxic resin components may continue for several days.
Heat-cured acrylic resin showed the least monomer leakage in both acidic and neutral solutions compared with the CAD/CAM material. Many studies were conducted to evaluate the amount of monomer leakage from heat-cured acrylic compared with that of other materials and all presented similar findings in which the heat-cured acrylic material showed less monomer leakage. This finding might be related to the high polymerization temperature needed to cure the acrylic material (Vallittu et al 1998; Shim and Watts 1999; Sideridou and Achilias 2005; Mohamed et al 2008; Chaves et al 2012; Nik et al 2014). In a recent study conducted to compare the residual monomer concentration and cytotoxic effect of three acrylic materials that were hot-cured or polymerized under pressure and at lower temperatures, the authors reported that the acrylic material polymerized at high temperatures has a lower residual monomer concentration, while self-curing materials polymerized at lower temperatures have a higher concentration of residual monomer, leading to a lower number of living cells that might trigger allergic reactions shortly after the new denture is delivered (Raszewski 2020).
CAD/CAM denture base acrylic resin is supplied as pre-polymerized blocks which are produced in industrially controlled conditions with standardized pressure and temperature and are known to have enhanced material-specific properties (McCabe and Walls 2013). As a result of the polymerization of PMMA blocks used for the milling of denture under high temperature and pressure, long polymer chains are formed leading to a higher degree of monomer conversion and lower values of residual monomer as well as minimal porosity (Kattadiyil et al 2013; Mörmann et al 2013; Murakami et al 2013 ; Nguyen et al 2014; Akin et al 2015; Kattadiyil et al 2015). In a recent study that aimed to evaluate the amount of monomer released from a CAD/CAM acrylic material when soaked in water, the results demonstrated that the CAD/CAM acrylic material released very little monomer. However, the amount released was not different from that released from conventionally heat-cured acrylic material (Steinmassl et al 2017). This finding agreed with ours when the two acrylic materials were soaked in neutral and basic salivary solutions. On the contrary, one study that instigated the mechanical properties including monomer leakage between heat cured and CAD/CAM denture base material found that CAD/CAD material leached lower amount of monomer compared to heat cure denture acrylic material and this variation was attributed to the method of polymerization under high pressure (Ayman 2017).
The presence of unreacted residual monomers in denture base acrylic resins is inevitable, and every effort should be applied in laboratory and clinical settings to reduce the exposure as much as possible (Rashid et al 2015). Generally, and regardless of the acrylic material type, lower pH values were associated with more monomer leakage. Because lower amounts of monomer leakage occurred from the heat-cured acrylic material in the acidic solution, this material might be the material of choice when treating patients who report a high intake of an acidic diet. Similarly, using acidic solutions as storage media for dentures before denture insertion might be warranted to eliminate larger amounts of monomer release.
The salivary pH value in the oral cavity changes continuously between acidic and basic based on the dietary intake of the patient. Consequently, it might be necessary to subject the same acrylic material to alter salivary pH values and study the effect of this parameter on monomer leakage. Similarly, acrylic materials are subjected to many other factors that might affect monomer leakage. These factors include enzymes in the oral cavity, cleanser agents, different brushing techniques, polymerization techniques, surface treatments and chewing forces. Further investigation is needed to study the effects of the combination of these factors on acrylic materials, particularly the newly introduced CAD/CAM materials.
CONCLUSION
Within the limitations of this study, acidic salivary pH values were associated with higher amounts of monomer leakage in both heat-cured and CAD/CAM denture acrylic materials. It might be recommended to immerse newly fabricated heat-cured and CAD/CAM acrylic dentures in an acidic solution to allow most unreacted monomers to leak before delivering the denture to the patient.
REFERENCES
Akay, C., Taniş, M.Ç. and Sevim, H., (2017). Effect of artificial saliva with different pH levels on the cytotoxicity of soft denture lining materials. The International Journal of Artificial Organs, 40(10), pp.581-588.
Akin, H., Tugut, F. and Polat, Z.A., (2015). In vitro comparison of the cytotoxicity and water sorption of two different denture base systems. Journal of Prosthodontics, 24(2), pp.152-155.
Alla, R.K., Sajjan, S., Alluri, V.R., Ginjupalli, K. and Upadhya, N., (2013). Influence of fiber reinforcement on the properties of denture base resins.
Al-Qarni, F.D., Goodacre, C.J., Kattadiyil, M.T., Baba, N.Z. and Paravina, R.D., (2020). Stainability of acrylic resin materials used in CAD-CAM and conventional complete dentures. The Journal of prosthetic dentistry, 123(6), pp.880-887.
Ayman, A.D., (2017). The residual monomer content and mechanical properties of CAD\CAM resins used in the fabrication of complete dentures as compared to heat cured resins. Electronic physician, 9(7), p.4766.
Batista, M.J., Lawrence, H.P. and de Sousa, M.D.L.R., (2014). Impact of tooth loss related to number and position on oral health quality of life among adults. Health and quality of life outcomes, 12(1), p.165.
Batista, M.J., Lawrence, H.P. and de Sousa, M.D.L.R., (2018). Oral health literacy and oral health outcomes in an adult population in Brazil. BMC Public Health, 18(1), p.60.
Bettencourt, A.F., Neves, C.B., de Almeida, M.S., Pinheiro, L.M., e Oliveira, S.A., Lopes, L.P. and Castro, M.F., (2010). Biodegradation of acrylic based resins: A review. Dental materials, 26(5), pp.e171-e180.
Bidra, A.S., Taylor, T.D. and Agar, J.R., (2013). Computer-aided technology for fabricating complete dentures: systematic review of historical background, current status, and future perspectives. The Journal of prosthetic dentistry, 109(6), pp.361-366.
Çakırbay Tanış, M., Akay, C. and Sevim, H., (2018). Cytotoxicity of long-term denture base materials. The International Journal of Artificial Organs, 41(10), pp.677-683.
Chaves, C.D.A.L., Machado, A.L., Vergani, C.E., de Souza, R.F. and Giampaolo, E.T.,(2012). Cytotoxicity of denture base and hard chairside reline materials: a systematic review. The Journal of Prosthetic Dentistry, 107(2), pp.114-127.
Choudhary, Ashish & Devanarayanan, Ashwin & Bali, Praful & Choudhary, Ekta & Vikram, Jay. (2016). Contact Allergy to Denture Resins and Its Alternative Options. International Journal of Oral Implantology & Clinical Research. 7. 40-44. 10.5005/jp-journals-10012-1152.
Cunha-Cruz, J., Hujoel, P.P. and Nadanovsky, P.A.U.L.O., (2007). Secular trends in socio-economic disparities in edentulism: USA, 1972–2001. Journal of Dental Research, 86(2), pp.131-136.
Degirmenci, K., Atala, M.H. and Sabak, C., (2020). Effect of Different Denture Base Cleansers on Surface Roughness of Heat Polymerised Acrylic Materials with Different Curing Process. Odovtos-International Journal of Dental Sciences, pp.281-289.
de Mendonça, A.F., Furtado de Mendonça, M., White, G.S., Sara, G. and Littlefair, D., (2016). Total CAD/CAM supported method for manufacturing removable complete dentures. Case reports in dentistry, 2016.
Dogan, O.M., Bolayir, G., Keskin, S., Dogan, A., BEK, B. and Boztug, A., (2007). The effect of esthetic fibers on impact resistance of a conventional heat-cured denture base resin. Dental materials journal, 26(2), pp.232-239.
Drozdz, K., Wysokinski, D., Krupa, R. and Wozniak, K., (2011). Bisphenol A-glycidyl methacrylate induces a broad spectrum of DNA damage in human lymphocytes. Archives of toxicology, 85(11), pp.1453-1461.
Du, Q., Fu, M., Zhou, Y., Cao, Y., Guo, T., Zhou, Z., Li, M., Peng, X., Zheng, X., Li, Y. and Xu, X., (2020). Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Scientific Reports, 10(1), pp.1-12.
Dye, B.A., Li, X. and Thornton-Evans, G., (2012). Oral health disparities as determined by selected healthy people 2020 oral health objectives for the United States, 2009-2010 (No. 100). US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics.
Emami, E., de Souza, R.F., Kabawat, M. and Feine, J.S., (2013). The impact of edentulism on oral and general health. International journal of dentistry, 2013.
Engler, M.L.P.D., Güth, J.F., Keul, C., Erdelt, K., Edelhoff, D. and Liebermann, A., (2020). Residual monomer elution from different conventional and CAD/CAM dental polymers during artificial aging. Clinical Oral Investigations, 24(1), pp.277-284.
Faltermeier, A., Rosentritt, M. and Müssig, D., (2007). Acrylic removable appliances: Comparative evaluation of different postpolymerization methods. American Journal of Orthodontics and Dentofacial Orthopedics, 131(3), pp.301-e16.
Gad, M.M., Fouda, S.M., Al-Harbi, F.A., Näpänkangas, R. and Raustia, A., (2017). PMMA denture base material enhancement: a review of fiber, filler, and nanofiller addition. International Journal of Nanomedicine, 12, p.3801.
Gautam, R., Singh, R.D., Sharma, V.P., Siddhartha, R., Chand, P. and Kumar, R., (2012). Biocompatibility of polymethylmethacrylate resins used in dentistry. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 100(5), pp.1444-1450.
Goiato, M.C., Freitas, E., dos Santos, D., de Medeiros, R. and Sonego, M., (2015). Acrylic Resin Cytotoxicity for Denture Base–Literature Review. Advances in clinical and experimental medicine: official organ Wroclaw Medical University, 24(4), p.679.
Gonçalves, T.S., Morganti, M.A., Campos, L.C., Rizzatto, S.M. and Menezes, L.M., (2006). Allergy to auto-polymerized acrylic resin in an orthodontic patient. American journal of orthodontics and dentofacial orthopedics, 129(3), pp.431-435.
Iça, R.B., Öztürk, F., Ates, B., Malkoc, M.A. and Kelestemur, Ü., (2014). Level of residual monomer released from orthodontic acrylic materials. The Angle Orthodontist, 84(5), pp.862-867.Nik, T.H., Shahroudi, A.S., Eraghihzadeh, Z. and Aghajani, F., 2014. Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. Journal of Orthodontics, 41(1), pp.30-37.
Ivković, N., Božović, D., Ristić, S., Mirjanić, V. and Janković, O., (2013). The residual monomer in dental acrylic resin and its adverse effects. Contemporary materials, 4(1), pp.84-91.
Nik, T.H., Shahroudi, A.S., Eraghihzadeh, Z. and Aghajani, F., (2014). Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. Journal of Orthodontics, 41(1), pp.30-37.
Kassebaum, N.J., Bernabé, E., Dahiya, M., Bhandari, B., Murray, C.J.L. and Marcenes, W., (2014). Global burden of severe tooth loss: a systematic review and meta-analysis. Journal of dental research, 93(7_suppl), pp.20S-28S.
Kassebaum, N.J., Smith, A.G.C., Bernabé, E., Fleming, T.D., Reynolds, A.E., Vos, T., Murray, C.J.L., Marcenes, W. and GBD 2015 Oral Health Collaborators, (2017). Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of dental research, 96(4), pp.380-387.
Kattadiyil, M.T., Goodacre, C. and Baba, N.Z., (2013). CAD/CAM complete dentures: a review of two commercial fabrication systems. Journal of the California Dental Association, 41(6), p.407.
Kattadiyil, M.T., Jekki, R., Goodacre, C.J. and Baba, N.Z., (2015). Comparison of treatment outcomes in digital and conventional complete removable dental prosthesis fabrications in a predoctoral setting. The Journal of prosthetic dentistry, 114(6), pp.818-825.
Kopperud, H.M., Kleven, I.S. and Wellendorf, H., (2011). Identification and quantification of leachable substances from polymer-based orthodontic base-plate materials. The European Journal of Orthodontics, 33(1), pp.26-31.
Kostić, M., Krunić, N., Najman, S., Nikolić, L., Nikolić, V., Rajković, J., Petrović, M., Igić, M. and Ignjatović, A., (2015). Artificial saliva effect on toxic substances release from acrylic resins. Vojnosanitetski pregled, 72(10), pp.899-905.
Koutis, D. and Freeman, S., (2001). Allergic contact stomatitis caused by acrylic monomer in a denture. Australasian journal of dermatology, 42(3), pp.203-206.
Lefebvre, C.A., Schuster, G.S., Marr, J.C. and Knoernschild, K.L., (1995). The effect of pH on the cytotoxicity of eluates from denture base resins. International Journal of Prosthodontics, 8(2).
Maeda, Y., Minoura, M., Tsutsumi, S., Okada, M. and Nokubi, T., (1994). A CAD/CAM system for removable denture. Part I: Fabrication of complete dentures. international Journal of Prosthodontics, 7(1).
Marsh, P.D. and Zaura, E., (2017). Dental biofilm: ecological interactions in health and disease. Journal of clinical periodontology, 44, pp.S12-S22.
Masri, R. and Driscoll, C.F. eds., (2015). Clinical applications of digital dental technology. Wiley-Blackwell.
Mohamed, S.H., Al-Jadi, A. and Ajaal, T., (2008). Using of HPLC analysis for evaluation of residual monomer content in denture base material and their effect on mechanical properties. Journal of Physical Science, 19(2), pp.127-135.
Mörmann, W.H., Stawarczyk, B., Ender, A., Sener, B., Attin, T. and Mehl, A.,( 2013). Wear characteristics of current aesthetic dental restorative CAD/CAM materials: two-body wear, gloss retention, roughness and Martens hardness. Journal of the mechanical behavior of biomedical materials, 20, pp.113-125.
Murakami, N., Wakabayashi, N., Matsushima, R., Kishida, A. and Igarashi, Y.,( 2013). Effect of high-pressure polymerization on mechanical properties of PMMA denture base resin. Journal of the mechanical behavior of biomedical materials, 20, pp.98-104.
Nakamura, M., Takahashi, H. and Hayakawa, I., (2007). Reinforcement of denture base resin with short-rod glass fiber. Dental materials journal, 26(5), pp.733-738.
Nguyen, J.F., Ruse, D., Phan, A.C. and Sadoun, M.J., (2014). High-temperature-pressure polymerized resin-infiltrated ceramic networks. Journal of dental research, 93(1), pp.62-67.
Oliveira, J.C.D., Aiello, G., Mendes, B., Urban, V.M., Campanha, N.H. and Jorge, J.H., (2010). Effect of storage in water and thermocycling on hardness and roughness of resin materials for temporary restorations. Materials Research, 13(3), pp.355-359.
Pusz, A., Szymiczek, M. and Michalik, K., (2010). Ageing process influence on mechanical properties of polyamide-glass composites applied in dentistry. Journal of Achievements in materials and manufacturing engineering, 38(1), pp.49-55.
Rashid, H., Sheikh, Z. and Vohra, F., (2015). Allergic effects of the residual monomer used in denture base acrylic resins. European journal of dentistry, 9(4), p.614.
Raszewski, Z., (2020). Influence of polymerization method on the cytotoxicity of three different denture base acrylic resins polymerized in different methods. Saudi Journal of Biological Sciences, 27(10), pp.2612-2616.
Sá, J.D., Vieira, F., Aroso, C.M., Cardoso, M., Mendes, J.M. and Silva, A.S., (2020). The Influence of Saliva pH on the Fracture Resistance of Three Complete Denture Base Acrylic Resins. International Journal of Dentistry, 2020.
Shim, J.S. and Watts, D.C., (1999). Residual monomer concentrations in denture-base acrylic resin after an additional, soft-liner, heat-cure cycle. Dental Materials, 15(4), pp.296-300.
Sideridou, I.D. and Achilias, D.S., (2005). Elution study of unreacted Bis‐GMA, TEGDMA, UDMA, and Bis‐EMA from light‐cured dental resins and resin composites using HPLC. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 74(1), pp.617-626.
Silva-Junior, M.F., Sousa, A.C.C.D., Batista, M.J. and Sousa, M.D.L.R.D., (2017). Oral health condition and reasons for tooth extraction among an adult population (20-64 years old). Ciência & Saúde Coletiva, 22, pp.2693-2702.
Singh, R.D., Gautam, R., Siddhartha, R., Singh, B.P., Chand, P., Sharma, V.P. and Jurel, S.K., (2013). High Performance Liquid Chromatographic Determination of Residual Monomer Released from Heat‐Cured Acrylic Resin. An In Vivo Study. Journal of Prosthodontics, 22(5), pp.358-361.
Steinmassl, P.A., Wiedemair, V., Huck, C., Klaunzer, F., Steinmassl, O., Grunert, I. and Dumfahrt, H., (2017). Do CAD/CAM dentures really release less monomer than conventional dentures?. Clinical oral investigations, 21(5), pp.1697-1705.
Tuna, E.B., Rohlig, B.G., Sancakli, E., Evlioglu, G. and Gencay, K., (2013). Influence of acrylic resin polymerization methods on residual monomer release. The journal of contemporary dental practice, 14(2), p.259.
Turssi, C.P., Hara, A.T., Magalhães, C.S.D., Serra, M.C. and Rodrigues Jr, A.L., (2003). Influence of storage regime prior to abrasion on surface topography of restorative materials. Journal of Biomedical Materials Research Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 65(2), pp.227-232.
Urban, V.M., Machado, A.L., Vergani, C.E., Giampaolo, E.T., Pavarina, A.C., de Almeida, F.G. and Cass, Q.B., (2009). Effect of water-bath post-polymerization on the mechanical properties, degree of conversion, and leaching of residual compounds of hard chairside reline resins. Dental Materials, 25(5), pp.662-671.
Vallittu, P.K., Ruyter, I.E. and Buykuilmaz, S., (1998). Effect of polymerization temperature and time on the residual monomer content of denture base polymers. European journal of oral sciences, 106(1), pp.588-593.
Zafar, M.S., (2020). Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers, 12(10), p.2299.
Zidan, S., Silikas, N., Haider, J. and Yates, J., (2020) Long-Term Sorption and Solubility of Zirconia-Impregnated PMMA Nanocomposite in Water and Artificial Saliva. Materials, 13(17), p.3732.