1Department of Microbiology, Maharani’s Science College for Women, Bengaluru – 560 001 Karnataka, India.
2Department of Microbiology, Gulbarga University, Kalaburagi – 585 106, Karnataka, India.
3Department of Biochemistry, Maharani’s Science College for Women, Bangalore-560 001, Karnataka, India.
4Department of Sericulture, Maharani’s Science College for Women, Bangalore-560 001
Corresponding author email: lvphaget@rediffmail.com
Article Publishing History
Received: 07/02/2020
Accepted After Revision: 15/03/2020
Amylases are a class of starch degrading enzymes catalyzing the hydrolysis of internal glycosidic bonds in polysaccharides and plays a crucial role with potential industrial and commercial applications. In the present study five bacterial isolates have been evaluated for the production of extracellular amylase and one potent isolate was selected based on maximum starch hydrolysis. Bacillus altitudinis GVK38 has been used for the production of amylase under submerged fermentation. B. altitudinis GVK38 showed maximum enzyme production of 728 U/mL at 45°C for 48 hours at pH 9.0. Further enhanced production was obtained by supplementing 1.0 % maltose as carbon source, 1.5 % Yeast extract as nitrogen source and 2.0 % salt concentration. The results of RSM reveals that F-value of 1.44, Lack of Fit F-value of 0.33, coefficient of determination (R2) for enzyme activity calculated as 0.9231, value of the adjusted determination coefficient, R2 was 0.2822. RSM depicts that the optimal level of the significant variables for the maximum amylase production were: Incubation period 72 hrs, pH – 8.00, temperature 45°C, NaCl Conc. -1.25%, maltose conc. – 1.25% and yeast extract conc. – 1.25%. The results of the study show that the isolate can be further exploited for commercial production of amylase.
Amylase, Bacillus altitudinis, 16S rRNA, RSM, Submerged fermentation.
Vishwanatha T, Keshavamurthy M, V. Kote N, Manjula A.C. Molecular Identification and Response Surface Methodological (RSM) Approach for Optimized Production of Amylase from Bacillus altitudinis GVK38. Biosc.Biotech.Res.Comm. 2020;13(1).
Vishwanatha T, Keshavamurthy M, V. Kote N, Manjula A.C. Molecular Identification and Response Surface Methodological (RSM) Approach for Optimized Production of Amylase from Bacillus altitudinis GVK38. Biosc.Biotech.Res.Comm. 2020;13(1). Available from: https://bit.ly/38SOSwy
Copyright © Vishwanatha et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Enzymes are biological catalysts, which regulate specific biochemical reactions. They speed up a reaction without being used up in the reaction. In recent past, chemical catalysts had been replaced by enzymes in various industrial applications (Keshavamurthy et al., 2018). Amylases are enzyme which hydrolyses starch molecules to give diverse products including dextrin and progressively smaller polymers composed of glucose units. Among the industrially important enzymes, amylases are considered to be the most prominent enzyme due to its wide area of potential application. Amylases are used in detergents, textiles, starch, baking and animal feed are the main industries, which use about 75% of industrially produced enzymes (Panneerselvam and Elavarasis, 2015). To meet the higher demands of these industries, low cost production of amylase is necessary. Bacillus sp. are one among the industrially important microorganisms used due to their rapid growth rates that lead to shorter fermentation cycles, their capacity to secrete proteins into extra cellular medium and general handling safety (Pandey 2000). In recent years, the potential of using microorganisms as biotechnological sources for the production of industrially relevant enzymes has stimulated interest in the exploration of newer and potential isolates (Alva et al., 2007). Amylases are widely distributed in nature and can be derived from various sources such as plants, animals and microorganisms (Reddy et al., 2003; Gopinath et al., 2017, Keshavamurthy et al., 2019).
Microbial enzymes have been generally favored for their easier isolation in high amounts, low-cost production in a short time, and stability at various extreme conditions, and their compounds are also more controllable and less harmful (Pandey et al., 2000). Amylases, biosynthesized by the bacteria, show unique characteristics such as thermophilic, thermotolerant, alkaline and acidophilic properties (Konsoula and Liakopoulou-Kyriakides 2007). The major advantage of using microorganisms for the production of amylases is the economical bulk production capacity and the fact that microbes are easy to manipulate to obtain enzymes of desired characteristics. Many microorganisms used in α-Amylases and β-amylases production include Bacillus subtilis, B. cereus, B. polmyxa, B. amyloliquefaciens, B. coagulans, B. subtilis, Lactobacillus, Escherichia, Proteus, B. lincheniformis, B. steriothermophilu, B,megaterium, Strepotmyces sp., and Pseudomonas sp. etc., (Gupta et al., 2003; Elmansy et al., 2018, Keshavamurthy et al., 2019).
Amylase substrates are widely available from cheap plant sources, rendering the potential applications of the enzyme more plentiful in terms of costs. To obtain maximum yield of an enzyme, development of a suitable medium and culture conditions is obligatory (Srivastava and Baruah, 1986). Starch or other sugars as a carbon source and ammonium salts or complex organic compounds as a nitrogen source are needed for bacterial growth and enzyme production (Ellaiah, 2002). Optimization of the various parameters and manipulations of media are one of the most important techniques used for the enhanced production of amylase in large quantities. To meet industrial demands, production of amylase in bacteria is known to depend mostly on metabolic state of the culture. Various physical and chemical factors such as temperature, pH, incubation period, carbon and nitrogen sources have been known to affect the production of amylase (Gangadharan, 2008).
The production of amylase by submerged fermentation (SMF) and solid-state fermentation (SSF) has been thoroughly investigated by many researchers and is affected by a variety of physiochemical factors. To meet the growing demands in the industry, it is necessary to improve the performance of the system and thus increase the yield without increasing the cost of production. Among the wide range of microbial species that secrete amylase, its production from bacteria is cheaper and faster than other microorganisms. The statistical experimental designs and mathematical methods have wide application in the field of microbial biotechnology. Response surface methodology (RSM) is one such method that is applied for modelling problems with the aim to optimize responses that were influenced by multiple variants. Performing statistically designed experiments, filling experimentally determined response data into a quadratic model, predicting response and checking significance of the model are the major steps involved in this process (Keshavamurthy et al., 2019).
RSM is advantageous for industrial purposes as it requires fewer numbers of experimental trials for prediction and quantification of combined interactions between the variables and hence eases the process of optimization. The 3D plots for response surface enables visualization of parameter interaction and it was often applied to satisfactory optimization of microbial enzyme production (Keshavamurthy et al., 2019). Hence to overcome all these challenges and to meet the demand a study was undertaken with the main aim of isolating the potent strain from the extreme environment and to use it in the production of amylase under submerged fermentation. Further the optimization of process parameter and media constituents were performed for enhanced production
MATERIALS AND METHODS
Sample collection and Isolation of Bacteria:Soil sample was collected from the Hutti gold mines (16o 11’ 45” North Latitude and 76o 38’ 31” East Longitude), Raichur district of Karnataka state, India. The bacteria were isolated by serial dilution plating method. The samples were inoculated onto nutrient agar plates and incubated at 37ºC for 24 h. The colonies obtained after incubation were further sub cultured and preserved under 4ºC for further use.
Screening and selection of strain for Optimization:The isolates were screened for amylase production bacterial colonies were screened on starch agar medium [g/L: Starch -10.0, Peptone – 5.0, NaCl – 2.0, MgSO4 – 0.2, Agar – 20.0, and pH 9.0. Inoculated plates were incubated at 37ᵒ C for 24 h. After incubation, the plates were flooded with iodine solution [g/L: Potassium iodide – 2.5, Gram’s iodine – 0.125]. Amylase positive bacterial strains were identified and recorded based on the clear zone formation around the bacterial growth. Out of five isolates, one strain of bacteria which produced maximum clear zone of hydrolysis for extracellular amylase, which was selected for further experimental studies.
Molecular identification of bacteria: Amylase positive strain Bacillus sp. GVK 38 was subjected for basic microscopic, biochemical, physiological and cultural characterization based on biochemical characteristics as per Bergey’s Manual of Systematic Bacteriology (Niall and Paul, 2009) The bacterial isolate was further identified by 16S rDNA sequence analysis using universal primers and genomic DNA as template. The genomic DNA of the isolate was extracted as described by (Roohi et al., 2012). The PCR amplified product was sequenced at Microbial Ecology Laboratory, National Centre for Cell Science, Pune. After DNA sequencing, the obtained results were subjected to BLAST analysis to compare with the sequence similarities. Phylogenetic tree was constructed with MEGA 6.0 software using neighbor-joining method (Tamura et al., 2011). Duly annotated partial nucleotide sequences of the novel bacterial strain was deposited with NCBI Genbank and accession number was obtained.
Production of amylase by submerged fermentation: Amylase producing bacteria was grown on the starch production media [g/L: Peptone – 5.0, Starch 10.0, NaCl-2.0, MgSO4-0.2, pH 9.0, incubated on a rotary shaker for 48 hours at 37ᵒ C. Enzyme was extracted by centrifuging the incubated broth at 8,000 rpm for 10 min at 4ᵒC. Supernatant was used as crude enzyme source. The experiment was carried out in 250 mL plugged Erlenmeyer flasks, each containing 100 mL sterile starch broth medium and inoculated with 1% of standard inoculum (2.3 × 106 CFU ml−1) for the tested bacterial isolate which was incubated at 45 °C on rotary shaker at 160 rpm for 48 h. The fermented medium was centrifuged at 10,000 rpm for 10 min in order to determine periodically the cell dry weight and amylases activity in the precipitate and supernatant, respectively (Yassien and Asfour, 2012).
Enzyme Assay: The amylase assay was measured by following the methodology proposed by Bernfeld (1955). Amylase activity was assayed in the reaction mixture containing 0.5 mL enzyme, 1mL of 1% starch as substrate, 1mL phosphate buffer and incubated at room temperature for 15 minutes. The reaction was arrested by adding 1mL DNS reagent. The inactivated reaction mixture was incubated on water bath for 10 minutes and, made up to 10 mL and absorbance was measured at 540 nm. Blank was prepared by immediate addition of 1mL DNS reagent to 0.5 mL enzyme, followed by the addition of 1mL starch and 1mL phosphate buffer. One unit of amylase activity is defined as the amount of enzyme required to release 1µg of reducing sugar (maltose) per ml per min under the above assay conditions.
Optimization of culture conditions and medium components for amylase production:
One Variable at a Time Approach (OVAT): Optimization of production of amylase by Bacillus altitudinis GVK38 was checked by varying the following physical and chemical parameters using one-variable-at-a-time-approach: Amylase production by B. altitudinis GVK38 was carried out in basal medium with different combinations of carbon and nitrogen sources (1% w/v) and 1% inoculum size at 37 °C for 72 h in a rotary shaker (120 rpm). The initial pH of the medium was adjusted to 7.0. Parametric optimization was performed with respect to incubation period (24 to 120 h), pH (6 to 10), temperature (30 to 60oC) were studied by using various levels of the test parameter and keeping the other parameters constant. The bacterium was grown in the production medium supplemented with different carbon sources (1% w/v) such as arabinose, fructose, glucose, lactose and maltose and nitrogen sources (1% w/v) like peptone, yeast extract, beef extract and ammonium chloride. At the end of the fermentation, the fermentation broth was centrifuged at 8,000 rpm for 10 min. The supernatant obtained by the centrifugation was used for measuring the amylase activity. The un-inoculated flasks served as controls. All the experiments were done in triplicates and the average of enzyme activity was taken for statistical analysis to know the significance of each factor.
Optimization of amylase production using Response surface methodology approach: Response surface methodology (RSM) is a well-accepted statistical technique which is able to design and optimize the experimental process that involves choosing the optimal experimental design and estimate the effect of the several factors independently and also their interactions simultaneously. RSM combined with Central Composite Design (CCD) was established using Design Expert software (Version 11.0, Stat-Ease Inc., Minneapolis, USA) to analyze and plot the response surface graphs. Five factors, namely, pH, temperature, maltose, yeast extract and NaCl were optimized for enhanced production of amylase using the isolate B. altitudinis GVK38. Based on CCD, the factors were analysed at two levels: -1, for low level, and +1, for high level. A total of twenty-nine (29) runs were performed to optimize the process parameters, and experiments were performed according to the experimental design matrix. The results were evaluated by applying the coefficient of determination (R2), analysis of variance (ANOVA) and response plots. Employing RSM, the most widely used second-order polynomial equation was developed to fit the experimental results and identify the relevant model terms:
Y=β0 ΣβiXi +ΣβiXiβij+ΣXiXj (1)
where Y is the predicted response; b0, bi, and bij are constant regression coefficients of the model; and Xi and Xj represent independent variables. The experimental design helps in investigating linear, quadratic and cross product effects of these factors and also centre points for replication (Kathiresan and Manivannan, 2006).
RESULTS AND DISCUSSION
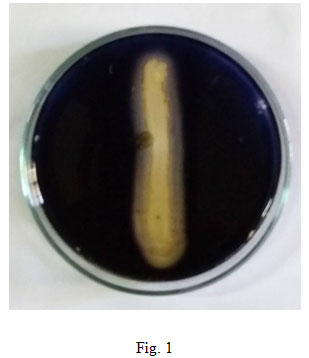
In the current study, 52 individual bacterial isolates were isolated from the soil sample collected from mining area. Amylase producing ability of five Bacillus species were checked on starch media and all were found to be positive, but Bacillus altitudinis GVK 38 was found to be the best amylase producer (fig. 1). Verma et al., (2011) found the maximum amount of amylase production in B. subtilis followed by B. megaterium, among nine strains tested which included B. cereus, B. megaterium and B. subtilis. But later during screening it was found that only three strains showed amylase activity on agar plate. The maximum amylase producing Bacillus altitudinis GVK 38 was taken for optimization studies through submerged fermentation by varying the temperature, pH, incubation period, carbon and nitrogen source, since the production of amylase enzymes are influenced by diverse physico-chemical and biological factors (Raj and Hemashenpagam, 2012).
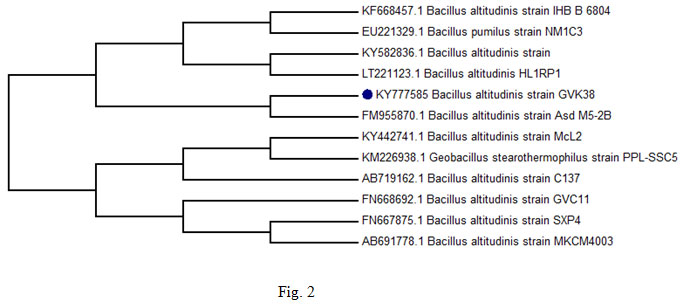
The strain GVK38 is gram positive rod, motile, spore former and was tentatively identified as Bacillus sp. based on its morphological and biochemical characteristics (Table 1). Identification of selected Bacillus altitudinis GVK 38 strain was identified on the basis of standard biochemical tests according to Bergey’s Manual of determinative Bacteriology. The occurrence of amylolytic organisms from the soil agrees with earlier reports of Rehana et al., (1989).The comparison of the 16S rRNA gene nucleotide sequence (1465 bp) of the strain Bacillus altitudinis GVK 38 with other 16S rRNA genes sequences of closely related strains from NCBI database showed that this isolate has 99 % sequence homology with B. altitudinis Asd M5-2B (Accession No. FM955870) (fig. 2). The phylogenetic tree, constructed by the neighbor-joining method indicated that the strain B. altitudinis GVK38 is affiliated with the genus Bacillus and closely related to B. altitudinis strain IHBB 6804 – Accession No. KF668457. The obtained nucleotide sequence of B. altitudinis GVK 38 was submitted to GenBank database and the accession number assigned is KY777585 (https://www.ncbi.nlm.nih.gov/ nuccore/KY777585).
Table 1. Morphological and biochemical characteristics of B. altitudinis strain GVK38
| Morphological Characteristics | Results |
| Gram staining |
Positive rods |
| Colour | Creamish white |
| Motility test | Motile |
| Spore |
Spore former |
| Physiological characteristics | |
| Catalase | Positive |
| Indole | Negative |
| Methyl red | Negative |
| Voges Proskauer | Positive |
| Citrate utilization | Positive |
| Oxidase reaction | Positive |
| Casein hydrolysis | Positive |
| Gelatin liquefaction | Positive |
| Starch hydrolysis | Positive |
| Nitrate reduction | Positive |
| Growth at 4o C | – |
| Growth at 45o C | + |
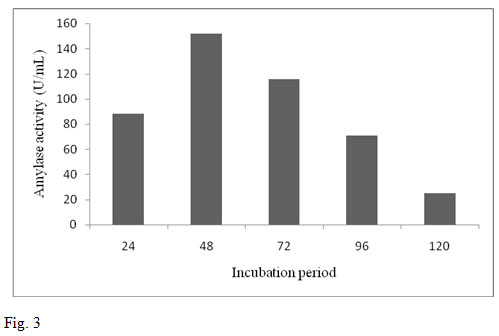
The enzyme production by the bacterial strain B. altitudinis GVK38 was studied in submerged fermentation. The significant amylase yield was obtained for the incubation period 48 h. It was observed that the enzyme production from the bacterium was found maximum at 48 h (152 U/mL). An increase in the enzyme production was observed from 24 h to 48 h. After 48 h of incubation a decreasing trend of enzyme activity was observed (fig. 3). The results are similar to the study conducted on α-amylase production by B. altitudinis in shake flask for different intervals of time (0 to 144 h) (Kumar et al., 2014).
Figure 1: Starch hydrolysis on starch agar plate by bacterial isolate B. altitudinis GVK38.
The production of enzyme was reached maximum at 48 h after inoculation. Further increase in incubation period however, did not show any significant increase in enzyme production rather it was decreased. This is because the cells would have reached decline phase with lowered enzyme synthesis. It might be also due to the depletion of the nutrients, death phase of organism or due to the production of amylase in the medium. This result was also supported by Oyeleke and Oduwole (2009) where the optimum incubation period on the yield of amylase enzyme was found at 48 h. Our result was also in agreement with the similar findings obtained for production of α-amylase from B. amyloliquefaciens (Gangadharan et al., 2008). The result revealed that after 48 h incubation decreased in enzyme yield might be due to the denaturation of enzyme caused by interaction with other components in the medium.
Figure 2: Phylogenetic analysis of 16S rDNA gene sequence data of isolate B. altitudinis GVK38 and of a number of related strains
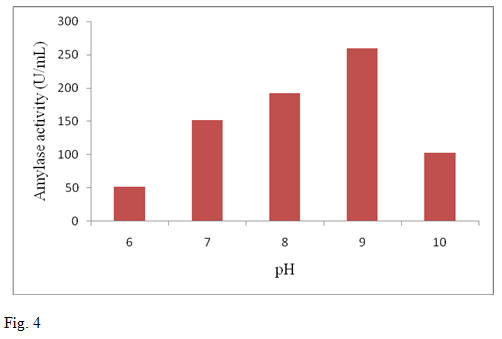
The pH of the production medium plays an important role in microbial growth and hence influences the enzyme production. Previous reports on amylase production indicate greater influence by pH (Parbat and Singhal, 2011; Sankaralingam et al., 2012). In the present investigation the enzyme activity for different pH ranging from 6.0-10.0 were determined by keeping the optimum incubation period and temperature conditions. The maximum amylase production was found at pH 9.0. Further increase in the pH decreased the activity of amylase. Amylase production by B. altitudinis GVK38 was found to be maximum at pH 9.0 (259.5 U/ml) (fig.4). When pH is altered below or above the optimum, the activity appears to be decreased or becomes denatured (Devi et al., 2012). Different organisms have different pH optima and decrease or increase in pH on either side of the optimum value results in poor microbial growth (Sankaralingam et al., 2012).
Figure 3: Effect of incubation period on amylase production
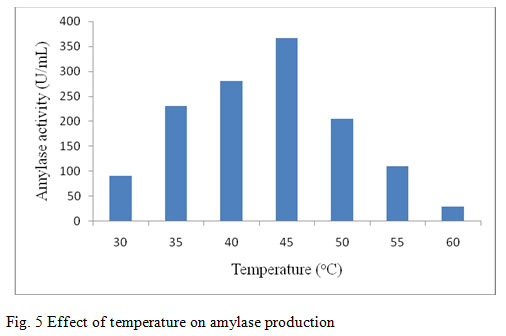
In this present work the amylase activity was studied for the temperature from 30°C – 60°C. It was observed in the present study that the maximum enzyme production from the bacterium was found to be maximal after 48 hours of time at 45°C (367 U/ml) (fig. 5). Previous report on optimum production of amylase by B. marini at 40°C indicates that as the temperature increased or decreased, there was gradual decrease in the enzyme activity (Ashwini et al., 2011). At 60°C, the production of amylase was extremely low. This might be due to inhibition of bacterial growth at high temperature and hence, enzyme formation was also prohibited. Similar finding was also reported by Riaz et al., (2003) when study was carried out on production of amylase by B. subtilis GCUCM-25 at 30 – 60°C. The production of the enzyme decreased with increased temperature. The effect of temperature on activity of amylase produced by B. megaterium was found to be maximum at 40°C followed by a sharp decrease in amylase activity at 50°C reported by Oyeleke and Oduwole (2009) which is similar to the present study. Jogezai et al., (2011) also reported the maximum production of α-amylase at 40°C by using B. subtilis. As the incubation temperature was increased, the production of the enzyme was decreased. Liu and Xu, (2008) reported that the strain B. aquimaris VITP4 exhibited maximum enzyme production at 40°C. Although growth was observed in the temperature range 30°C to 60°C, it was found maximum at 40°C indicating one-to-one correlation between enzyme production and biomass, which clarifies that the enzyme production is growth dependent.
Figure 4: Effect of pH on amylase production
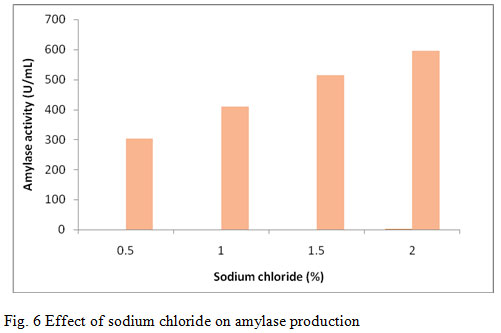
Sodium chloride (NaCl) is an important nutrient factor for growth and physiological activities. Various concentrations of NaCl such as 0.5%, 1.0%, 1.5%, and 2.0% were used to supplement the production media. Among the various concentrations the maximum amylase production (596 U/mL) was induced when the media was supplemented with 2.0 % (fig. 6). Vijayabaskar et al., (2012) reported 3% NaCl concentration was suitable for the amylase production . Ashwini et al., (2011) reported enzyme production at different concentrations of NaCl and found optimum enzyme yield at 4.5% NaCl concentrations. Further there was gradual decrease in enzyme production as the NaCl concentration was increased or decreased. Kokab et al., (2003) reported amylase production from B. subtilis having medium containing 2.0 % concentration of NaCl.
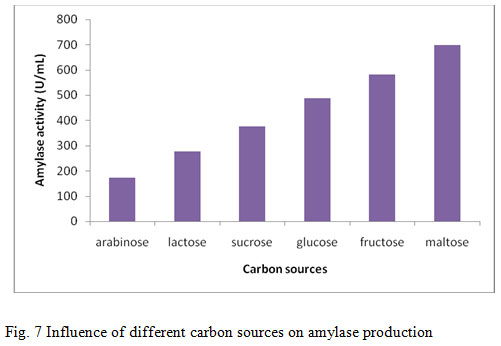
The effect of carbon sources on amylase production from B.altitudinis GVK38 was studied using different carbon sources such as arabinose, fructose, glucose, lactose, maltose and sucrose (1%) as supplement in the production media. The maximum amylase production was found when production medium was supplemented with maltose (698.5 U/mL) followed by fructose (582 U/mL) (Fig. 7). Least growth and amylase production have been recorded by lactose and arabinose. The addition of carbon source in the form of either monosaccharide or polysaccharides may influence the production of amylase enzyme. In this present study, the influence of maltose was found best carbon source than the other carbon sources. Similar finding was observed by Ashwini et al., (2011) when amylase production was optimized using different sugars at 1% (w/v) concentration. B. marini showed the maximum enzyme activity in the presence of starch as carbon source, whereas, the minimum enzyme activity was observed in the presence of dextrose. The decrease in the production of enzyme with other carbon sources may be due to catabolite repression. The finding in the present study was in agreement with earlier report where starch was observed as the best carbon source utilized by the organism Rameshkumar and Sivasudha, (2011). Similar result was also found by Goyal et al., (2005) that the soluble starch as the best carbon source supplement for amylase production by B. lichenifomis and Bacillus sp.I-3.
Figure 5: Effect of temperature on amylase production
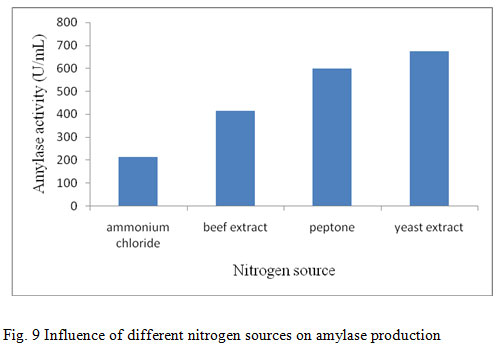
In the present study the supplementation of nitrogen sources on amylase production showed that yeast extract was found to be a better nitrogen source for the production of amylase (728 U/mL) (fig. 9). Yeast extract was the best nitrogen source for amylase production, probably due to its high content in minerals, vitamins, coenzymes and nitrogen components and when yeast extract was used as nitrogen source for B. stearothermophilus and S. albidoflavus respectively (Narayana and Vijayalakshmi, 2008; Roohi et al., 2011). The improvement of the nutritional value in the medium by the supplementation of organic and inorganic sources will also improve the growth of the bacterial culture and subsequently in the enzyme production. Similarly, enzyme production was more efficient in medium containing organic nitrogen sources, especially yeast extract as compared with inorganic nitrogen sources (Santos and Martins, 2003; Sourav et al., 2011).
Figure 6: Effect of sodium chloride on amylase production
Optimization using Response Surface Methodology (RSM): To examine combined effect of the independent variables starch (A), Incubation period (B), pH (C), Temperature (D), NaCl Conc. (E) Maltose conc. (F) Yeast extract on the activity of amylase from Bacillus altitudinis GVK38 under submerged fermentation, an experiment of 54 runs were designed and performed with an incubation period of 120 hrs. The Model F-value of 1.44 implies the model is not significant relative to the noise. There is a 43.58% chance that an F-value this large could occur due to noise. P-values less than 0.0500 indicate model terms are significant. In this case there are no significant model terms. Values greater than 0.1000 indicate the model terms are not significant. The Lack of Fit F-value of 0.33 implies the Lack of Fit is not significant relative to the pure error. There is a 77.76% chance that a Lack of Fit F-value this large could occur due to noise. Non-significant lack of fit is good. The coefficient of determination (R2) for enzyme activity was calculated as 0.9231. This indicated that the statistical model explained 92.31% of the variability in response and only 07.69% of variance was not explained by the model. Value of R2 near to 1.0 indicated that the model was strong and it can predict the response in a better and this supports our results (Box et al., 1978; Keshavamurthy et al, 2019).
Figure 7: Influence of different carbon sources on amylase production
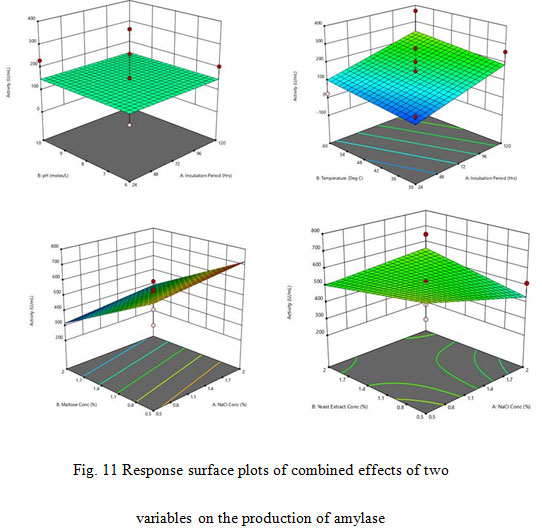
The adjusted R2 value corrects the R2 value for the sample size and for the number of terms in the model. The value of the adjusted determination coefficient, R2 was 0.2822. This implied a higher significance of the model applied for analysing the data (Cochran and Cox, 1957; Khuri and Cornell 1987). In the present study, the adjusted R2 value (0.2822) was lesser than the R2 value (0.9231). The smaller value of adjusted R2 as compared to that of the R2 may be due to the presence of multiple terms but small sample size. The interactive effects of independent variables on enzyme were studied by plotting 3D surface curves. The 3D curves of the calculated enzyme activity for the interactions between the variables are shown in Fig. 11. Optimal level of the significant variables for the maximum amylase production were: Incubation period 72 hrs, pH – 8.00, temperature 45°C, NaCl Conc. – 1.25%, maltose conc. – 1.25% and yeast extract conc. – 1.25%.
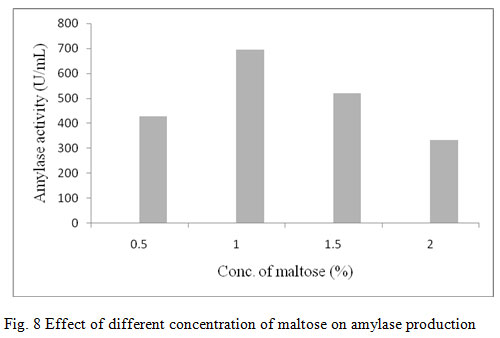
Figure 8: Effect of different concentration of maltose on amylase production
Figure 9: Influence of different nitrogen sources on amylase production
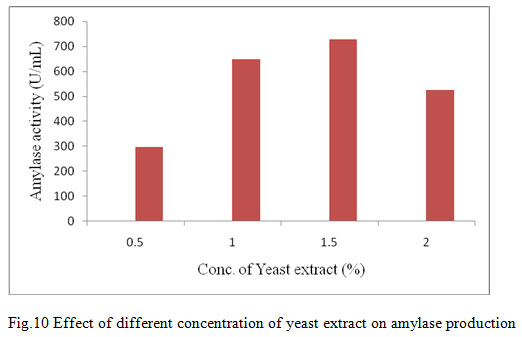
Figure 10: Effect of different concentration of yeast extract on amylase production
Figure 11: Response surface plots of combined effects of two variables on the production of amylase
RSM mediated optimization of amylase production from Bacillus sp. has previously been reported by many researchers. This method was previously used to evaluate the effect of pH, temperature and inoculum size on production of amylase from Bacillus sp by applying a full factorial central composite design (Zambare, 2011). In another report by Tanyildizi et al., (2005) on optimization of α-amylase production by Bacillus sp using RSM, combined interaction of starch, glycerine, peptone and yeast extract on the production of enzymes was evaluated. Optimized level of variables for the maximum α-amylase yield from Bacillus subtilis 168 were starch 2.55 g/l, yeast extract 8.4 g/l, sodium chloride 8.1% and 48 h of incubation (Samreen, 2011).
CONCLUSION
Bacillus altitudinis GVK38 (KY777585) isolated from mining environment showed a good amylolytic activity. The optimum yield of amylase was found at 40°C and 48 h and pH 9.0 under submerged fermentation using OVAT approach. Among the different carbon sources, starch supported highest amylase production followed by maltose under submerged fermentation. Yeast extract supported maximal amylase production from different nitrogen sources. A NaCl (2.0 %) concentration was observed for highest amylase production under submerged fermentation. Amylase produced by B. altitudinis GVK 38 was found to be potent since it could be active at a wide range of pH, temperature, carbon, nitrogen sources and sodium chloride concentrations under submerged fermentation. Therefore the selected strain of B. altitudinis GVK38 could be beneficial and further can be exploited for industrial purposes at large scale production.
Acknowledgments: Authors wish to acknowledge Dr. Yogesh Souche, Scientist, National Centre for Cell Science, Govt of India, Pune for sequencing and identification of Bacteria and Dr. Suresh Prabhu for RSM analysis.
Conflict of Interest: Authors declare that they have no conflict of interest in the publication.
REFERENCES
Alva, S., Anupama, J., Savla, J., Chiu, Y.Y., Vyshali, P., Shruti, M., Yogeetha B.S., Bhavya, D., Purvi, J., Ruchi, K., Kumudini, B.S., 2007. Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI 12 in solid state culture. African Journal of Biotechnology, 6, pp. 576-582.
Ashwini, K., Gaurav, K., Karthik, L., Bhaskara Rao, K.V., 2011. Optimization, production and partial purification of extracellular α-amylase from Bacillus sp. marini. Archives of Applied Science Research, 3, pp. 33-42.
Bernfeld, P., 1955. Amylases: and Method in Enzymology. Academic Press USA. 1: 149.
Box, G.E., Hunter, W.G., Hunter, J.S., 1978. Statistics for experimenters. John Wiley & Sons. New York.
Cochran, W.G., and Cox, M.E., 1957. Experimental design. Second edition. John Wiley and Sons. New York.
Devi, B., Unni, B.G., Wann, S.B., and Samanta, R., 2012. Immobilization of Partially Purified Alpha Amylase Enzyme Produced by Soil Borne Bacillus sp. Advances in applied Science research, 3, pp. 2739-44.
Elmansy, E.A., Asker, M.S., El-Kady, E.M. 2018. Production and optimization of α-amylase from thermo-halophilic bacteria isolated from different local marine environments. Bulletin of the National Research Centre, 42, pp. 31
Ellaiah, P., Adinarayana, K., Bhavani, Y., Padmaja, P., Srinivasulu, B., 2002. Optimization of process parameters for glucoamylase production under solid state fermentation by a newly isolated Aspergillus species. Process Biochemistry, 38, pp. 615-620.
Gangadharan, D., Sivaramakrishnan, S., Nampoothiri, K.M., Sukumaran, R.K., Pandey, A., 2008. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bio resource technology, 99, pp. 597-602.
Gopinath, S.C., Anbu, P., Arshad, M.K., Lakshmipriya, T., Voon, C.H., Hashim, U., Chinni, S.V., 2017. Biotechnological processes in microbial amylase production. BioMed research International, 5, pp. 235-245.
Goyal, A., Bandopadhyay, R., Sourdille, P., Endo, T.R., Balyan, H.S., Gupta, P.K., 2005. Physical molecular maps of wheat chromosomes. Functional and Integrative genomics. 5, pp. 260-3.
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V.K., Chauhan, B., 2003. Microbial α-amylases: a biotechnological perspective. Process biochemistry, 38:1599-616.
Jogezai, N., Raza, A., Abbas, F., Bajwa, M., Mohammad, D., Kakar, W., Saeed, M., Awan, A., 2011. Optimization of cultural conditions for microbial alpha amylase production. Journal of Microbiology and Antimicrobials, 3:pp. 221-7.
Kandra, L., 2003. α-Amylases of medical and industrial importance. Journal of Molecular Structure: Theochem, 666, pp. 487-98.
Kathiresan, K., and Manivannan, S., 2006. Amylase production by Penicillium fellutanum isolated from mangrove rhizosphere soil. African Journal of Biotechnology, 5(10).
Keshavamurthy, M., Vishwanatha, T., Suresh Kumar, M., and Subhaschandra, M Gaddad., 2018. Enhanced production of alkaline protease from novel bacterium Bacillus cereus GVK21 under submerged fermentation. Bioscience and Biotechnology Research Communication, 11(3), pp. 416-425.
Keshavamurthy, M., Vishwanatha, T., Suresh Kumar, M., Subhaschandra, M. Gaddad., 2019. Enhanced Production of Extracellular Alkaline Protease by Bacillus cereus GVK21 by Optimized formulations. International Journal of Pharmacy and Biological Science, 9(1), pp.1103-1113.
Khuri, A.I., and Cornell, J.A., 1987. Response Surfaces: Design and Analysis. New York USA.
Konsoula, Z., and Liakopoulou-Kyriakides, M., 2007. Co-production of α-amylase and β-galactosidase by Bacillus subtilis in complex organic substrates. Bio Resource Technology. 98, pp.150-157.
Kokab, S., Asghar, M., Rehman, K., Asad, M.J., Adedyo, O., 2003. Bio-processing of banana peel for α-amylase production by Bacillus subtilis. International Journal of Agriculture and Biosciences. 5, pp. 36-9.
Kumar, D.M., Rejitha, R., Devika, S., Balakumaran, M.D., Rebecca, A.I., Kalaichelvan, P.T., 2012. Production, optimization and purification of lipase from Bacillus sp. MPTK 912 isolated from oil mill effluent. Advances in Applied Science Research, 3:pp. 930-8.
Kumar, Y., Singh, P.K., Singh, A.K., Masih, H., Peter, J.K., Benjamin, J.C., Rath S., 2014. Production optimization of alpha amylase from Bacillus altitudinis. International Journal of Scientific engineering & Technology Research, 3, pp. 0564-73.
Liu, X.D., and Xu ,Y., 2008. A novel raw starch digesting α-amylase from a newly isolated Bacillus sp. YX-1: purification and characterization. Bioresourse Technology, 99: pp. 4315-20.
Narayana, K.J., and Vijayalakshmi, M., 2008. Production of Extracellular a-Amylase by Streptomyces albidoflavus. Asian Journal of Biochemistry, 3: pp. 194-197.
Niall, A.L., and Paul, D.V., 2009. Genus Bacillus. Bergey’s manual of systematic bacteriology. Second edition. Springer, New York, 21-128.
Oyeleke, S.B., Oduwole, A.A., 2009. Production of amylase by bacteria isolated from a cassava waste dumpsite in Minna, Niger State, Nigeria. African Journal of Microbiological Research, 3, pp. 143-6.
Pandey, A., Nigam, P., Soccol, C.R., Soccol, V.T., Singh, D., Mohan, R., 2000. Advances in microbial amylases. Biotechnology and Applied biochemistry, 31, pp.135-52.
Pandey, A., Soccol, C.R., Mitchell, D., 2000. New developments in solid state fermentation: International bioprocesses and products. Process biochemistry, 35, pp.1153-69.
Panneerselvam, T. and Elavarasis., 2015. Isolation of alpha – Amylase producing Bacillus subtilis from soil. International Journal of Current Microbiology and Applied Science, 4, pp. 543 – 552.
Parbat, R, and Singhal, B., 2011. Production of glucoamylase by Aspergillus oryzae under solid state fermentation using agro industrial products. International Journal of Microbiology Research, 2, pp. 204-7.
Raj, V., and Hemashenpagam, N., 2012. Production and medium optimization of amylase by Bacillus using fermentation methods. Journal of Microbiology and. Biotechnology Research, 2(4), pp. 481-84.
Rameshkumar, A., Sivasudha, T., 2011. Optimization of nutritional constitute for enhanced alpha amylase production using by solid state fermentation technology. International Journal of Microbiology Research, 2: pp. 143-8.
Reddy, N.S, Nimmagadda, A., and Rao, K.S., 2003. An overview of the microbial α-amylase family. African Journal of Biotechnology, 2, pp. 645-648.
Rehana, F., Venkatasubbaiah, P., Nand, K., 1989. Preliminary studies on the production of thermostable alpha-Amylase by a mesophilic strain of Bacillus licheniformis. Chemie, Mikrobiologie, Technologie der Lebensmittel, 12, pp. 8-13.
Riaz, N., Haq, I., Qadeer, M.A., 2003. Characterization of α-amylase by Bacillus subtilis. International Journal of Agricultural and Biological Engineering, 5, pp. 249-52.
Roohi, M. K., and Ahmad, I.Z., Arif, J.M., 2011. Production of cold-active extracellular a-Amylase by newly isolated microbacterium foliorum GA2 from gangotri glacier, Western Himalaya, India. Asian Journal of Biotechnology, 3: pp. 449-59.
Roohi, A., Ahmed, I., Iqbal, M., Jamil, M., 2012. Preliminary isolation and characterization of halotolerant and halophilic bacteria from salt mines of Karak, Pakistan. Pakistan Journal of Botany, 44, pp.365-370.
Samreen., Ahmad, W., Ijaz, B., Sarwar, M.T., Gull, S., Kausar, H., 2011. African Journal of Biotechnology, 10, pp. 2119-212.
Sankaralingam, S., Shankar, T., Ramasubburayan, R., Prakash, S., Kumar, C., 2012. Optimization of culture conditions for the production of amylase from Bacillus licheniformis on submerged fermentation. American-Eurasian Journal of Agricultural & Environmental Sciences, 12, pp.1507-13.
Santos, E. D., and Martins, M.L., 2003. Effect of the medium composition on formation of amylase by Bacillus sp. Brazilian Archives of Biology and Technology, 46, pp.129-34.
Sourav, B., Shilpi, B., Arijit, D., Sanchita, A., 2011. Utilization of sugarcane bagasse for solid-state fermentation and characterization of α-amylase from Aspergillus flavus isolated from Muthupettai Mangrove, Tamil Nadu, India. Australian Journal of Basic and Applied Science, 5, pp. 1012-22.
Srivastava, R.A., Baruah, J.N., 1986. Culture conditions for production of thermostable amylase by Bacillus stearothermophilus. Applied Environment Microbiology, 52, pp.179-84.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S., 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution, 28, pp. 2731-9.
Tanyildizi, M.S., Ozer, D., and Elibol, M., 2005. Process Biochemistry, 40: pp. 2291-2296.
Verma, V.M., Shekar Avasthi, A., Raj Gupta., Monika Singh., Kushwaha, A., 2011. Isolation, Screening and Characterization of Amylolytic Microorganisms. European Journal of Experimental Biology, 1, pp. 90-96.
Vijayabaskar, P., Jayalakshmi, D., Shankar, T., 2012. Amylase production by moderately halophilic Bacillus cereus in solid state fermentation. African Journal of Microbiology Research, 6: pp. 4918-26.
Yassien, M.A., Asfour, H.Z., 2012. Improved production, purification and some properties of α-amylase from Streptomyces clavifer. African Journal of Biotechnology,11:14603-11.
Zambare, V.P., 2011. Optimization of Amylase Production from Bacillus sp. using Statistics based Experimental Design. Emirates Journal of Food and Agriculture, pp. 37-47.