1Nanobiotechnology Research Centre, Baqiyatallah University of Medical Sciences, Tehran, Iran
2Clinical and Anatomical Pathologist at Tehran University of Medical Sciences, Imam Khomeini Hospital Complex, Tehran, Iran
3Radiology and Nuclear Medicine Department, School of Paramedical Sciences, Kermanshah University of Medical Sciences, Kermanshah, Iran
4Chemical Injuries Research Center, Baqiatallah University of Medical Sciences, Tehran, Iran
5Department of Pulmonology, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran
Corresponding author Email: r.nourani@yahoo.com
Article Publishing History
Received: 27/12/2018
Accepted After Revision: 12/02/2019
Pneumonitis is one of the most common consequences of exposure to high doses of ionizing radiation in lung tissue. It is associated with acute inflammation and infiltration of inflammatory cells, leading to massive release of several inflammatory mediators. Some studies have reported a risk for pneumonitis following exposure to a radiation disaster. In this experimental study, we aimed to evaluate possible mitigatory effect of alpha-tocopherol and nano-micelle curcumin on radiation pneumonitis in mice. 30 male mice were divided into 6 groups, including control, alpha-tocopherol or nano-micelle curcumin treatment, radiation, and radiation plus alpha-tocopherol or nano-micelle curcumin. Treatments were initially performed 1 day after irradiation and continued for 1 month. Irradiation was performed with 18 Gy using a cobalt-60 gamma rays source. After 8 weeks, mice were sacrifi ced and the lung tissues were removed for histopathological evaluation. Our study showed that pneumonitis was associated with was able to reduce infl ammatory cells infi ltration, edema, vascular and alveolar damage. Treatment with alpha-tocopherol could attenuated infl ammation markers, while it could not mitigate vascular and alveolar damage. By contrast, nano-micelle curcumin was able to reduce infl ammation, and vascular and alveolar damage. This study revealed that treatment with alpha-tocopherol or nano-micelle curcumin can mitigate radiation-induced pneumonitis in mice. These fi ndings may pave the way to mitigation of radiation toxicity after a radiation disaster such as nuclear explosion or radiological events.
Radiation; Lung; Pneumonitis; Alpha-Tocopherol; Nano-Micelles Curcumin
Asl P. A, Saffar H, Najafi M, Taheri R. A, Qazvini A, Nourani R. N. Mitigation of Radiation-Induced Pneumonitis in Mice Using Alpha-Tocopherol and Nano-Micelle Curcumin. Biosc.Biotech.Res.Comm. 2019;12 (1).
Asl P. A, Saffar H, Najafi M, Taheri R. A, Qazvini A, Nourani R. N. Mitigation of Radiation-Induced Pneumonitis in Mice Using Alpha-Tocopherol and Nano-Micelle Curcumin. Biosc.Biotech.Res.Comm. 2019;12 (1). Available from: https://bit.ly/2M959Hd
Copyright © Asl et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Pneumonitis is the most common frequent side effect of high dose ionizing radiation in lung tissue. It is characterized by acute inflammation in the arisen from inflammatory cells infiltration and thereby the release of several inflammatory mediators. A high dose of ionizing radiation has been shown to induce massive cell death followed by release of cytokines, inflammatory mediators, and reactive oxygen and nitrogen species (ROSs and RNSs, respectively). Chronic increased level of cytokines and production of free radicals lead to alveolar, vascular, bronchiolar damage, thereby interrupting normal function of lungs. This phenomenon may be observed in patients with various types of cancer in thoracic area such as non-small cell lung carcinoma (NSCLC), breast cancer, bronchial cancer, and etc. Moreover, mortality has been shown in association with lung injury among radioactive iodine- treated thyroid cancer patients with metastasis in the lungs, (Kwa et al. 1998, Rodrigues et al. 2004, Tsoutsou Koukourakis 2006, Hebestreit et al. 2011 and Najafi et al., 2018 ).
In addition to cancer patients, a body of evidence have suggested that pneumonitis may occur among people who were already exposed to acute ionizing radiation during nuclear or radiological disaster. Although bone marrow and gastrointestinal tract toxicity are the most common culprits for death following a radiation disaster, some studies have proposed that during whole body exposure to ionizing radiation, lung tissue may receive more than 7-8Gy; while lower parts of body may be affected by non-lethal doses, (Christofi dou-Solomidou et al. 2011; Christofi dou- Solomidou et al. 2017 and Yahyapour et al., 2018a).
Fortunately, stem cell therapy is able to prevent death caused by hematopoietic and gastrointestinal tract toxicity that occur following exposure to an acute radiation dose greater than 8Gy (Lataillade et al. 2007). Although this worthwhile therapeutic strategy can prevent early death, organ failure in lung following exposure to radiation is likely to occur. Additionally, there is piece of evidence indicating death caused by lung toxicity among people who were exposed to Chernobyl nuclear station explosion, (Deas et al. 2017; Yahyapour et al. 2018c). Experimental studies have proposed that treatment with some antioxidants and immunomodulatory agents may mitigate radiation injury in radiosensitive organs.
So, identifi cation of mitigatory effect of low toxic antioxidants and fl avonoids may be useful for to address the possible radiation disasters. Curcumin is a well-known herbal agent with potent anti-infl ammatory and antioxidant effects. Previous studies have shown considerable protective effect of curcumin against toxicity of ionizing radiation, (Jurenka 2009, Cho et al. 2013; Patil et al. 2015 Yahyapour et al. 2018b Bagheri et al. 2018).
One of the most important concerns about the curcumin is its low absorption in gastric owing to high lipophilicity. This issue has been tackled in nano-micelle form of curcumin (Li et al. 2015). Consequently, more effi ciency can be achieved with the same dose following treatment with nano-micelle form of curcumin. Alphatocopherol is another antioxidant that was used for mitigation of lung pneumonitis in the current study. It has shown a potent antioxidant effect which is able to protect and mitigate radiation toxicity (Ferreira et al. 2004; Singh et al. 2010). In this study, we aimed to illustrate possible mitigatory effect of nano-micelle form of curcumin and alpha-tocopherol on radiation-induced pneumonitis.
Material and Methods
Experimental design
All 30 male mice, purchased from Razi institute, Tehran, Iran; were kept under standard condition (temperature = 25C, humidity=55%, 12h light/12h dark). Then, they were divided into 6 groups (5mice in each). 1) G1 (control): mice who did not receive any drug or irradiation, and were taken only anesthesia drugs in a similar dose as other groups; 2) G2 (irradiation): mice who were irradiated locally in chest area, 3) G3 (alphatocopherol treatment): those received 200mg/kg/day alpha-tocopherol, 4) G4 (curcumin treatment): mice in this group were received 100mg/kg/day nano-micelle form of curcumin, 5) G5 (radiation plus alpha-tocopherol): mice were exposed to gamma rays, and after 24h, treatment with alpha-tocopherol was started, and 6) G6 (radiation plus curcumin): mice were exposed to gamma rays, and after 24h, treatment with nano-micelle form of curcumin was started. Finally, all mice were sacrificed 8weeks after irradiation and then the lung tissues were removed for histological evaluation.
Irradiation and drug administration
Alpha-tocopherol soft gel capsule was obtained from Nature Made and dissolved in olive oil. Each mouse orally received 1ml containing 6mg alpha-tocopherol (equal 200mg/kg). Then, treatment with alpha-tocopherol was started 1day after irradiation with the procedure of 5-time per week for 4 weeks. Nano-micelle form of curcumin was purchased from Exir Nano Sina, Mashahd, Iran, and was dissolved in water at a concentration of 1mg/ml. As mice drink 3 milliliter water daily which was equal to 100mg per kg per day. Treatment was started 24h after irradiation and continued for 1 month. Then, Irradiation was performed using a cobalt-60 source. Before this, mice were anesthetized using a combination of ketamine and xylazine (). After that, they were exposed to gamma rays source at supine position with a distance of 80cm. subsequently, irradiation of chest was done at a dose of 60cGy/min and other parts of the mice’s body were shielded using lead block.
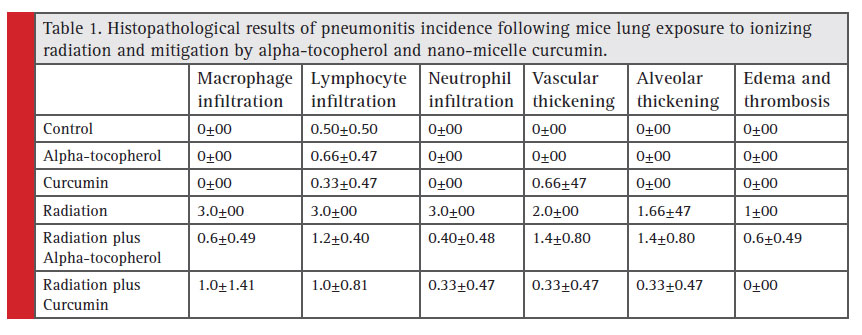 |
Table 1: Histopathological results of pneumonitis incidence following mice lung exposure to ionizing radiation and mitigation by alpha-tocopherol and nano-micelle curcumin. |
Histopathological evaluation
Following fi xation of lung tissues, all samples were embedded into a block of paraffi n. Then, they were cut by microtome to pieces with 4-micron thickness. Resultant lung pieces were located on the slides to be stained. Each slide was stained using hematoxylin and eosin (H&E). The stained slides were evaluated for morphological changes by a histopathologist. Pneumonitis markers including infi ltration of infl ammatory cells and alveolar and vascular damages were detected. All these procedures were performed in the pathology unit of Imam Khomeini hospital, Tehran University of Medical Sciences, Tehran, Iran.
Statically analyses
Results of histopathological evaluation were reported as grade 0 to3. Changes in each groups were calculated as mean ± standard deviation. Mann-Whitney test was used to evaluate the signifi cance in the groups (SPSS V16).
Results
Histopathological evaluations showed a signifi cant increase in pneumonitis markers following exposure to radiation. a drastic increase was also shown in the infi ltration of infl ammatory cells including macrophages, lymphocytes and neutrophils (p=0.008). Treatment with alpha-tocopherol caused signifi cant amelioration of these infl ammatory cells (p value of 0.018, 0.014, and 0.018 for mitigation of macrophages, lymphocytes, and neutrophils, respectively). When mice were treated with alpha-tocopherol after irradiation, only a low infi ltration of lymphocytes observed, with infi ltration of macrophages and neutrophils being similar to that in control group. Irradiation also lead to a moderate vascular thickening (p=0.008) and a mild alveolar thickening (p=0.01) and edema (p=0.008). However, treatment with alphatocopherol failed mitigate these parameters. Treatment with alpha-tocopherol alone did not cause any toxicity, and lung morphology in this group was similar to that in control group.
Treatment with curcumin nano-micelle has a potent mitigatory effect on radiation-induced pneumonitis. However, treatment with neither nano-micelle curcumin nor alpha-tocopherol led to signifi cant change in morphological properties of mice lung tissue. On the other hand, treatment with nano-micelle curcumin after irradiation could signifi cantly attenuate infi ltration of lymphocytes (p=0.037) and neutrophils (p=0.037), however, the difference in infi ltration of macrophages wasn’t signifi cant. administration of this drug also led to a remarkable attenuation of vascular thickening (p=0.037), edema, and thrombosis (p=0.025). However, difference in alveolar thickening between radiation group and radiation plus nano-micelle curcumin was not signifi cant.
Discussion
As previously mentioned, radiation-induced pneumonitis is the acute response of lung tissue which leads to respiratory impairments, and also may cause death in a few months. As results of this study showed, lung exposure to ionizing radiation caused severe infiltration of macrophages, lymphocytes and neutrophils, all of which resulting from massive DNA damage and cell death which in turn lead to the release of chemokines and recruitment of circulatory cells into the injured area (Mukaida et al. 1998). These cells are able to produce massive free radicals via a phenomenon named respiratory burst. Production of free radicals activates several signaling pathways, including redox reactions by prooxidant enzymes, that lead to chronic oxidative stress (Yahyapour et al. 2018d). Also, they are capable of releasing several pro-inflammatory and pro-fibrotic cytokines. Interactions between oxidative stress and infl ammatory cytokines plays a key role in development of late effects of radiotherapy and radiation disaster. targeting these interactions has been proposed as a strategy for mitigation of radiation injury (Farhood et al. 2018). With respect to the results, post irradiation-chronic infl ammation causes alveolar and vascular injury, edema, and thrombosis.
To date, some agents have been proposed for mitigation of radiation-induced lung injury in animal models. Targeting COX-2 by celecoxib has been shown to alleviate pneumonitis and increase survival following local chest irradiation (Hunter et al. 2013). renin-angiotensin system also has been proposed as a target for mitigation of lung pneumonitis (Medhora et al. 2012). Ghosh et al. showed that administration of captopril or losartan (which are angiotensin inhibitor) mitigates radiation pneumonitis and increases survival through the improved breathing, amelioration of vascular injury, and reduction of infiltration of inflammatory cells (Ghosh et al. 2009; Molthen et al. 2012). Similar results have been obtained with antioxidants. Mahmood et al. showed that treatment with genistein and/or Eukarion (EUK)-207, which have antioxidant effects, can mitigate the release of infl ammatory cytokines such as IL-1α, IL-1α, IL-6 and TNF-α, and reduce oxidative stress and activity of macrophages (Mahmood et al. 2011). BIO 300, a nanosuspension of genistein has shown similar results (Jackson et al. 2017). These studies show pivotal role of both free radical production and elevated activity of infl ammatory mediators in development of radiationinduced pneumonitis.
In current study we aimed to detect possible mitigatory effects of two natural anti-inflammation and antioxidant agents. Curcumin demonstrated potent anti inflammatory properties. However, it has low absorbance in intestine which may reduce its effi ciency. In recent years, several studies have used other forms of curcumin with high absorbance. For instance, Nanomicelle curcumin is a low cost form of curcumin with easy absorbance. Results of this study showed that treatment with nano-micelle curcumin can strongly mitigate inflammation, infiltration of inflammatory cells, thrombosis, and vascular and alveolar injury. Mitigatory effect of nano-micelle curcumin may be mediated by suppression of inflammatory mediators such as NF-kB which in turn leads to reduced release of inflammatory cytokines. Additionally, curcumin has antioxidant properties and is able to reduce activity of pro-oxidant enzymes such as COX-2 and iNOS.
Alpha-tocopherol was another agent we used for mitigation of lung pneumonitis. Treatment with Alphatocopherol reduced the serum level of cytokines such as G-CSF, IL-10, IL-6, IL-12, and FLT3 in peripheral blood mononuclear cells (PBMCs) (Singh et al. 2014). Moreover, it was shown to mitigate intestinal injury following exposure to radiation through suppression of apoptosis and enhancement of cell proliferation (Singh et al. 2013). This study showed that post-exposure treatment with alpha tocopherol can mitigate infi ltration of macrophages, lymphocytes and neutrophils. However, differences for alveolar and vascular was not signifi cant. It is possible that longer follow-up or treatment with higher dose of alphatocopherol would be benefi cial for a remarkable mitigation of radiation-induced lung pneumonitis.
Conclusion
This study showed that treatment with alpha-tocopherol or nano-micelle curcumin could mitigate radiationinduced pneumonitis in mice. However, alpha-tocopherol could not mitigate alveolar and vascular injury. Owing to the low toxicity of these agents, longer treatment or higher doses of alpha-tocopherol would be more effective. These findings may pave the way to mitigation of radiation toxicity after a radiation disaster such as nuclear explosion or radiological events.
References
Bagheri, Hamed, et al. (2018), Protection Against Radiation- Induced Micronuclei in Rat Bone Marrow Erythrocytes by Curcumin and Selenium L-Methionine (2018).
Cho, Y. J., et al. (2013), Curcumin attenuates radiation-induced inflammation and fi brosis in rat lungs, Korean J Physiol Pharmacol, 17 (4), 267-74.
Christofi dou-Solomidou, M., et al. (2017), Radiation Mitigating Properties of Intranasally Administered KL4 Surfactant in a Murine Model of Radiation-Induced Lung Damage’, Radiat Res, 188 (5), 491-504.
Christofi dou-Solomidou, M., et al. (2011), Dietary fl axseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice, BMC Cancer, 11, 269.
Deas, S. D., Huprikar, N., and Skabelund, A. (2017), Radiation exposure and lung disease in today’s nuclear world, Curr Opin Pulm Med, 23 (2), 167-72.
Farhood, B., et al. (2018), Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation J Cell Commun Signal. Ferreira, Paulo Renato, et al. (2004), Protective effect of alphatocopherol in head and neck cancer radiation‐induced mucositis: A double‐blind randomized trial, Head & Neck: Journal for the Sciences and Specialties of the Head and Neck, 26 (4), 313-21.
Ghosh, S. N., et al. (2009) Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis, Int J Radiat Oncol Biol Phys, 75 (5), 1528-36.
Hebestreit, Helge, et al. (2011) Pulmonary fi brosis in youth treated with radioiodine for juvenile thyroid cancer and lung metastases after Chernobyl’, European journal of nuclear medicine and molecular imaging, 38 (9), 1683.
Hunter, N. R., et al. (2013) Mitigation and treatment of radiation- induced thoracic injury with a cyclooxygenase-2 inhibitor, celecoxib Int J Radiat Oncol Biol Phys, 85 (2), 472-6.
Jackson, I. L., et al. (2017) BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fi brosis following high-dose radiation exposure in the C57L/J murine model’, Br J Pharmacol, 174 (24), 4738-50.
Jurenka, Julie S (2009) Anti-infl ammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research’, Alternative medicine review, 14 (2).
Kwa, Stefan LS, et al. (1998) Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients International Journal of Radiation Oncology* Biology* Physics, 42 (1), 1-9.
Lataillade, JJ, et al. (2007) New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy’.
Li, Jinglei, et al. (2015) Modifi ed curcumin with hyaluronic acid: combination of pro-drug and nano-micelle strategy to address the curcumin challenge’, Food Research International, 69, 202-08.
Mahmood, J., et al. (2011) Mitigation of radiation-induced lung injury by genistein and EUK-207’, Int J Radiat Biol, 87 (8),889-901.
Medhora, Meetha, et al. (2012) Radiation damage to the lung: mitigation by angiotensin converting enzyme (ACE) inhibitors Respirology (Carlton, Vic.), 17 (1), 66-71.
Molthen, R. C., et al. (2012) Mitigation of radiation induced pulmonary vascular injury by delayed treatment with captopril Respirology, 17 (8), 1261-8.
Mukaida, Naofumi, Harada, Akihisa, and Matsushima, Kouji (1998) Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions Cytokine & growth factor reviews, 9 (1), 9-23.
Najafi , M., et al. (2018) Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications Int J Radiat Biol, 94 (4), 335-56.
Patil, KarthiKeya, et al. (2015) Use of Curcumin Mouthrinse in Radio-Chemotherapy Induced Oral Mucositis Patients: A Pilot Study Journal of clinical and diagnostic research: JCDR, 9 (8),ZC59-62.
Rodrigues, George, et al. (2004) Prediction of radiation pneumonitis by dose–volume histogram parameters in lung cancer—asystematic review Radiotherapy and oncology, 71 (2), 127-38.
Singh, V. K., et al. (2013) Alpha-tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation Int J Radiat Biol, 89 (5), 334-45.
Singh, Vijay K, Brown, Darren S, and Kao, Tzu-Cheg (2010) Alpha-tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor’, International journal of radiation biology, 86 (1), 12-21.
Singh, Vijay K., et al. (2014) Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation combined injury in mice Journal of Radiation Research, 55 (1), 41-53.
Tsoutsou, Pelagia G and Koukourakis, Michael I (2006), ‘Radiation pneumonitis and fi brosis: mechanisms underlying its pathogenesis and implications for future research’, International Journal of Radiation Oncology Biology Physics, 66 (5), 1281-93.
Yahyapour, R., et al. (2018a) Targeting of Inflammation for Radiation Protection and Mitigation Curr Mol Pharmacol, 11 (3), 203-10.
Yahyapour, R., et al. (2018b) Radiation protection and mitigation by natural antioxidants and flavonoids; implications to radiotherapy and radiation disasters, Curr Mol Pharmacol.
Yahyapour, R., et al. (2018c), ‘Radiation-induced inflammation and autoimmune diseases’, Mil Med Res, 5 (1), 9.
Yahyapour, R., et al. (2018d), Reduction-oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics, Clin Transl Oncol, 20 (8), 975-88.