Department of Microbiology, Faculty of Biological Sciences,
University of Chittagong, Chattogram, Bangladesh
Corresponding author email: zobaid@cu.ac.bd
Article Publishing History
Received: 15/08/2021
Accepted After Revision: 22/09/2021
Mobile phone is an essential part of everyday life in modern days. Mobile phones act as vehicles for transmitting pathogenic bacteria due to lack of awareness and widespread use. This study aimed to investigate the bacterial contamination of mobile phones of different categories people at Chattogram city, Bangladesh. During the present study, 40 swab samples were collected from the mobile phones of students, businessmen, fishermen, and hospital patients for the isolation, identification of mobile phone associated bacteria, and their antibiogram. In our study, total viable count (TVC) was performed by the pour plate method and total coliform count (TCC) by the most probable number (MPN) method. Besides these, five selective media were used to isolate pathogenic bacteria from mobile phones and then identified. Antibiotic sensitivity assay was performed by disc diffusion method with 10 different antibiotics. Mobile phones of hospital patients (20165 cfu/ml) and students (1578 cfu/ml) showed the highest and lowest TVC respectively.
Coliform bacteria were detected from the mobile phones of 100% hospital patients, 90% from both businessmen, and fishermen but only 30% from students. Klebsiella pneumoniae and Pseudomonas aeruginosa were found the most prevalent bacteria but Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus sp., Bacillus sp., E. coli, Salmonella sp., Citrobacter sp., Serratia sp., Proteus sp., and Enterobacter sp. were also detected. Almost all the isolates were highly resistant to ampicillin, amoxicillin, rifampin, erythromycin and sensitive to ciprofloxacin, gentamicin, azithromycin, and tetracycline. Our findings ensure that mobile phones act as an important source of pathogenic organisms for humans and can serve as a vehicle for cross-transmission of microbiota. So, washing hands before and after handling food and also personal hygiene is very important.
Coliform, Klebsiella pneumoniae, Pseudomonas aeruginosa
Alam M. Z, Mantasha S. M. Microbial Contamination Analysis of Mobile Phones from Certain Users of Chattogram, Bangladesh. Biosc.Biotech.Res.Comm. 2021;14(4).
Alam M. Z, Mantasha S. M. Microbial Contamination Analysis of Mobile Phones from Certain Users of Chattogram, Bangladesh. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3onf385“>https://bit.ly/3onf385</a>
Copyright © Alam and Mantasha This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
A mobile phone is a device that may make and receive calls over a communication system whilst touring a good geographical area. It does so by connecting to a cellular network provided by a portable operator, allowing access to the general public telephone network (Suganya and Sumathy 2012). In every place and situation, it’s used for communication by different groups of individuals. For sending and receiving messages, photos, and videos mobile phone provide several additional services like SMS, email, internet, and MMS (Roy et al. 2013). It may also be used for business purposes, within the medical field, and in online banking and finance.
Mobile phone is a potential carrier of a variety of microorganisms (Dave and Shende 2015, Olsen et al. 2020). Research has been shown that mobile phones may well be a health hazard with ten thousand microbes living on each square of the phone (Kilic et al. 2009). People carried their mobile phones in hospitals, toilets, kitchens, etc. as a result they became loaded with thousands of microorganisms (Bhoonderowa et al. 2014, Bhardwaj et al. 2020). After handling fish, meat, and animals selected groups of individuals don’t wash their hands, as a result, contamination of mobile phones occurred. Moreover, mobile phones may be contaminated with E. coli and other enterobacteriaceae by hands washing water (Rather 2009).
Certain microbial species promptly colonized on the human body surface because it’s constantly in contact with environmental microorganisms (Prescott et al. 2005). Staphylococcus aureus is generally present on the skin and causes pimples, boils etc. Besides, it also causes other diseases like pneumonia and meningitis (Roy et al. 2013; Jamalludeen 2020). Irrational use of antibiotics is one of the most significant factors for the presence of emerging multi-drug resistant microorganisms everywhere. Poor drug quality and inadequate doses are also major reasons for the emergence of multi-drug resistant bacteria especially in developing countries like Bangladesh (Okeke et al. 1999). If this case continues, no dose or levels of antibiotics are going to be effective against bacterial diseases (Debnath et al. 2017). Antibiotic-resistant bacteria prompt high morbidity and mortality, patients need to stay a long time in hospital and causes higher treatment expenses (Olu-Taiwo et al. 2020).
In this research, an attempt was made to detect and characterize mobile phone associated bacterial pathogens among four categories of people living in Chattogram city, Bangladesh using microbiological techniques. Furthermore, the antibiotic susceptibility pattern of the isolates was also determined.
MATERIAL AND METHODS
The study was carried out in Chattogram city which is known as a seaport city and financial center in Bangladesh. A total of 40 mobile phone samples were collected from 4 categories of people living in Chattogram city such as 10 from students, 10 from fishermen, 10 from hospital patients, and 10 from businessmen. Sterile cotton swab was first moistened with normal saline and scratched over both surfaces of the tested mobile phones. The cotton ends of these swabs were cut off and soaked in 50 ml of sterile peptone water. The sample bottles were then preserved in an ice-fitted (4-8oC) basket to restrict bacterial growth and within 4-5 hours of the collection carried to the laboratory and preserved at 4oC until analysis.
Live microbial load on mobile phones can able to estimate by this method and the count represents the number of colony-forming units (cfu/ml) of the sample. TVC was conducted by means of the serial dilution agar plating method (Marzan et al. 2017). In this method, 1 ml of peptone water containing sample was inoculated into 9 ml of distilled water to get 10-1 dilution and then serially made up to 10-6 dilutions. One ml sample from each dilution was dispensed in the sterilized petriplate and then melted nutrient agar (about 45oC) was poured in each petriplate, mixed uniformly by rotated clockwise and anti-clockwise, and then allowed to solidify. After incubation at 37oC for 24 hours only 30-300 colonies containing plates were allowed for counting. The TVC was calculated according to the following formula:
cfu/ml= (Number of colonies) / (volume plated × dilution factor)
The presence of total coliform bacteria was assayed through the MPN technique according to the standard method (Clesceri et al. 1996). This test was carried out in three stages like presumptive, confirmed, and completed test. In presumptive test, for each sample, 3 sets of MacConkey broth containing screw cap tubes were prepared and in each tube Durham’s tube were used for the detection of gas formation by coliform bacteria. All the test tubes were incubated at 37°C for 48 hours for gas formation. For confirm test, brilliant green lactose bile broth containing test tubes were inoculated with positive presumptive test samples and incubated at 37oC for 48 hours for gas formation.
Complete test was performed for all the positive confirmed test samples. The positive samples were streaked on Eosin methylene blue containing plates and incubated for 24 hours at 37°C.Then selected nucleated colonies (with or without metallic sheen) were transferred to Lauryl tryptose broth tube and nutrient agar slant and then incubated at 37°C for 24-48 hours. After incubation, formation of gas, and in agar culture presence of Gram-negative, non-spore-forming, rod-shaped bacteria make sure the presence of coliform bacteria.
Five selective media were used to check microbial contamination of mobile phones. These were: Eosin Methylene Blue (EMB) agar (E. coli) and other coliform groups of bacteria, Xylose Lysine Deoxycholate (XLD) agar (Salmonella sp.), Salmonella-Shigella (SS) agar (Shigella sp.), Cetrimide agar (Pseudomonas sp.), and Mannitol salt agar (Staphylococcus sp.). Selective media were streaked by gas positive sample of MPN and after incubation, the grown colonies were transferred to nutrient agar slants and preserved for further microscopic, biochemical characterization, and antimicrobial susceptibility tests.
Kirby-Bauer method was used to determine the sensitivity and resistance of different antibiotics in-vitro (Bauer et al. 1996). This method can rapidly determine the efficacy of an antibiotic by the diameter of the zone of inhibition. A suspension of pure bacterial culture was prepared in peptone broth and incubated. The broth was spread by sterile cotton swab homogenously on the solidified Mueller-Hinton agar plate and then the antibiotic discs were placed. The plates were then kept at freeze for 30 minutes and then incubated for 24 hours. The used antibiotic discs were: gentamicin (10 µg), azithromycin (15 µg), ciprofloxacin (5 µg), ampicillin (10 µg), tetracycline (30 µg), erythromycin (15 µg), amoxicillin (15 µg), rifampicin (5 µg), chloramphenicol (30 µg), ceftriaxone (30 µg). After incubation, the diameters of the zone of inhibition were measured.
RESULTS AND DISCUSSION
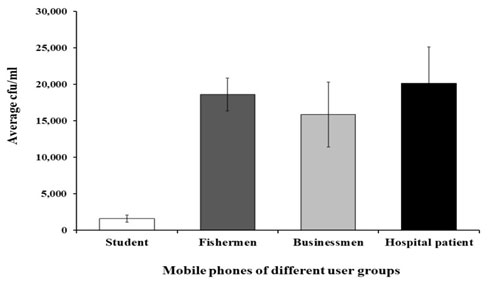
All the 40 samples were examined randomly and found 100% of mobile phones were contaminated with microbiota. The average cfu/ml was calculated and the highest average cfu/ml was observed in the mobile phones of hospital patients (20,165 cfu/ml) followed by fishermen (18,600 cfu/ml), businessmen (15,870 cfu/ml) and the lowest in students (1578 cfu/ml) mobile phones (Figure 1).This result indicated that students are handled their mobile phones carefully and kept them clean as a result carry comparatively less microorganisms, on the other hand, hospital patients should aware of the cleanliness of their mobile phones because of the highest TVC found on their mobile phones.
Otherwise, nosocomial infections will be spread by mobile phones. The results are in accordance with other findings who found that 99% healthcare workers phones were contaminated with pathogenic microbes and multi-drug resistant bacteria (Bhat et al. 2011; Huffman et al. 2020, Olsen et al. 2020; Simmonds et al. 2020). A study was conducted in Kashmir on which the highest TVC was found in animal handlers and lowest in veterinary surgeon’s mobile phones (Roy et al. 2013).
Figure 1: Average cfu/ml of mobile phones of different user groups. The presented
values are the mean and standard error of mean. N=40 (10 mobiles for each group).
Total coliform count includes aerobic and facultative anaerobic, non-spore forming, Gram-negative bacilli capable of growing with the fermentation of lactose and production of acid within 24 hours at 35-37oC. The presence of coliforms in collected samples indicates that mobile phones were contaminated with pathogenic organisms. These happen because people carry their phones in the toilet, kitchen, etc., and are not aware of the hygienic conditions of mobile phones. Thus, mobile phones become loaded with coliforms and other types of bacteria.
For total coliforms, the values ranged from 0 to 2400 MPN per 100 ml. In total, 77.5% of mobile phones of four different categories of people were found contaminated with coliform bacteria which indicated the poor hygienic condition of the mobile phones of the study area. These mobile phones can act as a carrier for coliform transmission. Some of these mobile phones can also be a cause of nosocomial infections. 22.5% of mobile phones were not contaminated with coliform which indicates a relatively good hygienic condition of these mobile phones.
Table 1. Results of MPN test for estimation of total coliform count according to WHO guidelines
| Mobile phones of different groups | Sample No. | MPN index / 100 ml | Average (%) in each groups | Mobile phones of different groups | Sample No. | MPN index / 100 ml | Average (%) in each groups |
| MM1 | 150 | MM21 | 28 | ||||
| MM2 | 23 | MM22 | 28 | ||||
| MM3 | 0 | MM23 | 21 | ||||
| MM4 | 0 | MM24 | 1100 | ||||
| Students | MM5 | 0 | 30% | Businessmen | MM25 | 210 | 90% |
| MM6 | 1100 | MM26 | 460 | ||||
| MM7 | 0 | MM27 | 23 | ||||
| MM8 | 0 | MM28 | 93 | ||||
| MM9 | 0 | MM29 | 20 | ||||
| MM10 | 0 | MM30 | 0 | ||||
| MM11 | >2400 | MM31 | 15 | ||||
| MM12 | 210 | MM32 | 75 | ||||
| MM13 | 0 | MM33 | 7 | ||||
| MM14 | 1100 | MM34 | 7 | ||||
| Fishermen | MM15 | 7 | 90% | Hospital patients | MM35 | 150 | 100% |
| MM16 | >2400 | MM36 | 460 | ||||
| MM17 | 120 | MM37 | 210 | ||||
| MM18 | 64 | MM38 | 150 | ||||
| MM19 | 460 | MM39 | 15 | ||||
| MM20 | 150 | MM40 | 460 |
In our result, the mobile phones collected from students were found less contaminated with coliforms (30%). On the contrary, the highest percentage of coliform contamination (100%) was found among mobile phones of hospital patients and 90% from businessmen and fishermen (Table 1). The presence of Gram-negative rod, Enterobacter aerogens, a number of coliform, indicates the possibility of the presence of fecal contamination on mobile phones (Dave and Shende 2015). Our data revealed that students are relatively more aware of the maintenance of hygienic conditions of mobile phones and hospital patients should handle their mobile phones carefully.
To check the mobile phones contamination, bacteria were isolated, identified, and then confirmed by using microscopic, cultural, and biochemical tests (Table 2). After 24 hours of incubation at 37oC, dark colonies with a green metallic sheen and dark colonies without green metallic sheen on EMB agar, colonies that appeared as red or entirely black were on XLD agar, colorless colonies and pink-colored colonies on SS agar, yellow and pink colored colonies on mannitol salt agar plates were picked and purified. The purified isolates were then subjected to Gram staining along with different morphological and biochemical tests to identify the bacterial strain. Compared with the standard description given in ‘‘Bergey’s Manual of Determinative Bacteriology’’ bacterial isolates were identified up to species level (Buchanan and Gibbons 1974).
From our study, a total of 12 different types of bacteria were isolated from 40 samples collected from students, fishermen, businessmen, hospital patients. We found average percentage of Klebsiella pneumoniae (75%) and Pseudomonas aeruginosa (58%) were the most prominent bacteria from all types of mobiles followed by Micrococcus sp. (35%), Staphylococcus aureus (25%), Salmonella sp. (25%), Bacillus sp. (10%), Proteus sp. (8%), Staphylococcus epidermidis (5%), Serratia sp. (5%), Citrobacter (3%), E. coli (3%), and Enterobacter sp. (3%) (Table 3). The study is in accordance with the findings of others in which S. aureus, P. aeruginosa, E. coli, S. typhi, and S. epidermidis were isolated on mobile phones of healthcare workers in Bangladesh (Debnath et al. 2017). From the mobile phones of dentists 52.52% Pseudomonas sp. were detected (Lee and Lee 2019) and a higher percentage of Klebsiella sp. was identified from mobile phones of healthcare university students (Olu-Taiwo et al. 2021) which supports our results.
In the mobile phones of all groups, Klebsiella pneumoniae was found with higher percentage, and the highest prevalence was found in fishermen, businessmen, and hospital patients (80%) and lowest in students (60%) (Table 3). Pseudomonas aeruginosa was also isolated from mobile phones of all groups with the highest prevalence in fishermen, businessmen, and hospital patients (60%) and lowest in students (50%). It has been proven that many pathogens particularly P. aeruginosa remain viable for months on inert surfaces (Kramer et al. 2006). The presence of Pseudomonas sp., K. pneumoniae, and E. coli on mobile phones need serious attention from the public as this organism is associated with hospital infections and may serve as a vehicle for the spread of nosocomial infections (Karabay et al. 2007).
Highest percentage of S. aureus was identified from hospital patients (50%) and lowest from fishermen (10%) mobile phones. S. aureus is a normal flora of humans, carried on hands, nose, mouth, skin, clothes, bed linen, and other human environments (Melnick and Edward 2004). In our study, S. epidermidis was only isolated from the mobile phones of fishermen (20%) whereas Serratia sp., Enterobacter sp., Salmonella sp., Proteus sp., Citrobacter sp. and E. coli was completely absent in the mobile phones of students.
Table 2. Microscopic and biochemical properties of the bacteria isolated from drinking
water sample of different schools in Chattogram city, Bangladesh
|
Bacterial Isolates
|
Shape | Gram stain | Indole | Citrate | Catalase |
TSI |
Oxidase | MR | VP | Nitrate R. | Urease | |||
| Butt | Slant | H2S | Gas | |||||||||||
| E. coli | Rods | – | + | + | + | A | A | – | + | – | + | + | + | + |
| K. pneumoniae | Rods | – | – | + | + | A | A | – | + | – | – | + | + | + |
| P. aeruginosa | Rods | – | – | + | + | K | K | – | – | + | – | – | + | – |
| S. aureus | Cocci | + | – | + | + | A | A | – | – | – | + | + | + | + |
| S. epidermidis | Cocci | + | – | – | + | A | A | – | – | – | – | + | + | + |
| Bacillus sp. | Rods | + | – | + | + | NC | A | – | – | – | – | + | + | – |
| Salmonella sp. | Rods | – | – | + | + | A | K | + | + | – | + | – | + | – |
| Proteus sp. | Rods | – | + | – | + | A | K | + | + | – | + | – | + | + |
| Citrobacter sp. | Rods | – | – | + | + | A | A | + | + | – | + | – | + | – |
| Enterobacter sp. | Rods | – | – | + | + | A | A | + | – | – | – | + | + | – |
| Micrococcus sp. | Cocci | + | – | – | + | NC | K | – | – | + | – | + | + | + |
| Serratia sp. | Rods | – | – | + | + | A | K | – | – | – | – | + | + | + |
TSI= Triple sugar iron test, A=Acidic (Yellow), K= Alkaline (Red), NC= No change, MR= Methyl red, VP= Voges-Proskauer, + = Positive/Present, – =Negative/Absent, R= Reductase, K– Klebsiella, S–Staphylococcus, P- Pseudomonas, E- Escherichia
Table 3. Comparison of percentages of isolated bacteria among samples collected from
mobile phones of students, fishermen, businessmen, hospital patients
| Isolated Bacteria | Students (%) | Fishermen (%) | Businessmen (%) | Hospital patients (%) | Average (%) |
| K. pneumoniae | 60 | 80 | 80 | 80 | 75 |
| P. aeruginosa | 50 | 60 | 60 | 60 | 58 |
| S. aureus | 20 | 10 | 20 | 50 | 25 |
| S. epidermidis | 0 | 20 | 0 | 0 | 5 |
| Micrococcus sp. | 60 | 30 | 40 | 10 | 35 |
| Bacillus sp. | 10 | 20 | 10 | 0 | 10 |
| Serratia sp. | 0 | 0 | 10 | 10 | 5 |
| Enterobacter sp. | 0 | 0 | 10 | 0 | 3 |
| Salmonella sp. | 0 | 30 | 10 | 60 | 25 |
| Proteus sp. | 0 | 10 | 0 | 20 | 8 |
| Citrobacter sp | 0 | 10 | 0 | 0 | 3 |
| E. coli | 0 | 0 | 10 | 0 | 3 |
E. coli is an indicator organism, so its presence on mobile phone indicated that other enterobacteriaceae might be present on mobile phones (Karabay et al. 2007; Tambekar et al. 2008). The highest percentage of Micrococcus sp. (60%) and Salmonella sp. (60%) was isolated from student’s and hospital patient’s mobile phones respectively (Table 3). The observations of the present study coincide with the findings of others (Roy et al. 2013). The mobile phones of students of Cape Coast University showed high levels of bacterial contamination like Bacillus sp. and Pseudomonas sp. (Tagoe et al. 2011). From the mobile phone of health workers, marketers, food vendors, lecturers, students different pathogens like S. aureus, Enterococcus feacalis, P. aeruginosa, E. coli, Klebsiella sp., Serratia sp., Proteus vulgaris, and Bacillus sp. were frequently isolated (Akinyemi et al. 2009; Kilic et al. 2009; Famurewa and David 2009; Al-Abdalall 2010; Singh et al. 2010; Gashaw et al. 2014; Olu-Taiwo et al. 2021).
Antibiotic sensitivity assay of 12 different isolates was performed by disc diffusion method, using 10 different types of antibiotics and the results are presented (Table 4). From the results of the antibiotic sensitivity test, it is observed that most of the organisms were found highly resistant to ampicillin, amoxicillin, erythromycin, and rifampin. On the contrary, most of the organisms were found highly sensitive to ciprofloxacin, gentamicin, azithromycin, and tetracycline.
Table 4. Antibiotic sensitivity profile of different bacteria from drinking water samples
| Bacterial isolates | AMP (10µ) | AMX (10µ) | ERY (15µg) | RMP (5 µg) | TET (30µg) | CRO (30µg) | CAP (30µ) | AZM (15µg) | CIP (5µg) | GEN (10µg) |
| K. pneumoniae | R | R | R | R | S | S | S | S | S | S |
| S. aureus | R | R | I | R | S | I | S | S | S | S |
| P. aeruginosa | R | R | R | R | R | I | R | S | S | S |
| S. epidermidis | R | R | R | R | I | I | I | I | S | S |
| E. coli | R | R | R | R | S | R | S | S | S | S |
| Enterobacter sp. | R | R | R | R | S | I | R | S | S | S |
| Serratia sp. | R | R | R | R | S | R | S | S | S | S |
| Citrobacter sp. | S | S | R | R | S | I | I | S | S | S |
| Micrococcus sp. | R | R | R | R | I | I | R | I | S | S |
| Bacillus sp. | R | R | R | R | S | I | I | S | S | S |
| Salmonella sp | R | I | R | R | S | S | S | S | S | S |
| Proteus sp. | S | S | S | S | S | S | S | S | S | S |
S-sensitive; R-resistant; I-intermediate; AMX-amoxicillin, AMP –ampicillin, ERY-erythromycin, RMP-rifampin, TET-tetracycline, CRO- ceftriaxon, CAP- chloramphenicol, AZM-azithromycin, CIP -ciprofloxacin, GEN-gentamicin, K– Klebsiella, S– Staphylococcus, P- Pseudomonas, E- Escherichia
In Bangladesh, for treatment purposes antibiotics are randomly used which are available in any pharmacy. Due to indiscriminate use (misuse, overuse, self-medications) of antibiotics, and not completing the dose suggested by physicians, the antibiotic-resistant bacteria was developed. By genetic recombination, resistant strains might be developed against antimicrobial agents (Buxton and Fraser 1977). In Kashmir, most of the pathogens like E. coli, K. pneumoniae, P. aeruginosa, E. faecalis, and S. aureus had already developed resistance to the most commonly used antibiotics including ampicillin.
In the valley, multi-drug resistant pneumonia and typhoid are common (Ahmad 2008). S. aureus, E. coli, and K. pneumoniae was found highly resistant to ampicillin by others which supports our results (Olu-Taiwo et al. 2021). Antibiotic drug resistance bacteria may cause high treatment failures, increased healthcare costs, and also increase morbidity and mortality (Brady et al. 2009; Neidell et al. 2012). So, our results revealed that pathogenic bacteria can harbor on mobile phones and some of the isolated bacteria become multi-drug resistant which will help us to select the most suitable antibiotics to fight against these organisms.
CONCLUSION
Mobile phone act as a vehicle for transmitting infectious agents. In the present study, coliform and other bacteria were highly present in the mobile phones of hospital patients which are alarming for the hospital patients and their visitors. On the contrary, students mobile phones were found less contaminated. In all the mobile phones, K. pneumoniae and P. aeruginosa were present with a huge percentage. Most of the organisms were found highly resistant to ampicillin, amoxicillin, erythromycin and rifampin and highly sensitive to ciprofloxacin, gentamicin, azithromycin, and tetracycline.
There are no restrictions for using mobile phones, as a result, pathogens carried by mobile phones cause diarrhea, skin infections, pneumonia, and meningitides. So, we should be aware of limiting mobile phone usage as it has a high risk for spreading infectious agents. Mobile phone users should follow and adopt cost-effective and simple hygienic measures for a safe and healthy life.
ACKNOWLEDGEMENTS
This research was done in the laboratory of the Department of Microbiology, University of Chittagong, Bangladesh. The authors become grateful to the Department of Microbiology.
Authors contribution: MZA designed the project, supervised, contributed in experiments and prepared the manuscript draft, editing and then reviewed the final manuscript. SMM carried out the laboratory experiments and contributed in manuscript draft. Both the authors read and approved the final manuscript.
Conflict of interest: The authors declare that they have no conflict of interests.
Submission declaration: The work has not been published previously, approved by another author and responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the copyright-holder.
REFERENCES
Ahmad, M. (2008). Addressing communicable diseases in Kashmir. Indian J. Practising Doctor, 3(4): 8-9.
Akinyemi, K.O., Atapu, A.D., Adetona, O.O., and Coker, A.O. (2009). The potential role of mobile phones in the spread of bacterial infections. J. Infect. Dev. Ctries, 3: 628-632.
Al-Abdalall, A.H.A. (2010). Isolation and identification of microbes associated with mobile phones in Dammam in eastern Saudi Arabia. J. Family Community Medicine, 17 (1): 11-14.
Bauer, A.W., Kirby, W.M.M., Sherris, J.C., and Turck, M. (1996). Antibiotic susceptibility testing by a standarized single disk method. American J. Clin. Pathol, 45(4): 493-496.
Bhardwaj, N., Khatri, M., Bhardwaj, S.K., et al. (2020). A review on mobile phones as bacterial reservoirs in healthcare environments and potential device decontamination approaches. Environ. Res, 186:109569.
Bhat, S.S., Hegde, S.K., and Salian, S. (2011). Potential of mobile phones to serve as a reservoir in spread of nosocomial pathogens. Online J. Health Allied. Sci, 10(2): 14-18.
Bhoonderowa, A., Gookool, S., and Biranjia-Hurdoyal, S. (2014). The importance of mobile phones in the possible transmission of bacterial infections in the community. J. Community Health, 39: 965-967.
Brady, R.R.W., Verran, J., Damani, N.N., and Gibb, A.P. (2009). Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J. Hospital Infection, 71(4): 295–300.
Buchanan, R.E., and Gibbons, N.E. (1974). Bergey’s Manual of determinative Bacteriology, 8th ed. Baltimore. The Williams and Wilkons co.
Buxton, A., and Fraser, G. (1977). Animal microbiology. Oxford, London, Edinburg, Melbourne, Blackwell scientist publication. 85-86.
Clesceri, L.S., Greenberg, A.E., and Eaton, A.D. (1996). Standard methods for the examination of water and wastewater. APHA, AWWA and WPCF, Washington DC.
Dave, M.S., and Shende, D.K. (2015). Isolation and identification of microbes associated with mobile phones in Durg district in Chattisgarh region, India. IOSR J. Env. Sci. Toxicology Food Technol, 1(6): 71-73.
Debnath, T., Bhowmik, S., Islam, T., and Chowdhury, M.M.H. (2017). Presence of multidrug resistant bacteria on mobile phones of healthcare workers accelerates the spread of nosocomial infections and regarded as a threat to public health in Bangladesh. J. Microscopy Ultrastructure, 148: 3-4.
Famurewa, O., and David, O.M. (2009). Cell phone: A medium of transmission of bacterial pathogens. World Rural Observations, 1(2): 69-72.
Gashaw, M., Abtew, D., and Addis, Z. (2014). Prevalence and antimicrobial susceptibility pattern of bacteria isolated from mobile phones of health care professionals working in Gondar town health centers. Hindawi Publishing Corporation ISRN Public Health.
Huffman, S., Webb, C., and Spina, S.P. (2020). Investigation into the cleaning methods of smartphones and wearables from infectious contamination in a patient care environment (I-SWIPE). Am. J. Infect. Control, 48(5):545–9.
Jamalludeen, N. (2020). Bacterial contamination associated with mobile phones used by students at Basrah medical college, Basrah, Iraq. 7e Med. J. Basrah University, 38(1): 58–66.
Karabay, O., Kocoglu, E., and Tahtaci, M. (2007). The role of mobile phones in the spread of bacteria associated with nosocomial infections. J. Infect. Dev. Ctries, 1: 72-3.
Kilic, L.H., Ozaslan, M., Karagoz, I.D., et al. (2009). The microbial coonisation of mobile phone used by healthcare staffs. Pak. J. Biol. Sci, 12(11): 882-884.
Kramer, A., Schwebke, I., and Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis, 6:130.
Lee, S.Y., and Lee, S.Y. (2019). Assessment of bacterial contamination of mobile phones of dentists and dental hygienists by Illumina MiSeq. Oral Biol. Res, 43(1): 60-65.
Marzan, L.W., Hossain, M., Mina, S.A., Akter, Y., and Chowdhury, A.M.M.A. (2017). Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong City, Bangladesh: bioremediation viewpoint. Egypt. J. Aquat. Res, 43: 65-74.
Melnick, J., and Edward, A. (2004). Medical Microbiology. 23th ed. New York, McGraw-Hill Professional.
Neidell, M.J., Cohen, B., and Furuya, Y., et al. (2012). Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clinical Infectious Diseases, 55(6): 807–815.
Okeke, I.N., Lamikanra, A., and Edelman, R. (1999). Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis, 5(1): 18.
Olsen, M., Campos, M., Lohning, A., et al. (2020). Mobile phones represent a pathway for microbial transmission: a scoping review. Travel Med. Infect. Dis, 35:101704.
Olu-Taiwo, M.A., Opintan, J.A., Codjoe, F.S., and Obeng F.A. (2020). Metallo-beta-lactamase-producing Acinetobacter spp. from clinical isolates at a tertiary care hospital in Ghana. BioMed. Res. Int, 1–8.
Olu-Taiwo, M., Laryea, C.A., Mykels, D.K., and Forson, A.O. (2021). Multidrug-resistant bacteria on the mobile phones and computer keyboards of Healthcare University students in Ghana. Canadian J. Infectious Diseases Medical Microbiol, 1-8.
Prescott, L.M., Harley, J.P., and Klein, D.A. (2005). Microbiology. 6th ed. New Delhi, Tim McGraw-Hill co.
Rather, T.A. (2009). Assessment of bacterial load in different drinking water sources in Srinagar city, M.V.Sc thesis, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, India.
Roy, S.S., Mishra, S.S., and Willayat, M.M. (2013). Isolation and identification of bacteria of public health importance from mobile phones of fish and animal handlers of Kashmir, India. African J. Microbiol. Res, 7(21): 2601-2604.
Simmonds, R., Lee, D., and Hayhurst, E. (2020). Mobile phones as fomites for potential pathogens in hospitals: microbiome analysis reveals hidden contaminants. J. Hosp. Infect, 104(2):207–13.
Singh, S., Acharya, S., Bhat, M., et al. (2010). Mobile phone hygiene: potential risks posed by use in the clinics of an Indian dental school. J. Dent. Educ, 74: 1153-1158.
Suganya, S., and Sumathy, J.H. (2012). Isolation and identification of bacteria from covered and uncovered mobile phones. Int. J. Env. Sci, 3(1): 44.
Tagoe, D.N., Gyande, V.K., and Ansah, E.O. (2011). Bacterial contamination of mobile phones. Central Microbiol, 65: 121-125.
Tambekar, D.H., Gulhane, S.P.B., Dahikar, S.G., and Dudhane, M.N. (2008). Nosocomial hazard of doctor’s mobile phones in hospitals. J. Med. Sci, 8: 73-76.


