1College of Life Science, Cancer Hospital and Research Institute, Gwalior (MP) India
2Department of Microbiology, Vikram University, Ujjain (MP) India
3Department of Bioscience, Rani Durgawati University, Jabalpur (MP) India
Corresponding Author Email : abhi.shri76@gmail.com
Article Publishing History
Received: 15/04/2025
Accepted After Revision: 17/06/2025
The search for other asparaginase sources like eukaryotic microorganisms can lead to an enzyme with less adverse effects. It has been observed that eukaryotic microorganisms like yeast and filamentous fungi have a potential for asparaginase production. L-asparaginase is generally used as a therapeutic agent in the treatment of cancer. It is commonly produced by bacteria. Analysis of Immobilized L-asparaginase kinetics prepared from fungi Aspergillus flavus. In this study, L-asparaginase from the fungus Aspergillus flavus was partially purified by a step purification method and then immobilized. The enzyme showed about 7.57-fold purification and 19.55% recovery.
The kinetic analysis of the immobilized enzyme showed optimum activity at 7.0 pH and 40 °C. Km value for free L-asparaginase was 5×10-6 M, while that for the immobilized one was 3.33×10-6M. The enzyme is more stable at neutral pH than at acidic and retains 85% of its activity after 53 days of storage. The findings of the present study also extend the idea of finding L-asparaginase potential in fungi and their application in translational medicine. As mentioned earlier, fungal asparaginase may be superior in comparison to bacterial enzyme, due to reduced adverse reactions associated with fungal enzyme, so the present study demands legitimate appraisal to unravel the biotechnological potential of fungal L-asparaginase and its immobilization.The immobilized enzyme can be an attractive candidate for biotechnological applications, including the management of cancer.
Aspergillus flavus, L-asparaginase, Anticancer, Immobilization, Kinetics.
Shrivastava A, Shrivastava A, Jain S. K , Singhal P. K. Kinetic analysis of immobilized L-asparaginase from Aspergillus flavus. Biosc.Biotech.Res.Comm. 2025;18(2).
Shrivastava A, Shrivastava A, Jain S. K, Singhal P. K. Kinetic analysis of immobilized L-asparaginase from Aspergillus flavus. Biosc.Biotech.Res.Comm. 2024;18(2). Available from: <a href=”https://shorturl.at/FY9eq“>https://shorturl.at/FY9eq</a>
INTRODUCTION
Anti-tumor enzyme L-asparaginase (L-asparagine aminohydrolase EC 3.5.1.1) produces L-aspartate and ammonia by hydrolysis of L-asparagine (Asselin et al., 1991). L-asparaginase is generally used as therapeutic agent in treatment of acute lymphoblastic lymphoma and other related malignancies (Capizzi et al., 1970). This enzyme reduces the concentration of intracellular L-asparagine, since tumor cells are unable to synthesize this amino acid and are selectively killed due to L-asparagine deprivation. The enzyme has been isolated from variety of microorganisms like bacteria, fungi, algae, plants and animals (Wriston and Yellin, 1973).
The enzymes isolated from E. coli and Erwinia carotovora are being commercially used in treatment of acute lymphoblastic leukaemia (Capizzi, 1993), despite the fact that bacteria are also positively associated with cancer (Khan and Shrivastava, 2010). Prolong administration of bacterial L-asparaginase can cause hypersensitivity in the patients, leading to allergic reactions and anaphylaxis (Reynolds and Taylor, 1993, Earl, 2009, Shrivastava et al., 2010, Shrivastava et al., 2012a Duarte et al 2024). The corresponding antibodies produced cause an anaphylactic shock or neutralization of the drug effect.
The search for other asparaginase sources like eukaryotic microorganisms can lead to an enzyme with less adverse effects. It has been observed that eukaryotic microorganisms like yeast and filamentous fungi have a potential for asparaginase production (Wade et al., 1971, Wiame et al., 1985, Pinheiro et al., 2001, Sarquis et al., 2004 Duarte 2024). The mitosporic fungal genera such as Aspergillus, Penicillium and Fusarium are commonly reported in scientific literature to produce asparaginase (Shrivastava et al., 2012b, Curran et al., 1985 Duarte et al 2024 ) and require legitimate appraisal for their asparaginase potential. The present paper reports kinetic analysis of immobilized L-asparaginase from Aspergillus flavus.
MATERIAL AND METHODS
Microorganism: Aspergillus flavus was obtained from the Department of Biotechnology, College of Life Science, Cancer Hospital and Research Institute, Gwalior (M.P.), India. It was maintained on Potato Dextrose Agar (PDA) [containing (gL-1) potato starch 200, glucose 20, agar 17, distilled water; pH 7.2] at 40C.
Production of L-asparaginase: Fungus was cultured on PDA petri plates for 96 hours at 370C and its newly growing mycelia was transferred on modified Czapek-Dox’s medium [Composition (gL-1): glucose 2, L-asparagine 10, KH2PO4 1.52, KCl 0.52, MgS04.7H2O 0.52, CuN03.3H2O trace, ZnS04.7H2O trace, FeS04.7H2O trace, agar 20; pH 6.2] and incubated in shaker incubator for 96 hours at 370C. The culture was filtered by Whatmann no.1 in line filter holder (pore size – 45 mm) and the supernatant (i.e. mycelia free broth) was used as a crude extract for assaying L-asparaginase activity.
Assay of L-asparaginase: Enzyme activity was estimated by Nesslerization method (Bilimoria, 1969). The reaction mixture contained 0.2 mM of Sodium acetate buffer (pH 5.2), 20 µM of L-asparagine and suitable dilution of enzyme extract. The mixture was incubated for the 15 minute at 370C. the reaction was stopped by addition of 0.1 ml of 15% trichloroacetic acid (TCA) and finally was centrifuged at 6000g for 10 minute. The resulting supernatant (0.5 ml) was dissolved in 4 ml ammonia-free distilled water and treated with 1 ml Nessler’s reagent (1N). The liberation of ammonia was determined at 450 nm in spectrophotometer. The ammonia content was estimated by a standard curve prepared from various dilutions of 4 mM Ammonium chloride solution.
The highest enzyme activity in each experiment was taken as the maximum L-asparatinase activity. The relative activity was determined compared to the maximum activity.

Total protein content was estimated according to Lowry’s method with BSA as standard (Lowry et al., 1951).
Purification of L-asparaginase: The purification was carried out using crude enzyme extract at 0-40C, unless otherwise mentioned. Finely powdered ammonium sulphate was added to the crude extract at the rate of 1 g minute-1. This solution was kept for overnight at 40C. L-asparaginase activity was associated with the fraction precipitated at 70-80 % saturation. The protein precipitate was collected by centrifugation at 15,000 g for 15 minutes. The supernatant was discarded and the pellet was dissolved in 500 µl Phosphate Buffer Saline (PBS). The resulting purified asparaginase preparation was stored in refrigerator and used for further experiments. Desalting of precipitated protein was performed by gel filtration chromatography with Sephadex G-25 in 0.05 M PBS buffer at pH 7.4. This desalted protein was further passed through Sephadex G-100 columns (1×10 cm) in the same buffer. The protein was eluted with the same buffer at a flow rate of 5 ml 5 min-1. L-asparaginase activity and total protein concentration was estimated at each step of purification process.
Immobilization of L-asparaginase in Calcium alginate complex: Immobilization of enzyme was performed as per the method of Yousuff and Al-Omair, 2008. Combination of Calcium and Sodium alginate mixture were prepared by adding 3 gm sodium alginate in 100 ml distilled water and autoclaved for 15 minute at 1200C. Then 3 ml of purified enzyme was mixed with 8 ml sodium alginate and stirred for 15 minutes for proper mixing. 1 ml glutaraldehyde was mixed in this solution and the resultant slurry was transferred to a dropping funnel with plastic tip to fall on 7% cold CaCl2 dropwise. This chilled CaCl2 solution was used for enzyme immobilization and led to formation of enzyme beads. These beads were left in CaCl2 solution for 20 minutes for hardening. Beads were collected after decanting supernatant and washed with distilled water and stored in refrigerator until further use.
Kinetic study of immobilized L-asparaginase
Effect of substrate concentration on the free and immobilized enzyme:
Varying substrate concentration ranging from 1.25, 2.5, 5.0, 10 and 20 µM were used and effect on activity of immobilized and free enzyme was observed through Nesslerization reaction (Bilimoria, 1969).
Effect of temperature on free and immobilized L-asparaginase:
Temperature profile of L-asparaginase was determined by performing enzymatic assay by previous method at various temperatures 5, 10, 20, 30, 40, 50 and 600C.
Effect of incubation time on free and immobilized L-asparaginase:
Incubation periods of routine enzymatic assay were altered for 5, 10, 15, 20, 25 minutes.
Effect of pH on free and immobilized enzyme:
The optimum pH for activity of free and Immobilized L-asparaginase was determined by 0.2 mM Sodium acetate buffer at pH ranging from 4, 5, 6, 7, 8, 9. Thermodynamic Properties of immobilized L-asparaginase:
Thermodynamic parameters of immobilized L-asparaginase including energy of activation (Ea), enthalpy of activation (ΔH), entropy of activation (ΔS) were calculated by standard methods ADDIN EN.CITE (. The activation energy of the reaction catalyzed by enzyme was determined from equation no.1,
Ea = – RT ln K (1)
Where R is gas constant and T is absolute temperature in kelvin.
The Gibbs free energy (ΔG) was estimated using the equation no.2,
ΔG = ΔH- TΔS (2)
Where, ΔH and ΔS are the enthalpy and entropy, respectively, which were determined using equation no.3,
lnK = ΔH/RT- ΔS/R (3)
Plot of lnK against 1/T gave a straight line whose slope was ΔH/RT, and the intercept was ΔS/R.
RESULTS
Purification of L- asparaginase from A. flavus was performed. The percentage yield and enzyme activity was observed at every step of purification. The data for purification of L-asparaginase is mentioned in following manner (Table 1).
Table 1. Purification of L-asparaginase from Aspergillus flavus
| Fraction | Volume | Total Enzyme Activity (IU.ml-1)* | Total Protein concentration (mg.ml-1) | Specific Activity (IU.mg-1)** | Purification
Fold § |
¶Yield (%) |
| Crude | 28 ml | 105.84 | 2.03 | 52.13 | 1 | 100 |
| Ammonium Sulphate | 23 ml | 40.04 | 15.07 | 2.65 | 0.05 | 37.80 |
| G-25 | 1 ml | 31.70 | 0.02 | 14.80 | 0.28 | 29.95 |
| G-100 | 1 ml | 20.70 | 0.05 | 394.66 | 7.57 | 19.55 |
* Enzyme Activity: Enzyme activity is defined as 1µM ammonia released per ml in one minute.
** Specific Activity: 1µM ammonia released per minute per mg of protein.
Purification fold: Specific activity of fraction/specific activity of homogenate
¶ Yield (%): Total activity of fraction/Total activity of homogenate X 100.
Effect of different parameters on kinetic activity of free and immobilized enzyme is presented in Figure 1-4.
Figure 1: LineweaverBurk plot for L-asparaginase from Aspergillus flavus.
Km values were found as 5×10-6 M and 3.33×10-6 M for free and immobilized L-asparaginase, respectively.
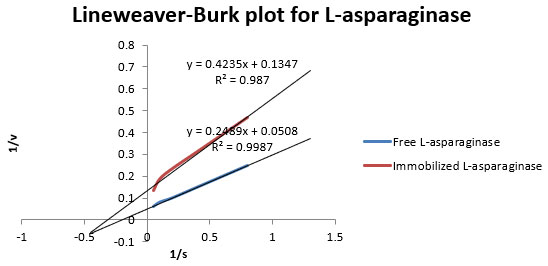
Figure 2: Effect of temperature on relative activity of free and immobilized L-asparaginase from Aspergillus flavus.
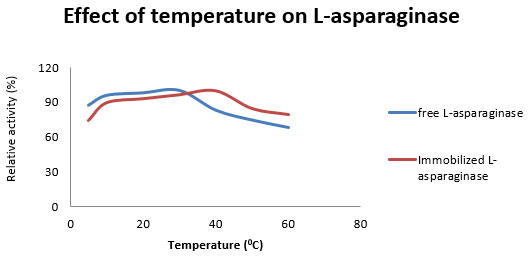
Figure 3: Effect of pH on relative activity of immobilized and free L-asparaginase from Aspergillus flavus.
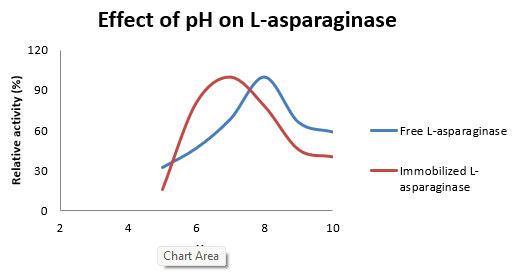
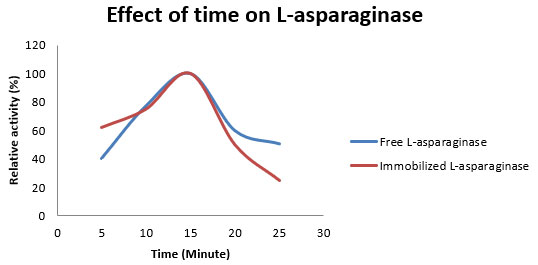
Figure 4: Effect of time on relative activity of free and immobilized L-asparaginase from Aspergillus flavus.
Functional stability of L-asparaginase was also measured during the study. L-asparaginase activity was found 14.98 IU/ ml at the time of bead preparation, while the reproducibility of average activity was maintained despite significant storage with 12.84 IU/ ml after 53 days of bead preparation. Thus, immobilized enzyme maintained about 86% of its activity in comparison to its initial status.
Thermodynamic parameters: Thermodynamic parameters of immobilized enzyme were estimated (Zeng et al., 2009, Pogaku et al., 2012).
Table 2. Thermodynamic properties of immobilized L-asparaginase
| Sr. No. | Thermodynamic parameter | Value |
| 1 | Activation energy (Ea) | 5.13 KJ/ M |
| 2 | Enthalpy of activation (ΔH) | 2.52 KJ/ M |
| 3 | Entropy of activation (ΔS) | 5.09 KJ/ K/M |
DISCUSSION
Bacterial L-asparaginases show many side effects and allergic reactions. An attempt was made to partially purify L-asparaginase from A. flavus which is eukaryotic in nature. L-asparaginase production from A. flavus was already reported by researchers (Soniamby et al., 2011). Two-step purification through ammonium sulphate precipitation followed by Sephadex G-100 gel filtration chromatography was performed (Table 1). Purification fold was 7.57 and total yield was 19.55%. This purification fold was lower in comparison to some previous studies (Oza et al., 2009). Purification was followed by immobilization and kinetic studies of immobilized and free enzyme. Immobilization of the enzyme increased several beneficial kinetic parameters, including thermal stability, reduced Km, and increased specific activity. Surprisingly, the kinetic study indicated that Km value of the immobilized bead was 1.5 times lower compared to the free enzyme. Low Km value is related to its degree of effectiveness against tumors (Schwartz et al., 1966). Therefore, it can be assumed that immobilized A. flavus asparaginase will be superior in efficacy against tumors in comparison to free enzyme (Figure 1). Although, some other studies also showed that Km value decreases with immobilization in some enzymes (Ertan et al., 2006).
During analysis of optimum temperature for enzyme activity, it was observed that optimum temperature was 40 0C and 20 0C for immobilized and free enzyme respectively. Another important serendipitous observation was 4.8 fold increased specific activity of immobilized enzyme at 40 0C. Effect of temperature also showed that immobilization increases the thermal stability of asparaginase (Figure 2). These results are in accordance with other studies performed on different enzymes where immobilization increases stability of enzyme (Mateo et al., 2000, Grazu et al., 2005). Furthermore, study of pH indicated that pH 7 was optimum for immobilized enzyme in compare to free enzyme which showed maximum activity at pH 8. Similar to increase in L-asparaginase specific activity with temperature after immobilization, immobilization also increased specific activity with pH by 1.73 fold (Figure 3). Increase in optimum pH, temperature for maximum activity was also observed in other study analyzing immobilization of oxalate oxidase enzyme (Pundir et al., 1999). Increase in specific activity of immobilized asparaginase suggests favorable confirmation changes after immobilization leading to increase in specific activity of enzyme as evidenced in other studies (Chen et al., 2009).
Alteration in kinetic parameters of immobilized enzyme in comparison to free enzymes are reported probably due to structural modifications in active sites resulted through this process (Liburdi et al., 2012) Although immobilization affects kinetic parameters of enzymes, but we did not find any difference in optimum incubation temperature for enzyme activity. During the study, 15 minute was the optimum incubation time for both free and immobilized enzyme (Figure 4).
An important objective of enzyme immobilization is to make it as a reusable biocatalyst. This situation demands long term stability of immobilized enzyme. Our immobilized enzyme maintained 85% of initial enzyme activity despite 53 days of storage. This observation further appraises superiority of A. flavus immobilized enzyme in comparison to free L-asparaginase. Although this study gives significant insights towards potential of A. flavus asparaginase and its immobilized derivative, but proper and careful studies are required for application of this enzyme.
The thermodynamic parameters of immobilized L-asparaginase were evaluated to understand catalytic activity of enzyme. This study of asparaginase catalyzed reactions was a favorable reaction in direction of product formation and could be helpful in its industrial scale production (Table 2). This study also extends the idea of finding L-asparaginase potential in fungi and their application in translational medicine. As mentioned earlier, that fungal asparaginase may be superior in comparison to bacterial enzyme, due to reduced adverse reactions associated with fungal enzyme, so present study demand legitimate appraisal to unravel biotechnological potential of fungal L-asparaginase and its immobilization.
Author Conflict of Statement: No conflict of interest.
Data Availability Statement: The data are available on request from the corresponding author.
Funding status: Not applicable.
Ethical Consideration Not applicable.
REFERENCES
Asselin, B. L., Lorenson, M. Y., Whitin, J. C., Coppola, D. J., Kende, A. S., Blakley, R. L. & Cohen, H. J. 1991. Measurement Of Serum L-Asparagine In The Presence Of L-Asparaginase Requires The Presence Of An L-Asparaginase Inhibitor. Cancer Res, 51, 6568-73.
Bilimoria, M. H. 1969. Conditions For The Production Of L-Asparaginase 2 By Coliform Bacteria. Appl Microbiol, 18, 1025-30.
Capizzi, R., Holcenberg Js 1993. Asparaginase. In: Holland, J., Frei, E, Bast, Rc Jr Et Al (Ed.) Cancer Medicine. 3 Ed. Philadelphia: Lea & Febiger.
Capizzi, R. L., Bertino, J. R. & Handschumacher, R. E. 1970. L-Asparaginase. Annu Rev Med, 21, 433-44.
Chen, B., Lei, C., Shin, Y. & Liu, J. 2009. Probing Mechanisms For Enzymatic Activity Enhancement Of Organophosphorus Hydrolase In Functionalized Mesoporous Silica. Biochem Biophys Res Commun, 390, 1177-81.
Curran, M. P., Daniel, R. M., Guy, G. R. & Morgan, H. W. 1985. A Specific L-Asparaginase From Thermus Aquaticus. Arch Biochem Biophys, 241, 571-6.
Duarte SM, AK Carvalho Silva, Borges KRA, CB Cordeiro et al (2024) Fermentation Vol 10 No 5 226
Earl, M. 2009. Incidence And Management Of Asparaginase-Associated Adverse Events In Patients With Acute Lymphoblastic Leukemia. Clin Adv Hematol Oncol, 7, 600-6.
Ertan, F., Yagar, H. & Balkan, B. 2006. Some Properties Of Free And Immobilized Alpha-Amylase From Penicillium Griseofulvum By Solid State Fermentation. Prep Biochem Biotechnol, 36, 81-91.
Grazu, V., Abian, O., Mateo, C., Batista-Viera, F., Fernandez-Lafuente, R. & Guisan, J. M. 2005. Stabilization Of Enzymes By Multipoint Immobilization Of Thiolated Proteins On New Epoxy-Thiol Supports. Biotechnol Bioeng, 90, 597-605.
Khan, A. A. & Shrivastava, A. 2010. Bacterial Infections Associated With Cancer: Possible Implication In Etiology With Special Reference To Lateral Gene Transfer. Cancer Metastasis Rev, 29, 331-7.
Liburdi, K., Straniero, R., Benucci, I., Vittoria Garzillo, A. M. & Esti, M. 2012. Lysozyme Immobilized On Micro-Sized Magnetic Particles: Kinetic Parameters At Wine Ph. Appl Biochem Biotechnol.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. 1951. Protein Measurement With The Folin Phenol Reagent. J Biol Chem, 193, 265-75.
Mateo, C., Abian, O., Fernandez-Lafuente, R. & Guisan, J. M. 2000. Increase In Conformational Stability Of Enzymes Immobilized On Epoxy-Activated Supports By Favoring Additional Multipoint Covalent Attachment*. Enzyme Microb Technol, 26, 509-515.
Oza, V. P., Trivedi, S. D., Parmar, P. P. & Subramanian, R. B. 2009. Withania Somnifera (Ashwagandha): A Novel Source Of L-Asparaginase. J Integr Plant Biol, 51, 201-6.
Pinheiro, I., Araujo, J. M., Ximenes, E. C. P. A., Pinto, J. C. S. & Alves, T. L. M. 2001. Production Of L-Asparaginase By Zymomonas Mobilis Strain Cp4. Biomaterial And Diagnostic Bd, 06, 243-244.
Pogaku, R., Jagannathan, K. & Gujjula, R. 2012. Evaluation Of Activation Energy And Thermodynamic Properties Of Enzyme Catalyzed Transesterification Reaction. Adv Chem Engg Sci, 2, 150-154.
Pundir, C. S., Thakur, M., Goyal, L. & Bhargava, A. K. 1999. Immobilization Of Sorghum Leaf Oxalate Oxidase Onto Alkylamine And Arylamine Glass. Chin J Biotechnol, 15, 129-38.
Reynolds, D. R. & Taylor, J. W. 1993. The Fungal Holomorph: Mitotic, Meiotic And Pleomorphic Speciation In Fungal Systematics : Proceedings Of An International Symposium, Newport, Oregon, 4-7 August 1992, Cab International.
Sarquis, M. I. M., Oliveira, E. M., Santos, A. S. & Costa, G. L. 2004. Production Of L-Asparaginase By Filamentous Fungi. Mem Inst Oswaldo Cruz, 99, 489-492.
Schwartz, J. H., Reeves, J. Y. & Broome, J. D. 1966. Two L-Asparaginases From E. Coli And Their Action Against Tumors. Proc Natl Acad Sci U S A, 56, 1516-9.
Shrivastava, A., Khan, A., Jain, S. K. & Singhal, P. K. 2012a. Bacterial Asparaginase: A Potential Therapeutic Agent For Treatment Of Acute Lymphoblastic Leukemia. In: Khan, A. A. (Ed.) Bacteria And Cancer. Netherlands: Springer Science Publisher.
Shrivastava, A., Khan, A., Shrivastav, A., Jain, S. K. & Singhal, P. K. 2012b. Partial Purification Of L-Asparaginase From Penicillium Digitatum. Preparative Biochem. Biotech., In Press.
Shrivastava, A., Khan, A. A., Jain, S. K., Singhal, P. K., Jain, S., Marotta, F. & Yadav, H. 2010. Biotechnological Advancement In Isolation Of Anti-Neoplastic Compounds From Natural Origin: A Novel Source Of L-Asparaginase. Acta Biomed, 81, 104-8.
Soniamby, A., Lalitha, S. & Praveesh, B. 2011. In Vitro Antioxidant And Anticancer Activity Of L-Asparaginase From Aspergillus Flavus (Kufs20). Asian J Pharm Clin Res, 4, 174-177.
Wade, H. E., Robinson, H. K. & Phillips, B. W. 1971. Asparaginase And Glutaminase Activities Of Bacteria. J Gen Microbiol, 69, 299-312.
Wiame, J. M., Grenson, M. & Arst, H. N., Jr. 1985. Nitrogen Catabolite Repression In Yeasts And Filamentous Fungi. Adv Microb Physiol, 26, 1-88.
Wriston, J. C. & Yellin, T. O. 1973. L-Asparaginase: A Review. Adv Enzymol Relat Areas Mol Biol, 39, 185-248.
Zeng, H., Liao, K., Deng, X., Jiang, H. & Zhang, F. 2009. Characterization Of Lipase Immobilized On Mg-Al Hydrotalcide For Biodeisel. Process Biochem, 44, 791-798.


