Dr.N.G.P Arts and Science College, Coimbatore-48 India.
Article Publishing History
Received: 15/07/2023
Accepted After Revision: 25/09/2023
The modernization of the society has made our environment face a series of changes. One among them is the enzymatic method produced by microorganisms from any waste. Considering the fruit waste globally several industrial uses have been fulfilled by the fruit waste. The most abundant enzyme now a days is protease from papaya waste isolated from Bacillus spp. Alkaline protease has been choosen for the detergent industries widely since it has pH range over 7.5. It performs more effectively in decomposing proteins. Since, it is stable over wide temperature and pH range.
The aim of the study was to isolate alkaline protease enzyme from fruit waste and its applications as detergent against three different stains such as blood stain, banana stain and tea stain. Papaya fruit waste was used to extract protease enzyme to create a useful detergent for stain removal. The enzyme’s activity was tested at different pH and temperature, and the best results were obtained from three samples. The study suggests that alkaline protease enzyme from papaya waste is effective in removing protein stains and could help manage solid waste while being produced at lower temperature.
Alkaline Protease,Bacillus Species.,
Indhumathi T, Mahavidhya R, Mavuniq S, Shri V. P. Isolation of Alkaline Protease from Fruit Waste and its Application as Detergent. Biosc.Biotech.Res.Comm. 2023;16(3).
Indhumathi T, Mahavidhya R,Mavuniq S, Shri V.P. Isolation of Alkaline Protease from Fruit Waste and its Application as Detergent. Biosc.Biotech.Res.Comm. 2023;16(3). Available from:<ahref=”http://surl.li/lxxqq“>http://surl.li/lxxqq</a>
INTRODUCTION
Fruit and vegetable waste is the ever growing global question. Several techniques have been developed to facilitate the waste into possible sources of energy and helps in reducing the environmental pollution. Globally, the overall production of fruits and vegetables is about 675 million metric tons annually and out of the which 1.3 billion ton wastage is produced. India alone produces 86.602 million metric tons of fruits and vegetables and constitute about 5.6 million ton of waste annually.
Manyof the garbage are removed in ecologically unsustainable manner or the minimum quantity of waste was enzymatically decomposed with microorganisms due to their ability to secrete enzymes (Elvira C et al., 1998 Chao et al., 2017). .Papaya is a popular fruit consumed worldwide and its well known for food and nutritional values. It is used in food industries, detergent industries, chewing gum industries etc. As a result, the industries generate huge amounts of papaya peel (PP) and seeds as by-products, which are typically considered a waste, and thus discarded, but that can be converted into many value-added products (Pathak et al., 2019).
Now a days enzymes play a key role in industrial areas. The two major enzymes from papaya include lipase and protease. Commercial proteases account for nearly 60% of the total industrial enzyme market (Katsuhisa et al., 2007). Bacillus sp is the most important group of bacteria that are involved in the the enzyme industry and this bacterium is also known to produce proteolytic enzymes quite effectively (Patel 1985).Although many other classes of enzymes are currently in industrial use, the focus of this paper is on alkaline proteases from bacterial species. This is because the organisms producing enzymes capable of catalysing the reactions at the extremes of pH above neutrality (Arunachalamand & Saritha 2009).
The genus Bacillus is vital for commercially important alkaline protease which is active at alkaline pH ranging between 9 and 11 .The detergent industry consumes alkaline proteases most abundantly (Moon, & Parulekar 1991). The largest application of protease is in laundry detergents and leather industry, where they remove protein based stains from clothes and dehairing purpose, respectively. As the modern world focuses on ecofriendly products and product output, subsequently more chemical processes are being replaced by enzymatic methods (Manavalan et al., 2020 Bektas et al 2023).
Protease is one of the important enzymes used in textile industry. The presently available proteases are not sufficient to meet industrial demands. Hence; there is continuous search for new proteases with novel characteristics for industrial application from diverse bacteria isolates. Microbes from varied habitats have been examined by many researchers to obtain the industrially suitable proteases. This present study was to discuss about the isolation of alkaline protease from the fruit waste papaya peel and discuss the stain removal (detergent activity) against the different stains as made.
MATERIAL AND METHODS
Isolation and characterization of microorganism: Protease producing isolates were analysed from the papaya peel using a skim milk agar plates after incubation. Among the isolates, Bacillus species exhibited a prominent clear zone. Pure culture was obtained for the best three isolates of clear zone after the incubation period of 24-48 hrs at 370C. This was selected for the study of protease production. This was maintained at 40C in skim milk agar plates[10]. The morphological and physiological properties of the isolate were investigated according to Bergey’s manual of determinative bacteriology (Towatana 1999 & Becerra et al., 2016).
The three bacterial isolates were inoculated separately to three conical flasks containing 100 ml nutrient broth and incubated over 2-3 days period at 37⁰ C in a rotary shaker. Nutrient broth without bacterial inoculation kept as control. The contents of the flasks were collected in a centrifuge tube and it was centrifuged at 10000 rpm for 10 minutes at 4⁰ C in order to get the cell free supernatant containing crude enzyme.
Quantitative analysis of protease enzyme activity: Protease activity in the culture supernatant was determined a by using casein as a substrate. A mixture of 500 μl of 1% (w/v) of casein in 50 mM phosphate buffer, pH 7 and 200 μl crude enzyme extract were incubated in a water bath at 40°C for 20 minutes. After 20 minutes, the enzyme reaction was terminated by the addition of 1 ml of 10% (w/v) trichloroacetic acid (TCA) and was kept at room temperature for 15 minutes. Then, the reaction mixture was centrifuged to separate the unreacted casein at 10,000 rpm for 5 minutes. The supernatant mixed with 2.5 ml of 0.44M Na2CO3. 1 ml of 3-fold diluted FollinCiocalteus phenol reagent was added (Lu et al., 2018).
The resulting solution was incubated at room temperature in the dark for 30 minutes and absorbance of the blue colour developed was measured at 660 nm against a reagent blank using a tyrosine standard (Sony & Potty 2016). This was modified as Lowry’s method (Lowry et al., 1951). One unit of protease is defined as the amount of enzyme that releases 1 μg of tyrosine per ml per minute under the standard conditions of supernatant solution.
Enzyme activity was calculated by the formula
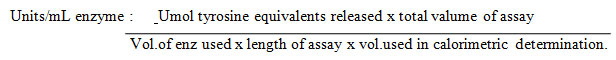
Effect of pH on enzyme activity: The culture was incubated at 370C for 48hrs and the pH was adjusted using different buffers ranging from 3-9. The OD values of enzyme activities were taken in spectrophotometer at 600nm (Badhe et al., 2016).
Effect of Temperature on enzyme activity: To study the optimum temperature where an enzyme shows its maximum activity were exposed to different temperature ranging from 40C – 1000C. After incubation the OD values were taken in spectrophotometer at 600nm (Olajuyigbe et al., 1980).
Stain removal activity with crude enzyme: The three sample broths were taken for the washing activity. Application of protease enzyme by isolated organism as a detergent additive was studied (Kalapana et al., 2008). The washing test is performed with the three prepared broth samples for three different stains.
Table 1. Activity performed with different dilutions against different stains
| Stains | Tap water
(C) |
Broth 10-5 | Broth 10-7 | Broth 10-9 | Detergent
+ Broth |
Detergent |
| Blood stain | 20ml | 20ml | 20ml | 20ml | 0.2g in 20ml
of tap water, 10-5,10-7,10-9 |
0.2g in 20ml tap water |
| Banana stain | 20ml
|
20ml | 20ml | 20ml | 0.2g in 20ml
of tap water, 10-5,10-7,10-9 |
0.2g in 20ml tap water |
| Tea stain | 20ml | 20ml | 20ml | 20ml | 0.2g in 20ml
of tap water, 10-5,10-7,10-9 |
0.2g in 20ml tap water |
Partial Purification Method: Based on this washing test the best stain removal test sample medium 10-5was identified and done partial purification for the sample by saturated Ammounium sulphate method (Mark et al., 2002).
Characterization of Proteins Using SDS-PAGE analysis: Electrophoresis is the process of migration of charged molecules in response to an electric field.Proteins have a net charge at any pH other than their isoelectric point (pI), thus when placed in an electric field, proteins will migrate towards the electrode of the opposite charge. This principle is used to separate molecules of differing charges (Sanbrook et al., 1989).
Stain removal capability after partial purification: The washing test activity is done again after the partial purification method to identify the capacity of the enzyme in stain removal ability. The three different stains were tested against the sample 10-5 medium. The stain removal of the enzyme was studied along with the detergent powder. The stain removal was studied under the following ways in table 2
Table 2. Activity performed with effective broth dilution against different stains
| Stains | Tap water | Broth 10-5 | Detergent + Broth | Detergent |
| Blood stains | 20ml | 20ml | 0.2g in 10ml tap water and
10ml broth |
0.2g in 20ml tap water |
| Banana stain | 20ml | 20ml | 0.2g in 10ml tap water and
10ml broth |
0.2g in 20ml tap water |
| Tea stain | 20ml | 20ml | 0.2g in 10ml tap water and
10ml broth |
0.2g in 20ml tap water |
RESULTS AND DISCUSSION
Isolation and characterization of microorganism: Out of 86 isolates screened the best hydrolysis zone were obtained for three isolates after 48hrs of incubation at 370C . The zone of isolation of three three isolates were named as S1,S2,S3. The zone of hydrolysis on skim milk agar is shown in Fig.1 Their morphological and physiological study for casein hydrolyse study as as shown in table 2 and 3.
Estimation of Protease activity: The maximum enzyme activity was given by the sample test 1 which showed its higher activity at 0.45nm. The enzyme acidity gradually increased as the substrate concentration also increased and also there was a gradual decrease in the test 2 and 3 with its substrate concentration. Table 4 shows the test samples enzyme activity.
Optimization of growth using Temperature and pH: The enzyme showed the best activity between temperature range 250C and 450C with maximum activity at 370C. As the graph shown in (Fig 2) the enzyme was constant and stable at 370C. At 450C and above it started to decrease its activity and get completely inactivated by 120 min at the further temperatures. And in the same way the enzyme showed the reasonable activity between the pH 8 and 11 with maximum activity at 10 as shown in (Fig 3) which shows enzyme was stable at 10. At above pH 11 the enzyme started to reduce its activity after few minutes and get inactivated by 90min at further pH.
Stain removal activity with crude enzyme: A good detergent additive protease enzyme must have a cabaple to remove the protein stains by cleaving the protein bond breakage. Therefore, the effects of various oxidizing agents and surfactants on the protease enzyme were studied.stability of enzyme towards these stains were also analysed with the incubation period of 60min and 120mins (Kumar; Takagi 1999 & Joo 2005). protein stability has commercially wide applications in detergent industry has been reported in many studies (Kumar & Bhalla 2004) Oxidizing agents are major ingredientsof modern bleach based detergents. Alkaline protease from bacillus shows extreme stability towards oxidizing agents (Nadeem et al., 2013).
By consisdering these facts the washing test were perfomed for the blood stain, banana stain,tea stain with crude enzyme of three isolates were washed after the incubation period of 2hrs .The best results were obtained from the sample 1 isolate as shown in (Fig 2,3 and 4).
Partial purification and molecular weight determination: To obtain proteins from cell free culture filtrate the sample 1 isolate were partially purified with ammonium sulphate at 75%(w/v)saturation by adding powdered Ammonium sulphate slowly with continuous stirring . The yield of protein was 2.30mg/mL, with a total activity of 415.26U/mL per min. This yield of partially purified protein was undergone for molecular weight determination by SDS -PAGE analysis and the result was shown in the( Fig 5)
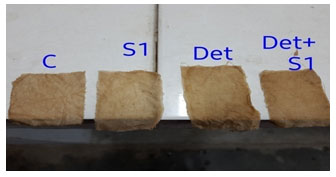
Washing test with partially purified enzyme: The enzyme with extreme stability towards oxidizing agent is commercially significant as the peroxidise are common ingredients of bleach based detergents. The enzymes prepared are mostly stored under low temperatures which will prevent its denaturation if the enzymes can withstand room temperature it is very useful in lifetime of a detergent product. So by consisdering these issues the partially purified enzyme sample 1 isolate has withstand its room temperature and also removed the blood stain, banana stain, tea stain. Thus this reveals its use in detergent industry. The washing test performed is shown in ( Fig 6,7, and 8.)
Isolation and characterization:
Growth of Bacillus sp., in different dilutions

Table 3. shows the results of Morphological study
| SI.No | Gram staining | Negative staining | Spore staining |
| S1 | Positive | Short chains | Endo spores |
| S2 | Positive | Short chains | Endo spores |
| S3 | Positive | Short chains | Endo spores |
Table 4. shows the results of Physiological stud
Casein hydrolyse test
| Sample | Growth | Zone of inhibition(mm) |
| S1 | Yes | 15mm |
|
S2 |
Yes |
11mm |
|
S3 |
Yes |
9mm |
Table 5. Estimation of protein by Lowry’s method
| Test tube | Tyrosine
Vol. Conc. |
Alkaline
reagent |
Cond. | Dis.
water |
Folins
phenol |
Cond. | OD at 660nm |
| B | – – | 2 | Incubation | 2.00 | 0.2 | Keep | 0.00 |
| S1 | 0.05 5 | 2 | in | 1.95 | 0.2 | in | 0.25 |
| S2 | 0.10 10 | 2 | dark | 1.90 | 0.2 | dark | 0.33 |
| S3 | 0.15 15 | 2 | room | 1.85 | 0.2 | room | 0.40 |
| S4 | 0.20 20 | 2 | for | 1.80 | 0.2 | for | 0.53 |
| S5 | 0.25 25 | 2 | 10 mins | 1.75 | 0.2 | 30mins | 0.60 |
| T1 | 1.00 | 2 | 1.00
|
0.2 | 0.45 | ||
| T2 | 1.00 | 2 | 1.00 | 0.2 | 0.37 | ||
| T3 | 1.00 | 2 | 1.00. | 0.2 | 0.33 |
Graph 1: shows the growth rate in pH values
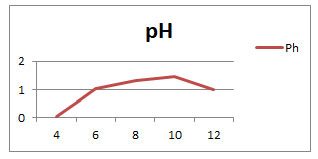
Graph 2: shows the growth rate in Temperature values
Washing test with crude enzyme:
Blood stain
Figure 2(a): Stained blood clothes in S1 sample.

Figure 2(b) : After 2 hrs the washing activity shows best in S1 sample.
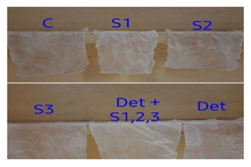
Banana stain:
Figure 3(a) : Stained banana clothes in S1 sample.

Figure 3(b) : After 2 hrs the washing activity shows best

Tea stain:
Figure 4 (a) : Stained tea clothes in S1 sample

Figure 4(b) : After 2 hrs the washing activity shows best.

Partial purification and molecular weight determination
Lane -1 : Marker, Lane -2 : Crude lane, Lane -3 : Partial Purified lane Marker : 10kda , 50kda, 75kda, 100kda
Figure (5) : SDS-PAGE analysis of isolated protease from papaya peel waste.
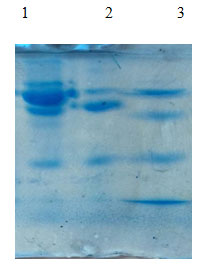
- Molecular weight of crude proteins :
1st band – 82kda, 2nd band- 68kda, 3rd band- 12kda
- Molecular weight of partially purified proteins :
1stband -81kda, 2nd band- 47kda, 3rd band – 10kda
Washing test with partially purified enzyme :
Blood stain
Figure (6a) Blood stained clothes purified protein

Figure 6(b) : Washing activity after 2hrs proved best partially results in 10-5

Banana stain
Figure (7a): Banana stain clothes in partially purified protein

Figure (7b): Washing activity after 2hrs proved best results in 10-5

Tea stain :
Figure (8a): Banana stain clothes in partially purified protein

Figure (8b): Washing activity after 2hrs proved best results in 10-5
CONCLUSION
Papaya fruit waste was used to extract protease enzyme to create a useful detergent for stain removal. The enzyme’s activity was tested at different pH and temperature, and the best results were obtained from three samples. Washing activity was performed with three different stains, and the best sample was identifies as 10 – 5 broth, showing the best stain removal activity after 2 hours.The sample was partially purifies and tested again, confirming the presence of protein by SDS – PAGE analysis with a specific molecular weight The study suggests that alkaline protease enzyme from papaya waste is effective in removing protein stains and could help manage solid waste while being produced at lower temperature.
ACKNOWLEDGEMENTS
The authors acknowledge the use of instrumentation and infrastructure facilities provided by DST-FIST and DBT- under Star College Scheme, Ministry of Science and Technology, Goverment of India for the successful completion of the project. We thank the host institution Dr. N.G.P Arts and Science College and its management for rendering all the facilities and support.
REFRENCES
Arunachalamand C K. Saritha (2009). Protease enzyme: an eco-friendly alternative for leather industry. Indian Journal of Science and Technology Vol.2 No. 12 ISSN: 0974- 6846.
Badhe,p.,Joshi, M.,Advarekar,R. (2016). Optimized production of extra cellular protease by Bacillus subtilis from degraded abattoir waste .J BioSci Biotechnol. 9 (5),29-31.
Becerra SC, Roy DC, Sanchez CJ, Christy RJ, Burmeister DM (2016). An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue. BMC Res Notes. 12;9:216. doi: 10.1186/s13104-016-1902-0. PMID: 27071769; PMCID: PMC4828829.
Bektas, K Inan Aleyna Nalcaoğlu, Esma Ceylan (2023) Isolation and characterization of detergent-compatible amylase-, protease-, lipase-, and cellulase-producing bacteria Brazilian Journal of Microbiology volume 54, pages725–737
Chao Ji., Chui-Xue Kong, Zi-Li Mei, Jia (2017). A Review of the Anaerobic Digestion of fruit and vegetable waste. Appl Biochem Biotechnol. 183(3):906-922.
Elvira C, Sampedro L, Benitez E, Nogales R (1998). Vermicomposting of sludges from paper mill and dairy industries with Eisenia anderi: A Pilot – Scale study. Biores. Technol 63: 205-211.
Gupta, R., Beg Q. & Lorenz, P. (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Applied Microbiology and Biotechnology 59 pg15-32.
Sony and V. P. Potty (2016). Quantitative Estimation of Protease Produced by Bacterial Isolates from Food Processing Industries. International Journal of Engineering Research & Technology (IJERT). volume 5 isue 10.
Joo, H.S., C.S (2005). Production of protease from a new alkalophilic Bacillus sp.I-312 grown on soya meal: optimization and properties. Process Biochem,40(3),1263-1270.
Kalapana Devi, M.,Raseedha Banu, A., Gnanaprabhai,G. R., Pradeep,B.V., Palaniswamy, M. (2008). Purification and characterization of alkaline protease enzyme from native isolate Aspergillus niger and its compatibility with commercial detergents.Ind J Sci Technol,1 (7), 1-6.
Katsuhisa Saeki, Katsuya Ozaki, Tohru Kobayashi (2007). Detergent alkaline proteases: enzymatic properties, genes, and crystal structures, Journal of Bioscience and Bioengineering,Volume 103, Issue 6 , Pages 501-508, ISSN 1389-1723.
Kumar, C. G., Takagi, H (1999). Microbial alkaline protease: from a bioindustrial view point . Biotechnol Adv; 179(7),561-594
Kumar,D., Bhalla, T.C (2004). Purification and characterization of a small size protease from Bacillus sp. APR-4 Ind j exp boil, 42(5),515-521.
Lowry,O.H.,N.J.Rosebrough,A.L.Farr, and R.J.Randall (1951). protein measurement with folin phenol reagent.J.Biol.chen. 193: 256-275.
Lu Z, Guo W, Liu C (2018). Isolation, identification and characterization of novel Bacillus subtilis. J Vet Med Sci. 24;80(3):427-433. doi: 10.1292/jvms.16-0572. Epub 2018 Jan 23. PMID: 29367516; PMCID: PMC5880821.
Manavalan T, Manavalan A, Ramachandran S, Heese K (2020). Identification of a Novel Thermostable Alkaline Protease from Bacillus megaterium-TK1 for the Detergent and Leather Industry. Biology (Basel). 16;9(12): PMCID: PMC7765983.
Moon, S.H. and S.J. Parulekar. (1991). A parametric .study of protease production in batch and fed batch cultures of Bacillus firmus. Biotechnol. Bioengg., 37: 467-483.
Nadeem, M., Qazi, J. I., Syed, Q., Glusher, M. (2013). Purification and characterization of alkaline protease from Bacillus licheniforms UV-9 for detergent formulations. Songklanakarin J Sci Technol. 35(2), 187-195.
Olajuyigbe, F.M., Falade,A. M. (2014). Purification and partial characterization of serine alkaline mettaloprotease from Bacillus brevis MWB-01. Bioresources and Bioprocessing. 1980,1-10.
Page, Mark & Thorpe, Robin. (2002). Purification of IgG by Precipitation with Sodium Sulfate or Ammonium Sulfate. 10.1385/1-59259-169-8:983.
Patel, P.R., (1985). Enzyme isolation and purification. In: Biotechnology: Applications and Research. Technomic Publishing Co. Inc., USA, pp: 534-564.
Pathak, P.D., Mandavgane, S.A. & Kulkarni, B.D (2019). Waste to Wealth: A Case Study of Papaya Peel. Waste Biomass Valor 10, 1755–1766.
Sanbrook,J.,Fritsch,E.F., and Maniatis,T. (1989). Molecular cloning: a laboratory manual Newyork.
South Asian J (2018). Waste Utilization of Fruits and Vegetables. Food Technol. Environ., 4(1): 605-615 [605].
Towatana NH, Painupong A and Suntinanaler P (1999). Purififi cation and characterization of an extracellular protease from alkaliphilic and thermophilic Bacillus species PS719. J Biosci Bioeng 87:581–587.


