Department of Doctoral Pharmacy, Mazandaran University of Medical Sciences (Mazums) Sari, Iran
Corresponding author Email: g.vahdati@aftermail.ir
Article Publishing History
Received: 27/12/2018
Accepted After Revision: 25/03/2019
In this research study, it has been attempted to synthesize mPEG-PCL dual copolymers and use their nanoparticles for tamoxifen anticancer drug delivery. Tamoxifen is a non-steroidal anti-estrogen that also has weak estrogenic effects. Tamoxifen is used to treat breast cancer and infertility dependent on oligomenorrhea or secondary amenorrhea. This drug inhibits estradiol receptors and, using it, ovulation involves the occupation of estrogen receptors and eliminates the inhibitory effect of the hormone and thereby stimulates the release of the gonadotrophin-releasing hormone from the hypothalamus. In order to increase the local concentration of tamoxifen in Estrogen Receptor (ER) positive breast cancer, we have prepared and characterized nanoparticle formulation using methoxy poly (ethylene glycol) –Poly (ϵ-caprolactone) (mPEG-PCL). In this study, PEG-PCL copolymers were synthesized from mPEG and ϵ-caprolactone (ϵ-CL) using Sn(oct)2 as catalyst by ring opening polymerization. The copolymers were prepared and characterized by 1H-NMR, FTIR, DSC and GPC. From the results, it was clear that nanoparticles showed sustained release behavior for a long period of time. These results suggest that the nanostructure of mPEG-PCL nanoparticles can be used as a promising
candidate for sustained release of tamoxifen.
Nanoparticles, Mpeg-Pcl, Tamoxifen, Controlled Drug Delivery
Vahdati S. G. F. Investigations on the Development of Biodegradable Nanoparticles for Anti-Cancer Drug. Biosc.Biotech.Res.Comm. 2019;12(1).
Vahdati S. G. F. Investigations on the Development of Biodegradable Nanoparticles for Anti-Cancer Drug. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2VPgWtT
Copyright © Vahdati, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Nanotechnology has enormous potential in various fields, used in the development of insecticides, pharmaceuticals (Manjili et al, 2018), and electronics. Nowadays, nanoparticles have found widespread use that can be mentioned in several ways: dermal delivery, as antimicrobial and anticancer agents, carries peptide and protein medications such as insulin, as well as carriers of anti-inflammatory and respiratory drugs. Also, some biodegradable polymer-derived drug delivery systems, such as nanoparticles delivering anticancer agents, are commercially available (Sajadi Tabassi, 2012). PCL-PEGPCL is a thermo- sensitive copolymer which is more fl exible than PCL-PEG diblock copolymer for drug loading and drug delivery, (Kesente, 2017, Zamani et al, 2018).
Biodegradable polymeric materials are ideal carrier systems for biomedical applications. Features like controlled and sustained delivery, improved drug pharmacokinetics, reduced side effects and safe degradation make the use of these materials very attractive in a lot of medical fi elds, with dermatology included (Bunker, 2012). Biodegradable polymers have several advantages over non-biodegradable polymers for controlled drug delivery. It does not require surgical use after use, which is the most important advantage in a drug that has no surgical complications. Natural and synthetic biodegradable polymers have good properties, such as biocompatibility, biodegradability and high mechanical resistance. They have a negligible toxicity, and their destruction is non-toxic. Due to their mechanical properties, they are suitable for a wide range of specifi cations. Natural biodegradable polymers such as gelatin, albumin, chitosan, hyaluronic acid, and biodegradable synthetic polymers such as Poly(-caprolactone) PCL, PEO, and aliphatic thermoplastic polyester, such as Poly(lactic acid) and poly(lactid-co-glycolid), are fully investigated for drug delivery systems, (Verhoef, 2013).
Polyethylene glycol is a non-ionic polyether that has excellent biocompatibility. Ethylene oxide is non-toxic and approved by the FDA. Polyethylene glycol is a neutral polymer with end hydroxyl groups that has poor hydrogen bands, PEG molecules can be added to drug carriers through a number of different routes, including covalently.When PEG is covalently attached to the protein, it is biologically stored, and when linked to covalently, PEG can help solubilize other molecules. PEG is soluble in water and a few organic solvents. One of the biggest benefi ts of PEG for drug delivery systems is its ability to stay long in the body (Jenkins et al, 2016). Poly (ethylene glycol) (PEG) has been used on the outer surface of polymeric micelles, due to its benefi cial characteristics of pharmacokinetic of the micelles; i.e., longcirculating characteristics and signifi cant tumor accumulation. Considering its properties such as nontoxicity, hydrophilicity, solubility in water and organic solvents, and absence of antigenicity and immunogenicity, PEG could be used for many clinical applications (Kelley et al, 2016).
Polycaprolactone is an aliphatic polyester and its monomeric form -caprolactone is a biodegradable and biodegradable, non-toxic polymer having a semi-crystalline structure and a melting point of about 59-64° C, hydrophobic, as well as a low glass transition temperature of about -60 °C, is also synthesized by loop polymerization, (Danafar et al, 2012).
Copolymers are in fact polymers that are polymerized by two or more different monomers and have different properties of homopolymers. Today, due to the various properties of copolymers, attention has been paid to their application in various fi elds, especially drug delivery. Among thesecopolymers, it can be noted that mPEG-PCL copolymers can, due to the duality, create a variety of polymer particles by varying the proportion of hydrophobic to hydrophilic. The obvious properties of these copolymers are their biodegradability, which in the body turns into nontoxic absorbent metabolites. These properties have made these polymers a lot of potential for applications in areas of nanotechnology, tissue engineering and drug chemistry. The nanoparticles obtained by these copolymers, due to their core-shell property, have the potential to store the drug in the nucleolar nucleus (PCL) and the shell (PEG) has a dual role in particle stabilization and bioassay distribution. This copolymer is used to create drug carrier systems, slow release. Copper mPEG-PCL is used with dual-mode behavior to form core-shell core membranes. This polymer carrier, as a carrier of hydrophobic drugs, increases circulation time in the blood and reduces the absorption of nanoparticles by the liver, (Rychter et al, 2018).
Copolymer PCL-PEG-PCL was synthesized by loop polymerization method at 120 ° C and 24 hours by Keng-Lun Chang et al (Rychter et al, 2018). Then, the copolymer nanoparticles were used as a lauric acid carrier for the treatment of acne vulgaris. The size of the nanoparticles was about 24-24 nm. Hiremath in a research paper, prepared polyacroclactone injectable microspheres loaded with tamoxifen by solvent evaporation and in the presence of surfactant (PVA) for the treatment of breast cancer. They produced microspheres with a percentage of surfactant 0.4% and 0.8% with varying levels of tamoxifen and pcl. When 0.4% PVA was used, the mean size of 44-48 nm and the loading rate of the drug in microspheres were higher compared with 0.8% PVA was used, the size of the microspheres was 26-28 nm and the drug loading was lower than the first one. This is due to the reduction in the tension between the organic phase and the blue phase during the fabrication of nanoparticles and the increase in viscosity in the final blue phase.
Copolymer mPEM-PCL was synthesized by Wichuda et al (2011) using methoxypoly (ethylene glycol) and poly (caprolactone) and the Sn (Oct) 2 catalyst, and the nanoparticles of this copolymer were prepared using nanotube and without any surfactant preparation. Became According to TEM analysis, spherical nanoparticles have a smooth surface. The mean particle size and glass transition temperature decreased and the melting temperature signifi cantly increased with increasing mPEG / PCL ratio. Authors have reviewed the two drugs Ibuprofen and Indomethacin in PCL-PEG-PCL triloclopropylmer nanoparticles and the effect of nicotinic acid on the production of nanoparticles. Increasing the amount of nicotinic acid increases the particle size and increases the hydrophobicity of the polymer, increasing drug loading for both drugs compared to nicotine-free nanoparticles. Shakiba et al (2017) in a study to achieve the controlled release of ATRA, PCL-PEG-PCL copolymer with average molecular weight of 5.34 kDa was synthesized. The nanosized micelles were prepared from copolymer by nano-precipitation method. In the case of 10% drug to polymer ratio, more than 80% of the drug was released within 3 days, whereas for ratio of 2% more than 90% of the drug was released within 3 h. The cytotoxic study performed by MTT assay showed that H1299 survival percent decreased signifi cantly (P≤0.05) after exposure to drug-loaded micelles, while no proliferation inhibition effect was observed by either free ATRA or blank PCL-PEG-PCL micelles.
Gavasane and Pawar (2014) believed that polymers are becoming increasingly important in the fi eld of drug delivery. The pharmaceutical applications of polymers range from their use as binders in tablets to viscosity and fl ow controlling agents in liquids, suspensions and emulsions. Polymers are classifi ed as biodegradable and nonbiodegradable. Biodegradable polymers have been widely used in biomedical applications because of their known biocompatibility and biodegradability. The present communication gives an overview of the different biodegradable polymers that are currently being used in the development of controlled drug delivery system.
Materials and Methods
Stannous 2-ethyl-hexanoate [Sn (Oct) 2], Dichloromethane, Petroleum ether, Chloroform, Ethanol, Methanol, Acetone, ϵ-Caprolactone, Methoxyethylene glycol, Tetrahydrofuran, Methoxyethylene oxide, Calcium Hydride, PVA, all of which were used with high purity from Merck and Sigma, and also tamoxifen citrate from Iran Hormone Inc. The copolymer of mPEG-PCL was synthesized by loop-polymerization of caproclactone-free monomer in the presence of dry methoxy-polyethylene glycol as a primer and catalysts of tinctanate (reaction 1).
In this way, the amount of 3 g of mPEG (MW = 5000) is introduced into a balloon of a span and dried under vacuum and at a temperature of 80 ° C for 24 hours.
The 6 g is taken from a monomeraproclactone in a balloon for 1 week in its calcium hydrogen hydride after the end of the desired period by the Buchner funnel and the vacuum pump. mPEG and caprolactone in a spatula, then add the Sn (oct)2 catalyst at 0.01 milloles per mMEG of mPEG hydroxyl group to the contents of the balloon. Then the vacuum balloon is placed in the bath of oil.
Under nitrogen atmosphere and magnetic stirring, the reaction temperature was adjusted to 150 ° C and the reaction time was allowed for 12 hours. Then cool the system, dissolve the copolymer into chloroform, and dissolve in cold ether. Collect the precipitate with filter paper and dry it.
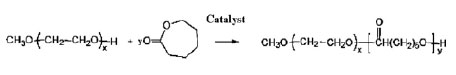
Reaction (1) mPEG-PCL Copolymer Synthesis Route
Analysis of copolymers mPEG-PCL
1HNMR has been used to determine the copolymer structure of mPEG-PCL. 1HNMR spectra were taken with Bruker 400MHz device at 25 ° C. Dieter chloroform (CDCL3) has been used as solvent and tri-methyl ketone (TMS) as the internal standard. The spectra taken in (CDCL3) were used to determine the molecular weight of synthetic copolymers by using sub-surface integration of various chemical groups. The ratio of lactone to ethylene glycol in synthetic copolymer can be calculated using Equation 1.
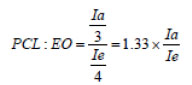
In this equation, Ia is the integral of the subliminal surface associated with PCL, which appears in the range of 1.5-1.6 ppm in the spectrum, and Ie is the integral of the PEG methylene group substrate, which is about 3.6-3.7 ppm, is observed.
When the PCL / EO ratio is synthesized in copolymer, PCL polymerization degree is calculated by considering the degree of polymerization of PEG starting from Equation 2. Finally, the molecular mass of copolymer is obtained using equation 3.
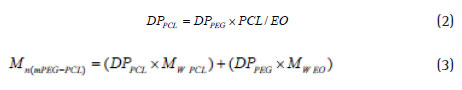
FT-IR Infrared Spectroscopy
To confi rm the mPEG-PCL copolymer formation, the FT -IR spectrum has been used. The FT-IR spectra of various specimens were recorded using a Bruker spectrometer (FT-IR).
Gel Permeation Chromatography (GPC)
The average molecular mass and mPEG-PCL copolymer molecular mass distribution are analyzed by chromatographic gel analysis. For the GPC analysis, the German Knauer machine was used. The sample was completely dissolved in pure tetrahydrofuran and, after being injected into the column, and compared with the calibration graph, drawn using standard polystyrene; the molecular mass and the synthesized PolyDispersity copolymer were calculated.
Differential Scaling Calorimeter (DSC)
DSC analysis was used to study the thermal behavior of mPEG-PCL copolymers and their nanoparticles. The samples were heated at a temperature of 10 °C / min and measured in a range of 25-200 °C using a Mettler Toledo, Star SW 9.30, manufactured by England.
Analysis of the morphology of nanoparticles by Scanning Electron Microscopy (SEM)
SEM (Scanning Electron Miroscopy) is used to capture specimens. In this way, a layer of gold is placed on the sample and the electron beam is emitted. Then, they shrink two or three electron beam concentrating lenses so that when they come in contact with the specimen diameter it is between 10-2 nm. Then several pictures are taken from the sample. For SEM imaging, the XL30 was used as an XL series manufactured by Philips and Hitachi S4160 Cold Field Emission.
Study of drug release in vitro at pH = 40.5
To study drug release, place 10 mg of suspension of nanoparticles in 2 ml of buffer and place in a 12000 cut off bag and place it in a container containing 13 ml phosphate buffer (pH = 4.50). Place the bag containing the dialysis bag in a 37-°C chiccroncobacterial device and remove 1 ml from the outer solution of the dialysis bag at specifi ed times, and replaced with fresh buffer, and the amount of drug released by the UV spectrophotometer measured at 250 nm wavelengths. Each of the measurements is repeated three times, and the average of these three data is used to calculate the amount of drug released.
Results and Discussion
Investigating the FT-IR spectrum of the mPEG-PCL copolymer In Fig. 1, the FT-IR spectrum of mPEG-PCL is shown. From the mPEG-PCL Copolymer FT-IR spectrum, it can be seen that the absorption band at the frequency of 1108 cm-1 and 1240 is related to the tensile bond (C-O). The sharp and severe absorbing strip at a frequency of 1722 cm-1 shows the existence of steric carbonyl groups (C = O), indicating the formation of mPEG-PCL copolymer. Methylene hydrone (-CH2-) adsorb two C-H adsorbents, which are related to the symmetric and asymmetric adsorbent adsorption, which absorbs the fi rst in 2869 cm-1 and the second in 2943 cm-1
Investigating the 1HNMR spectrum of mPEG-PCL copolymer
After reviewing the raw materials used in synthesis to confirm the synthesis of copolymer, the structure. As can be seen, the peak in the area of 3.38 ppm and 3.64 is related to the methoxy group and the methyl group PEG. Peak 1.3 ppm (2H, g), 1.6 ppm (4H, f), 2.2 ppm (2H, e), ppm 4.06 (2H, h) 3.4 ppm (3H,a) respectively corresponding to group -(CH2) 3 -,(- (CH2) 2-OCCH2-) – and (-CH2OOC-) caprolactone. The weight of the molecular weight calculated with 1HNMR is 21000 g / mol.
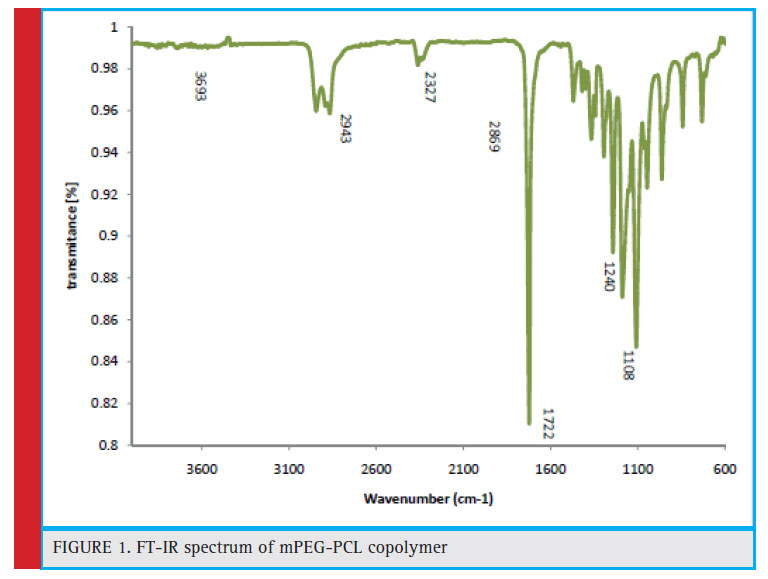 |
Figure 1: FT-IR spectrum of mPEG-PCL copolymer |
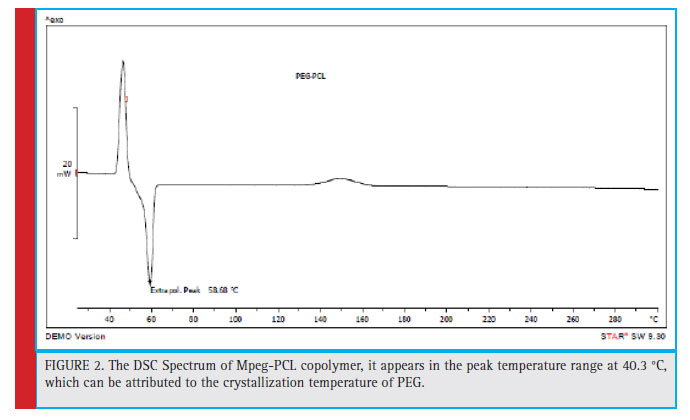 |
Figure 2: The DSC Spectrum of Mpeg-PCL copolymer, it appears in the peak temperature range at 40.3 °C, which can be attributed to the crystallization temperature of PEG. |
Investigating the Differential scanning calorimeter (DSC) spectrum of mPEG-PCL copolymer
Thermal analysis can be defi ned as measuring the properties of a polymer sample against temperature variations. The most important and fi nal factor in the study of polymers is to measure and measure their thermal stability. Usually weight loss is used to measure thermal stability. Weight variations are recorded against a rise in temperature or against a rise in time at constant temperature. Differential Schematic Thermometry (DSC) is a method in which the difference in thermal energy input to the sample and reference material is measured as a function of temperature. The mPEG-PCL copolymer DSC shows in Fig. 2, the peak of the sample is in the range of 67.42 °C, which is a thermoforming courier. Previous studies have shown that PEG and PCL hemopolymers, both of which are semi-crystalline polymers, have a melting point of 62 °C and 60 °C, respectively. However, in the copolymer spectrum, the thermal event belongs to the melting point of PEG and PCL. In addition, it appears in the peak temperature range at 40.3 °C, which can be attributed to the crystallization temperature of PEG.
Investigating the GPC spectrum associated with mPEGPCL copolymer
The molecular mass of the weighted mass and molecular weight distribution of mPEG-PCL copolymer is investigated by analysis of gel chromatography. The sample is dissolved in the tetrahydrofuran and, after being injected into the column, and compared to the calibration graph compiled using standard polystyrene, the molecular mass and the synthesized poly-dispersed copolymer are calculated. The molecular mass distribution diagram is indicated in Fig. 3, which represents a peak at 7.02 seconds after sampling. After placing this time on the Exell chart, the value of mPEG = 5000 g / mol is the mass of the molecular weight of 24663 g/ mol.
Analysis and measuring the size of the nanoparticles
At this stage, after preparing the nanoparticles, the size of the nanoparticles prepared from the DLS sample was taken. According to the measurement of the size of the nanoparticles (Fig. 4), the mean particle size without drug loading was 184.4 nm with PDI = 0.418 obtained.
The average size of the polymer nanoparticles that was loaded onto the drug was investigated by the particle size chart, shown in Fig. 5. According to the size chart of nanoparticles, the average nanoparticle size of 151.7% was obtained with PDI = 0.328. Due to the lack of drug-loading nanoparticles at 184.4 nm, drug loading has not signifi cantly changed the size of the nanoparticles, which can be a reason for absorbing the drug into nanoparticles.
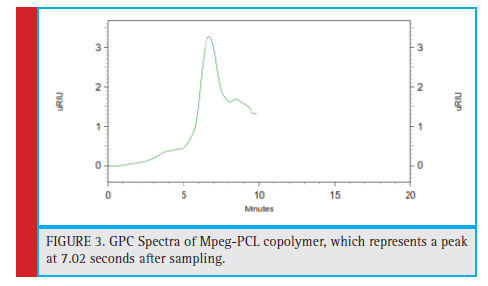 |
Figure 3: GPC Spectra of Mpeg-PCL copolymer, which represents a peak at 7.02 seconds after sampling. |
SEM Analysis of Polymer Nanoparticles Loaded with Tamoxifen
The morphology of nanoparticles was investigated using SEM and its images are depicted in Fig. 6.After the preparation of nanoparticles loaded with tamoxifen, a SEM photo was taken, depicted in Figure 6. The SEM image shows that nanoparticles have a spherical shape and approximately one shape with a size of 234 nm. As can be seen, the average size of nanoparticles from a photographed image is well-matched with the size of the DLS.
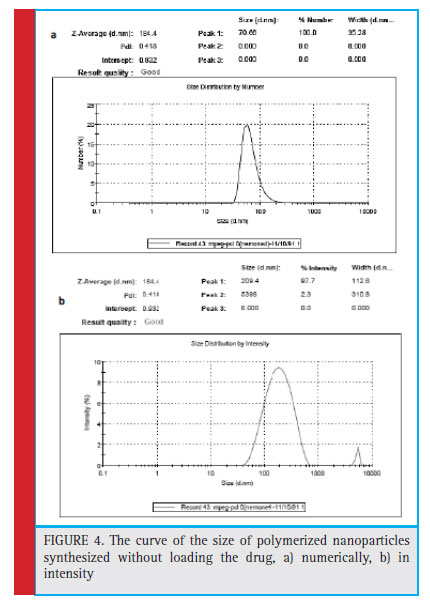 |
Figure 4: The curve of the size of polymerized nanoparticles synthesized without loading the drug, a) numerically, b) in intensity |
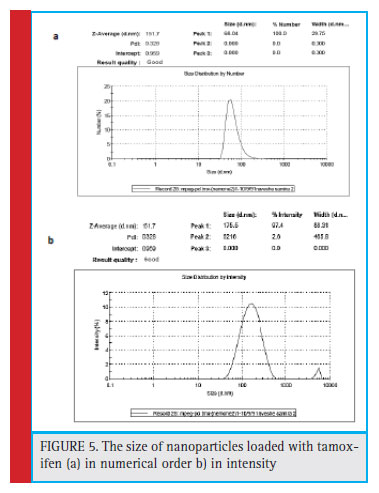 |
Figure 5: The size of nanoparticles loaded with tamoxifen (a) in numerical order b) inntensity |
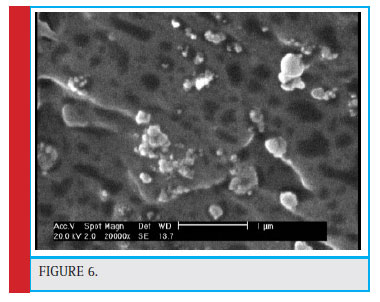 |
Figure 6 |
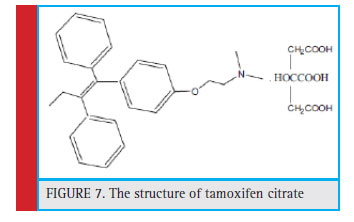 |
Figure 7: The structure of tamoxifen citrate |
FT-IR spectra of polymeric nanoparticles mPE-PCL loaded with tamoxifen
Regarding the structure of tamoxifen citrate (Fig. 7) and the FT-IR spectrum, the average absorption bands in 1590 cm-1 and 1471 cm-1 are related to the aromatic ring group. Absorption band in 2828 cm-1 of the unsaturated stretch C-H3 group, absorption band of 2960 cm-1 of the CH3 group. The 1217 cm-1 band represents the tensile C-O group, and the 1300 cm-1 band represents the C-N stretching group. The band in the 703 cm-1 region of the Ortho substrate and the 3010 cm-1 band represents the OH strain group (Fig. 8)
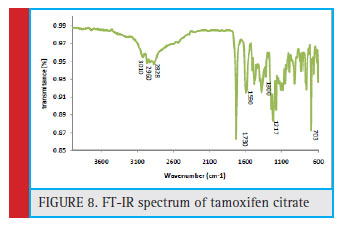 |
Figure 8: FT-IR spectrum of tamoxifen citrate |
Comparison of the range of nanoparticles loaded with the mPEG-PCL copolymer spectrum and tamoxifen spectrum showed drug loading in nanoparticles. The existence of a band of 1602 cm -1 shows the aromatic ring in both of the two spectra of tamoxifen and nanoparticles. The 1728 cm-1 band in two spectra of copolymer and nanoparticles represents the carbonyl tensile group. The 1300 cm-1 band is available in two spectra of tamoxifen and nanoparticles that specify the C-N group. The C = O group has a tensile strength of 1217 cm -1 in two spectra of tamoxifen and nanoparticles. The presence of bands of 2868 cm-1 and 2940 cm-1 in two spectra of copolymers and nanoparticles, indicating methylene tensile CH groups. The results from the following spectrum (Figure 9) showed that tamoxifen drug was loaded onto polymer nanoparticles.
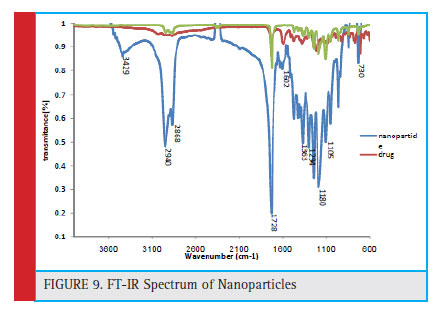 |
Figure 9: FT-IR Spectrum of Nanoparticles |
DSC spectrum of tamoxifen citrate
The DSC for tamoxifen shows that the peak from the specimen is in the range of 145.43 °C, which is a thermosetting peak and represents the melting point of tamoxifen citrate (Figure 10).
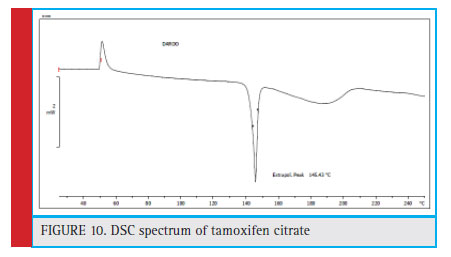 |
Figure 10: DSC spectrum of tamoxifen citrate |
Tamoxifen measurement
Figure 11 shows the tamoxifen curve at a maximum wavelength of 250 nm. Which shows very good linear citizenship of the concentration of tamoxifen with its adsorption in an organic environment. The linear equation obtained from this graph has been used to estimate the concentration of tamoxifen in assessing the drug loading rate in the drug release analysis.
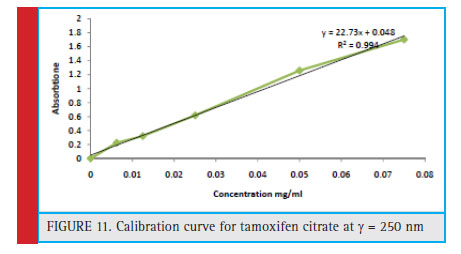 |
Figure 11: Calibration curve for tamoxifen citrate at λ=250 nm |
Determination of tamoxifen loading and encapsulation in mPEG-PCL copolymer micelles according to the calcifi cation curve of tamoxifen
According to the following calculations, the amount of drug loaded and encapsulated was 12.909% and 70.025%, respectively. The reason for loading the drug in tamoxifen in the hydrophobic nucleus of the nanoparticles (the PCL portion) is the hydrophobic nature of the drug.
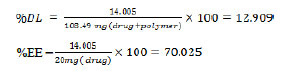
According to the following calculations, if the percentage of precipitation of nanoparticles loaded withtamoxifen is 100%, the theoretical loading of the drug should be about 16.66%.
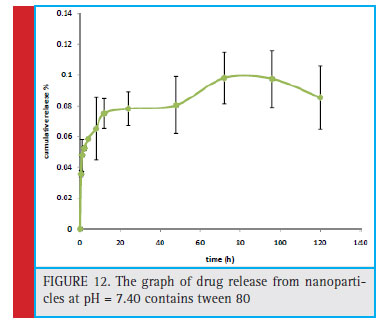 |
Figure 12: The graph of drug release from nanoparticles at pH = 7.40 contains tween 80 |
![]()
Based on the results obtained and the proximity of theoretical and empirical results, the degree of encapsulation of the drug in nanoparticles is determined. The mPEG-PCL nanoparticles show a high ability to encapsulate tamoxifen.
Reviewing the drug release pattern from nanoparticles synthesized at pH = 7.40
Figure 13 shows the release of tamoxifen from nanoparticles of mPEG-PCL in a phosphate buffer with pH = 7.40 contain thiamine. Tamoxifen citrate is a highly hydrophobic drug. Therefore, phosphate buffer containing 0.01 (w / v) Tween 80 was used to increase the solubility of the drug. According to the chart, in the fi rst 72 hours, 9.82% of the tamoxifen drug was released from the nanoparticles.
In this research work, it was fi rst mPEG-PCL polymer in the laboratory synthesized. Subsequently, nanoparticles containing tamoxifen were prepared by a single emulsion method, after which the size and surface profile of them were evaluated. Finally, drug release from nanoparticles was investigated at various pH levels. In this collaborative research, mPEG-PCL copolymer was synthesized with a mPEG / PCL ratio of 1.3, and its structure and composition were confi rmed by 1HNMR and FT-IR. The thermal behavior of copolymer synthesized by DSC was investigated and the molecular mass of the copolymer synthesized by GPC was determined. In the FT-IR spectrum, the mPEG-PCL copolymer exhibits a sharp absorption band at a frequency of 1722 cm-1, which confi rms the presence of carbonyl steary groups (C = O) and shows a severe peak in 2963 cm-1 representing ethylene groups of PEG that can confi rm copolymer synthesis. The results of mPEGPCL copolymer synthesis showed that the copolymer was completely synthesized. The DSC of mPEG-PCL copolymer indicates that the sample courtesy is in the range of 67.42 ° C, which is a thermosetting peak and represents the melting point of PCL and PEG.
The polymer was used to make biodegradable nanoparticles based on polyethylene glycol and polycaprolactone. Copolymer micelles mPEG-PCL were prepared by single emulsion method and tamoxifen drug was loaded. The particle size was about 151.7 nm and particle zeta potential was 13.9 mv. The drug loading effi ciency in nanoparticles and the drug release profi le in vitro were measured by UV spectroscopy.
The results showed that the loading and encapsulated drug was 12% and 70%, respectively, indicating high drug loading in nanoparticles. Data from drug release from nanoparticles in different environments also showed that the drug was controlled and released from nanoparticles over a long period of time. Overall, it can be concluded from the current research that pharmaceutical-containing mPEG-PCL nanoparticles can be considered as a good option for controlled release and tamoxifen in the treatment of cancer. Generally, the results of this research work provide valuable information for the development of new pharmaceutical systems and the production of high-strength polymers and a higher volume-to-volume ratio in the structure of nano-materials, especially mPEG-PCL-based nanoparticles.
References
Alex Bunker (2012). Poly (Ethylene Glycol) in Drug Delivery, Why Does it Work, and Can We do Better? All Atom Molecular
Dynamics Simulation Provides Some Answers. Physics Procedia. Volume 34, 2012, Pages 24-33.
Chao. C, Ming. H, Chun. H. (2011) The Nanoparticles for Combating Acne Vulgairs: In-vitro Effi cacy of Lauric Acid- Loaded PCL-PEG-PCL on Propion bactrium acne, International Conference on Nanotechnology and Biosensors, IPCBEE 2 (2011) IACSIT Press, Singapore.
Chen, Y., Chen, X., Chen, Y., Wei, H., Lin, S., Tian, H. Gu, X. (2018). Preparation, characterisation, and controlled release of sex pheromone-loaded MPEG-PCL diblock copolymer micelles for Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE, 13(9), e0203062. http://doi.org/10.1371/journal.pone.0203062
Danafar H, Rostamizadeh K, Davaran S, Hamidi M. Preparation and characterization of PLA-PEG-PLA tri-block copolymer polymersomes as a novel carrier for lisinopril. Res Pharm Sci. 2012;7(5):S398.
Ebrahim Shakiba, Saeedeh Khazaei, Marziyeh Hajialyani, Bandar Astinchap, Ali Fattahi (2017). Preparation and in vitro characterization of retinoic acid-loaded poly(ϵ-caprolactone)-poly(ethylene glycol)-poly(-caprolactone) micelles . Research in Pharmaceutical Sciences, December 2017; 12(6): 465-478
Gavasane AJ, Pawar HA (2014) Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview. Clin Pharmacol Biopharm 3:121.doi:10.4172/2167-065X. 1000121
Hiremath. G.J, kusm. D.V. Tamoxifev. Loaded poly(ϵ-caprolactone) based injectable microspheres for breast cancer, International Journal of Pharmacy and Pharmaceutical Sciences. 2 (2010) 0975-1491.
Jenkins SI, Weinberg D, Al-Shakli AF, Fernandes AR, Yiu HHP, Telling ND, Roach P, Chari DM (2016). ‘Stealth’ nanoparticles evade neural immune cells but also evade major brain cell populations: Implications for PEG-based neurotherapeutics. J Control Release. 2016 Feb 28;224:136-145. doi: 10.1016/j. jconrel.2016.01.013. Epub 2016 Jan 11.
Kesente, M., Kavetsou, E., Roussaki, M., Blidi, S., Loupassaki, S., Chanioti, S., Detsi, A. (2017). Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering, 4(3), 75. http://doi. org/10.3390/bioengineering4030075
Manjili H K, Malvandi H, Mousavi M S, Attari E, Danafar H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG– PCL micelles and its pharmacokinetic study. Artifi cial cells, nanomedicine, and biotechnology. 2018; 46(5), 926–936. 10.1080/21691401.2017.1347880
Gou M, Wei X, Men K, Wang B, Luo F, Zhao X, Wei Y, Qian Z PCL/PEG copolymeric nanoparticles: potential nanoplatforms for anticancer agent delivery. Curr Drug Targets. 2011 Jul 1; 12(8):1131-50.
Rancan, F., Blume-Peytavi, U., & Vogt, A. (2014). Utilization of biodegradable polymeric materials as delivery agents in dermatology. Clinical, Cosmetic and Investigational Dermatology, 7, 23–34. http://doi.org/10.2147/CCID.S39559
Rychter M, Baranowska-Korczyc A, Milanowski B, Jarek M, Maciejewska BM, Coy EL, Lulek J (2018). Cilostazol-Loaded Poly(ϵ-Caprolactone) Electrospun Drug Delivery System for Cardiovascular Applications. Pharm Res. 2018 Jan 16;35(2):32. doi: 10.1007/s11095-017-2314-0.
Sajadi Tabassi SA, Khodaverdi E, Hadizadeh F, Rashid R. Preparation of hydrogels based on inclusion complexes of the triblock copolymer PCL-PEG-PCL with ϵ-cyclodextrin (ϵ-CD). Res Pharm Sci. 2012;7(5):S975.
Syed A.A. Rizvia Ayman M. Saleh (2016). Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceutical Journal. Volume 26, Issue 1, January 2018, Pages 64-70
Suksiriworaponga. J, Sriphab. K, Kreuterc. J, Junyapraserta. V.B, (2012) Functionalized (poly(-caprolactone))2-poly(ethylene glycol) nanoparticles with grafting nicotinic acid as drug carriers, International Journal of Pharmaceutics. 423 562- 570.
Verhoef, J. J. F., & Anchordoquy, T. J. (2013). Questioning the Use of PEGylation for Drug Delivery. Drug Delivery and Translational Research, 3(6), 499–503.
Wichuda. N, Mangkorn. S-A, Yodthong. B. (2011) Biodegradable Blend Nanoparticles of Amphiphilic Diblock Copolymers Prepared by Nano-Precipitation Method, Journal of Biomaterials and Nanobiotechnology. 2 561-566.


